Introduction
Ovarian cancer is one of the most serious malignant
tumors in the female reproductive system. In the worldwide more
than 125,000 cases died of ovarian cancer (1), while in China, the incidence of ovarian
cancer has risen by 30% in recent years (2). At present, the primary clinical problem
is to improve early diagnosis of patients and the efficacy of
ovarian cancer treatment. In recent years, surgery combined with
drug treatment has improved patient prognosis, however the 5-year
survival rate of advanced ovarian cancer remains <30% (3,4).
Therefore, there is a strong imperative to search for novel
therapeutic strategies to treat ovarian cancer.
Efforts are being made to develop a novel
anti-cancer drug from natural compounds. Some compounds used in
traditional Chinese medicine are known to exhibit antitumor
activity with few side effects (5).
Gambogic acid (GA), which is used in traditional medicine, exerts
its anti-tumor effect through various mechanisms (6). Previous studies have suggested that GA
induces cell cycle arrest and apoptosis (7), inhibits the activity of telomerase,
angiogenesis and tumor metastasis, and decreases resistance to
multiple chemotherapy drugs (8,9). The
effect of GA is thought to be highly selective, in one study it was
found to induce apoptosis only in tumor cells (10). However, the mechanism of action of GA
on tumor cells remains unclear.
Evidence from previous studies suggests that the
NF-κB signaling pathway is closely related to tumor formation via
different mechanisms including cell apoptosis, cell cycle arrest,
cell differentiation and migration (11). The NF-κB family consists of the
transcription factors RELA/NF-κBp65 (p65), RELB, c-Rel, NF-κB1
(p50), and NF-κB2 (p52), of which p65 is the most widely studied
member. In resting cells, p65 remains in the cytoplasm combined
with the inhibitory protein IκB. However, some factors such as
mitogen are able to stimulate the movement of p65 into the nucleus,
where it promotes the transcription and translation of important
genes that regulate cell proliferation and apoptosis (12). The aim of the present study was to
investigate whether p65 exerts an important role in the action of
GA on ovarian cancer tumors.
Materials and methods
Cell culture
Human ovarian adenocarcinoma cancer cells from the
SKOV3 cell line were obtained from the cell bank of the Shanghai
Institute of Biochemistry and Cell Biology (Shanghai, China). Cells
were cultured in the RPMI 1640 medium (Invitrogen; Thermo Fisher
Scientific, Inc. Waltham, MA, USA) including 10% fetal bovine serum
(FBS; HyClone; GE Healthcare Life Sciences, Logan, UT, USA) in an
environment of 5% CO2 at 37°C. For cell cycle analysis,
the cells were synchronized to G0 by serum starvation
for 72 h prior to treatment with GA.
Reagents
Propidium iodide (PI) and RNAase used in the cell
cycle analysis were obtained from Sigma-Aldrich (St. Louis, MO,
USA). The cell counting kit-8 (CCK-8) was purchased from Dojindo
Molecular Technologies Inc. (Kumamoto, Japan). The p65 DNA activity
kit was purchased from Cayman Chemical Company (Ann Arbor, MI,
USA). Caspase-3, caspase-9, p65, phospho-p65 and β-actin antibodies
were obtained from Cell Signaling Technology, Inc. (Danvers, MA,
USA).
Cell survival analysis
The cell count kit analysis was detected by the
protocol of methods as described previously (13). i) After cultivation in an incubator,
the cell suspension was inoculated in 96-well plates (100 µl/well),
ii) 10 µl of CCK-8 reagent was added to each well. iii) The culture
plate was incubated in the incubator for 2 h. iv) The absorbance
value was measured at 450 nm using the Multiskan™ GO Microplate
Spectrophotometer (Thermo Fisher Scientific, Inc.). v) Calculation
of cell survival rate was carried out using the formula: Cell
survival (%) = (Vdrug - Vcontrol)
/(Vdrug=0 - Vcontrol) × 100%.
Cell cycle and apoptosis analysis
SKOV3 cells were treated with varying concentrations
of GA for 24 h. After the cells were harvested, 70% alcohol was
used to fix the cells overnight. The fixed cells were washed with
phosphate-buffered saline (PBS), then 150 µl RNAase (0.1 mg/ml) was
added for 30 min at 4°C. Following this, 120 µl PI (0.1 mg/ml) was
added and annexin V staining was carried out for 10 min, at 4°C.
Finally, the cells were analyzed for DNA content distribution by
flow cytometry (FCM).
