Introduction
Angiogenesis is a complex multistep biological
process in which new blood vessels are formed (1). Angiogenesis is required in numerous
normal physiological processes (including wound healing,
embryogenesis and normal ovarian function) as well as in the
pathogenesis of many disorders (including malignant tumors)
(2). Angiogenesis is regulated by a
balance between pro- and anti-angiogenic molecules. However, in
cancer, angiogenesis is dysregulated (2,3). In normal
tissue, new blood vessels are predominantly well differentiated,
while in tumors, both differentiated and undifferentiated new blood
vessels are observed. In cancer patients, a poor prognosis is
correlated with poor differentiation of new blood vessels (4,5).
Angiogenesis is crucial for tumor growth. Therefore,
creating therapies to target angiogenesis is a popular area of
investigation. A number of anti-angiogenic drugs have been
developed, including monoclonal antibodies and synthetic tyrosine
kinase inhibitors (6,7). Anti-angiogenic agents are approved for
use in various types of cancer. Numerous patients have benefited
from these inhibitors (8). One of
these inhibitors, endostatin, has been observed to significantly
suppress angiogenesis and tumor growth in a number of cancers,
including Lewis lung cancer (9).
However, endostatin, as well as other angiogenesis inhibitors,
exhibits host toxicity, possibly due to the role angiogenesis plays
in normal tissues.
Anti-angiogenic therapies are capable of improving
chemotherapy efficacy by causing ‘vessel normalization’ in tumors
(8). It has been observed that
anti-angiogenic therapies transiently normalize the tumor
vasculature (10,11). For example, recominant human
(rh-)endostatin (Endostar) normalizes the tumor vasculature and
microenvironment in Lewis lung carcinoma (12). However, the mechanism of vessel
normalization is unclear. We hypothesized that vessel normalization
may result from the different responses of differentiated and
undifferentiated tumor blood vessels to the anti-angiogenic
therapies. Currently, the effects of anti-angiogenic therapies on
differentiated or undifferentiated vessels have not been
determined.
Tumor angiogenesis is measured by microvessel
density. Two blood vessel markers, CD31 and CD34, are used to
reveal different characteristics of the tumor vasculature. CD31 is
expressed in all microvessels (i.e. undifferentiated and
differentiated) (13,14), while CD34 is highly expressed in the
normal vascular endothelium, and is therefore a sensitive marker of
differentiated, well-formed vessels (15,16).
In the present study, we investigated the response
of differentiated and undifferentiated vasculature to rh-endostatin
in Lewis lung carcinoma by measuring the microvessel density. We
also observed the normalization of tumor vessels using two-photon
confocal microscopy.
Materials and methods
Cell culture and animal model
The Lewis lung carcinoma cell line was purchased
from the Laboratory of Immunology, Shandong Academy of Medical
Sciences, Jinan, China, and maintained in 10% fetal bovine
serum.
Forty female specific pathogen-free C57BL/6 mice
(Vital River Laboratories Ltd., Beijing, China) were selected for
this study. The mice were 5–7 weeks of age and weighed 17–19 g. The
mice were housed in groups of three and kept on a 12 h light/dark
cycle with free access to food and water. To establish tumors,
2×106 Lewis lung carcinoma cells were subcutaneously
injected into the right flank of each mouse. All experiments were
approved by the Institute Animal Care and Committee of Shandong
University.
Treatment protocol
Rh-endostatin was provided by Shandong
Simcere-Medgenn Bio-pharmaceutical Company (Yantai, China). Once
the tumors reached a length of 6–7 mm, the mice were randomly
assigned to four groups. Subcutaneous injections of rh-endostatin
were administered daily at 5, 25 and 50 mg/kg for 14 days.
Physiological saline was administed to the control group. Body
weights were recorded daily. Tumor volumes were estimated daily
using the formula 0.52 × length (mm) × width (mm2), in
which the length and perpendicular width were measured by calipers
(12). After 14 days, the mice were
sacrificed and the tumors were harvested, fixed in 10% formalin,
and embedded in paraffin for subsequent experiments.
Intravital microscopy
To visualize functional blood vessels in the C57BL/6
mice, fluorescein isothiocyanate-dextran (FD2000S; Sigma-Aldrich,
St. Louis, MO, USA) was injected following the 14-day rh-endostatin
treatment. The dye was injected immediately prior to observation
using two-photon confocal microscopy (17). The two-photon confocal microscope was
comprised of an FV 300 laser confocal microscope (Olympus
Corporation, Tokyo, Japan) with a 60X objective and photomultiplier
tubes. A Ti:Sapphire laser source (Coherent, Inc., Santa Clara, CA,
USA) with an excitation wavelength of 900 nm was used in the
two-photon experiments. Normal skeletal muscle vessels were also
observed as the normal control.