Western blot analysis
SKOV3 cells were harvested following treatment with
varying concentrations of GA. Protein separation was carried out by
sodium dodecyl sulphate-polyacrylamide gel electrophoresis
(SDS-PAGE) as described previously (14,15). The
specific antibodies caspase-3 (1:800) and caspase-9 (1:800) came
from the Apoptosis Antibody Sampler kit (cat. no. 9915T; Cell
Signaling Technology, Inc.), the specific antibodies p65 (1:800)
and phospho-p65 (1:800) came from the NF-κB p65 Antibody Sampler
kit (cat. no. 4767T; Cell Signaling Technology, Inc.), and were
used to detect the protein levels of indicated cells. β-actin
served as an internal reference.
Analysis of p65 DNA activity
p65 DNA activity analysis was performed following
the previously defined protocol (16). i) 10 µg of nuclear extract was added
to the cell culture plate, which had already been incubated with
100 µl of p65 DNA sequence overnight at 4°C). ii) Each well was
washed five times with 200 µl wash buffer. iii) 100 µl diluted goat
anti-rabbit secondary antibody (1:1,500; cat. no. 7074; Cell
Signaling Technology, Inc.) was added for 1 h. iv) Each well was
washed five times with 200 µl wash buffer. v) 100 µl of developing
solution per well was added for 45 min. vi) 100 µl of stop solution
per well was added for 5 min. vii) Absorbance was measured at 450
nm.
Murine xenograft model
Equal numbers of SKOV3 cells (2×106) were
harvested at the log growth phase. Tumor xenografts were
established by subcutaneous fat pad injections into athymic mice.
The tumor volumes were monitored as described previously (17). After sixty days, the mice were
sacrificed.
GA therapy was initiated ten days after subcutaneous
fat pad injections were performed. 3.2 µM of GA was administered to
the mice intraperitoneally every 2 days. All animal procedures were
performed according to the 1998 Guide for the Care and Use of
Medical Laboratory Animals (Ministry of Health, Beijing, China),
and with the ethical approval of the Dongda Hospital Animal Care
and Use Committee (Xi'an, China; permit no. SYXK 2013–0024) and the
Ethical Committee of Southeast University (Nanjing, China; permit
no. 2013008).
Statistical analysis
All statistical analyses were performed using Graph
Pad software (version 5; GraphPad Software Inc., La Jolla, CA,
USA). A one-way ANOVA statistical test was also carried out.
P<0.05, was considered to indicate a statistically significant
difference.
Results
GA inhibited the growth of ovarian
cancer cells in a dose and time dependent manner
The SKOV3 cells were treated with varying
concentrations of GA (0, 0.8, 1.6, 3.2, 6.4, 12.8, 25.6, 51.2 µM).
After 24 h, the cell survival rate was analyzed using the CCK-8
assay. The results demonstrated that GA inhibited SKOV3 cell growth
in a dose-dependent manner (Fig. 1A).
The SKOV3 cells were then treated with 3.2 µM GA, and cell survival
rate was analyzed at set times (0, 30, 60, 120 and 240 min) by
CCK-8. GA was also found to inhibit SKOV3 cell growth in a
time-dependent manner (Fig. 1B).
GA arrested the cell cycle in ovarian
cancer cells
To further study the mechanism of GA on the SKOV3
cells, cell cycle analysis was performed using FCM. The cells were
treated with varying concentrations of GA (0, 3.2, and 6.4 µM).
After 24 h, the cells were harvested. The results of the cell cycle
analysis indicated that a higher proportion of the cells treated
with GA were arrested in the S-phase compared to control cells
(Fig. 2). This demonstrates that
treatment of GA inhibited cell growth by arresting ovarian cancer
cells in the S-phase.
GA increased the activity of caspase-3
and caspase-9 in ovarian cancer cells
To study whether caspase activity was involved in
the GA-induced growth arrest in SKOV3 cells, protein levels of
active caspase-3 and active caspase-9 were measured before and
after GA treatment. The preceding results suggested that the
vehicle could not exert an influence on the survival of SKOV3
cells, therefore western blot analysis was performed to compare the
levels of active caspase-3 and caspase-9 before and after GA
treatment (Fig. 3). The results
obtained show that GA treatment increased the expression of active
caspase-3 and active caspase-9, whilst overall capsase-3 and
capsase-9 levels remained unchanged, demonstrating that GA
inhibited cell growth by upregulating caspase-3 and caspase-9
activity.
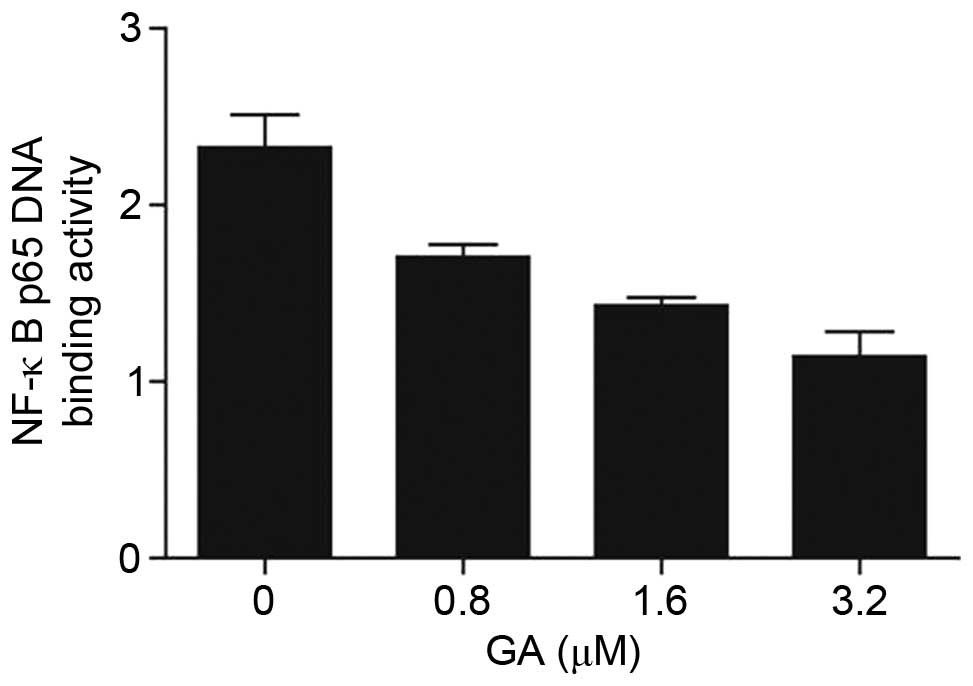
GA inhibited p65 DNA binding activity
in ovarian cancer cells
Evidence from previous studies suggests that the
NF-κB signaling pathway is closely related to tumor formation via
different mechanisms including cell apoptosis, cell cycle arrest,
cell differentiation and migration (11). In the current study, phosphorylated
p65 (p-p65) expression decreased after treatment with GA.
Furthermore, p65 DNA binding activity markedly decreased when
treated with increasing concentrations of GA (Fig. 4). Taken together, these results
suggest that GA inhibited the cell survival of ovarian cancer cells
by suppressing p65 DNA binding activity.
GA suppressed tumor growth in
vivo
Finally, tumor xenograft experiments were conducted
in nude mice in order to detect whether GA could suppress tumor
growth in vivo. The results showed that the tumors in
GA-treated mice were significantly smaller than in control mice or
mice treated with a vehicle (Fig. 5;
P<0.05). These results indicated that GA inhibited tumor growth
in a mouse model.
Discussion
Due to the high incidence of patient relapse and
adverse reactions caused by the chemotherapy drugs currently used
to treat ovarian cancer, discovering new therapeutic strategies to
improve treatment efficacy is important. Our previous study
demonstrated that ovarian cancer was a serious cancer of the female
reproductive system (18,19). However, some compounds used in
traditional Chinese medicine may play an important role in treating
cancer. GA is one such compound with multiple targets that exhibits
anti-tumor activity (20,21). In the current study, GA was identified
to inhibit the growth of ovarian cancer tumors both in vivo
and in vitro. Furthermore, GA also inhibited the growth of
ovarian cancer tumors by arresting the cell cycle. The results
indicate that GA inhibits p65 DNA binding activity, thus inhibiting
cell proliferation in ovarian cancer tumors.
Future studies should focus on uncovering the
mechanism of action of GA on ovarian cancer tumors. Normal cell
survival depends on the cell cycle operating normally and is
controlled by the precise mechanism of cell cycle regulators
(22). Tumor growth is thought to
arise from dysregulation of the cell cycle.
GA induced apoptosis in tumor cells with few adverse
reactions, unlike radiotherapy and chemotherapy. The present study
suggests that GA may be important in regulating the cell cycle of
tumor cells by arresting at S-phase. Additionally, these results
also suggest that some compounds used in traditional Chinese
medicine may be candidates for the development of novel therapeutic
strategies for a variety of diseases.
GA exhibits antitumor activity within the scope of
the effective dose and seems to cause few side effects in the
hemopoietic system and does not seem to adversely affect the immune
function of normal cells. These advantages are currently not
possessed by other tumor chemotherapy drugs. In the current study,
GA inhibited the cell survival of SKOV3 cells by arresting the cell
cycle in G2/M phase and inducing apoptosis, suggesting that GA
could be developed as a novel treatment of ovarian cancer.
NF-κB is a class of dimer transcription factor that
recognizes and binds to DNA. Stimulated of the cells by various
internal and external factors activated NF-κB, promoting the
expression of a series of anti-apoptotic genes. Previous studies
have demonstrated that the NF-κB signaling pathway plays a crucial
role in the development of various tumors (23–25).
However, it remains unknown whether GA acts on ovarian cancer tumor
cells by stimulating the NF-κB signaling pathway, to induce
apoptosis. The current study demonstrated that GA inhibited p65 DNA
binding activity, suggesting p65 plays a key role in the mechanism
of action of GA.
In conclusion, the present study demonstrates that
GA inhibited the growth of ovarian cancer in vivo and in
vitro by arresting the cell cycle and inducing apoptosis. The
results indicate that GA may act by inhibiting p65 DNA binding
activity, thus inhibiting cell proliferation in ovarian cancer
tumors.
Acknowledgements
The present study was supported by the National
Natural Scientific Foundation of China (grant nos. 30872999;
81372480); the Six Talents Peak of Jiangsu province of China,
(grant no. 2010-ws-062) Revitalize (grant no. RC2011033). The
project was also funded by the Wuxi hospital management center
medical technology development fund (grant no. YGM1401), and the
Wuxi science and technological developmental project (grant no.
CSE31N1427), to defend the key talent's subsidy project in science
and education of department of public health of Jiangsu
Province.
References
|
1
|
Perets R, Wyant GA, Muto KW, Bijron JG,
Poole BB, Chin KT, Chen JY, Ohman AW, Stepule CD, Kwak S, et al:
Transformation of the fallopian tube secretory epithelium leads to
high-grade serous ovarian cancer in Brca; Tp53; Pten models. Cancer
Cell. 24:751–765. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jianrong He, Xi Gao and Zefang Ren: The
distributed characteristic of female breast and ovarian cancer in
the global. Chinese Cancer. 18:169–172. 2009.(In Chinese).
|
|
3
|
Yang D, Sun Y, Hu L, Zheng H, Ji P, Pecot
CV, Zhao Y, Reynolds S, Cheng H, Rupaimoole R, et al: Integrated
analyses identify a master microRNA regulatory network for the
mesenchymal subtype in serous ovarian cancer. Cancer Cell.
23:186–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang N, Li WW, Li JP, Liu JY, Zhou YC,
Zhang Y, Hu J, Huang YH, Chen Y, Wei LC and Shi M: Comparison of
concurrent chemoradiotherapy followed by radical surgery and
high-dose-rate intracavitary brachytherapy: A retrospective study
of 240 patients with FIGO stage IIB cervical carcinoma. Onco
Targets Ther. 7:91–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tianmin X, Weiqin C, Shuying W, Yang L and
Manhua C: Protection of ovarian function during chemotherapy for
ovarian cancer. Eur J Gynaecol Oncol. 35:562–565. 2014.
|
|
6
|
Wang J and Yuan Z: Gambogic acid
sensitizes ovarian cancer cells to doxorubicin through ROS-mediated
apoptosis. Cell Biochem Biophys. 67:199–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang F, Zhang W, Guo L, Bao W, Jin N, Liu
R, Liu P, Wang Y, Guo Q and Chen B: Gambogic acid suppresses
hypoxia-induced hypoxia-inducible factor-1α/vascular endothelial
growth factor expression via inhibiting phosphatidylinositol
3-kinase/Akt/mammalian target protein of rapamycin pathway in
multiple myeloma cells. Cancer Sci. 105:1063–1070. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wen J, Pei H, Wang X, Xie C, Li S, Huang
L, Qiu N, Wang W, Cheng X and Chen L: Gambogic acid exhibits
anti-psoriatic efficacy through inhibition of angiogenesis and
inflammation. J Dermatol Sci. 74:242–250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Xiang W, Wang M, Huang T, Xiao X,
Wang L, Tao D, Dong L, Zeng F and Jiang G: Methyl jasmonate
sensitizes human bladder cancer cells to gambogic acid-induced
apoptosis through down-regulation of EZH2 expression by miR-101. Br
J Pharmacol. 171:618–635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi X, Chen X, Li X, Lan X, Zhao C, Liu S,
Huang H, Liu N, Liao S, Song W, et al: Gambogic acid induces
apoptosis in imatinib-resistant chronic myeloid leukemia cells via
inducing proteasome inhibition and caspase-dependent Bcr-Abl
downregulation. Clin Cancer Res. 20:151–163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu Y, Wan Y and Huang C: The biological
functions of NF-kappaB1 (p50) and its potential as an anti-cancer
target. Curr Cancer Drug Targets. 9:566–571. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bock FJ, Peintner L, Tanzer M, Manzl C and
Villunger A: P53-induced protein with a death domain (PIDD): Master
of puppets? Oncogene. 31:4733–4739. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fei M, Lu M, Wang Y, Zhao Y, He S, Gao S,
Ke Q, Liu Y, Li P, Cui X, et al: Arsenic trioxide-induced growth
arrest of human hepatocellular carcinoma cells involving FOXO3a
expression and localization. Med Oncol. 26:178–185. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu M, Fei M, Cheng C, Wang Y, He S, Zhao
Y, Gao S, Ke Q, Li P, Cui X and Shen A: Mutant p27 (Kip1) and its
potential effect as hepatocellular gene therapy. Arch Med Res.
39:573–581. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu M, Ma J, Xue W, Cheng C, Wang Y, Zhao
Y, Ke Q, Liu H, Liu Y, Li P, et al: The expression and prognosis of
FOXO3a and Skp2 in human hepatocellular carcinoma. Pathol Oncol
Res. 15:679–687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gui J, Yue Y, Chen R, Xu W and Xiong S:
A20 (TNFAIP3) alleviates CVB3-induced myocarditis via inhibiting
NF-κB signaling. PLoS One. 7:e465152012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Wang Y, Xiang J, Ji F, Deng Y,
Tang C, Yang S, Xi Q, Liu R and Di W: Knockdown of CRM1 inhibits
the nuclear export of p27 (Kip1) phosphorylated at serine 10 and
plays a role in the pathogenesis of epithelial ovarian cancer.
Cancer Lett. 343:6–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu M, Zhao Y, Xu F, Wang Y, Xiang J and
Chen D: The expression and prognosis of FOXO3a and Skp2 in human
ovarian cancer. Med Oncol. 29:3409–3415. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu M, Xiang J, Xu F, Wang Y, Yin Y and
Chen D: The expression and significance of pThr32-FOXO3a in human
ovarian cancer. Med Oncol. 29:1258–1264. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao W, You CC, Zhuang JP, Zu JN, Chi ZY,
Xu GP and Yan JL: Viability inhibition effect of gambogic acid
combined with cisplatin on osteosarcoma cells via
mitochondria-independent apoptotic pathway. Mol Cell Biochem.
382:243–252. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu N, Hui H, Yang H, Zhao K, Chen Y, You
QD and Guo QL: Gambogic acid inhibits angiogenesis through
inhibiting PHD2-VHL-HIF-1α pathway. Eur J Pharm Sci. 49:220–226.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ng AJ, Walia MK, Smeets MF, Mutsaers AJ,
Sims NA, Purton LE, Walsh NC, Martin TJ and Walkley CR: The DNA
helicase recql4 is required for normal osteoblast expansion and
osteosarcoma formation. PLoS Genet. 11:e10051602015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mumblat Y, Kessler O, Ilan N and Neufeld
G: Full length semaphorin-3C is an inhibitor of tumor
lymphangiogenesis and metastasis. Cancer Res. 75:2177–2186. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Q, Liu Z, Ren J, Morgan S, Assa C and
Liu B: Receptor-interacting protein kinase 3 contributes to
abdominal aortic aneurysms via smooth muscle cell necrosis and
inflammation. Circ Res. 116:600–611. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qu Y, Zhang X and Wu R: Knockdown of NF-κB
p65 subunit expression suppresses growth of nude mouse lung tumor
cell xenografts by activation of Bax apoptotic pathway. Neoplasma.
62:34–40. 2015. View Article : Google Scholar : PubMed/NCBI
|