Immunohistochemistry
Four-micron serial sections were cut from the blocks
of each mouse tumor. The slides were deparaffinized in xylene and
rehydrated through a series of graded alcohol. High-temperature
antigen retrieval was performed in a citrate salt antigen repair
solution for 10 min in a microwave oven. After cooling to room
temperature, the slides were incubated in blocking serum for 30
min. Primary anti-CD31 antibody (1:100, rat monoclonal, ab56299;
Abcam, Cambridge, MA, USA) and anti-CD34 antibody (1:50, rat
monoclonal, ab8158, Abcam) were applied. The slides were incubated
overnight at 4°C in a high humidity chamber. Certain sections were
incubated in phosphate-buffered saline as a negative control. After
washing, the tissue sections were treated with biotinylated
goat-anti-rat secondary antibody (Zhongshan Biotechnology Company,
Beijing, China) and further incubated with streptavidin-horseradish
peroxidase complex for 20 min. Sections were finally stained with
diaminobenzidine and counterstained with hematoxylin and eosin.
Microvessel density
Microvessel density (MVD) was determined according
to the method described by Huang and Chen (12). Briefly, two researchers independently
assessed MVD. Any CD31+ or CD34+ endothelial
cells or cell clusters that were clearly separated from the
surrounding tumor and stromal cells were counted as microvessels.
The sections were screened at lower magnifications (x100) to
identify five vascularized areas. Within the selected areas,
microvessels were counted under high magnification (x400). The MVD
was the average number of microvessels in the five fields.
Discrepancies were resolved through discussion and reviewing the
section.
Statistical analysis
Data were expressed as the means ± standard error.
The Statistical Package for the Social Sciences (SPSS) version 19.0
(IBM SPSS, Armonk, NY, USA) was used for statistical analysis.
Statistical significance was determined by one-way analysis of
variance. The least significant difference test was applied for
multiple means comparisons. P<0.05 was considered to represent a
statistically significant difference.
Results
Rh-endostatin inhibits tumor growth in
a dose-dependent manner
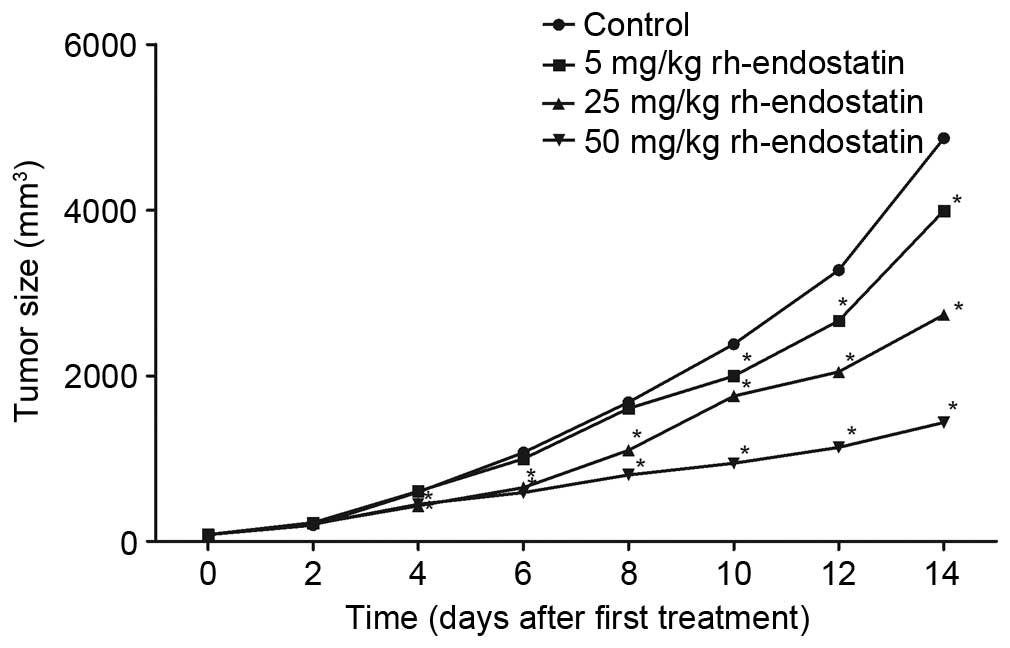
Following the 14-day treatment with rh-endostatin,
tumor growth was inhibited (Fig. 1).
Tumor volumes were significantly reduced in the rh-endostatin
groups compared with those in the control group (3,991.5±761.9,
2,735.1±558.3 and 1,433.9±275.3 mm3, respectively, in
the 5, 25 and 50 mg/kg groups vs. 4,869.8±990.0 mm3 in
the control group; P<0.05). Treatment with 50 mg/kg
rh-endostatin was significantly more effective in tumor growth
inhibition than the other two doses (P<0.05). This data confirms
that rh-endostatin effectively inhibits tumor growth.
Rh-endostatin normalizes the
architecture of the tumor vasculature
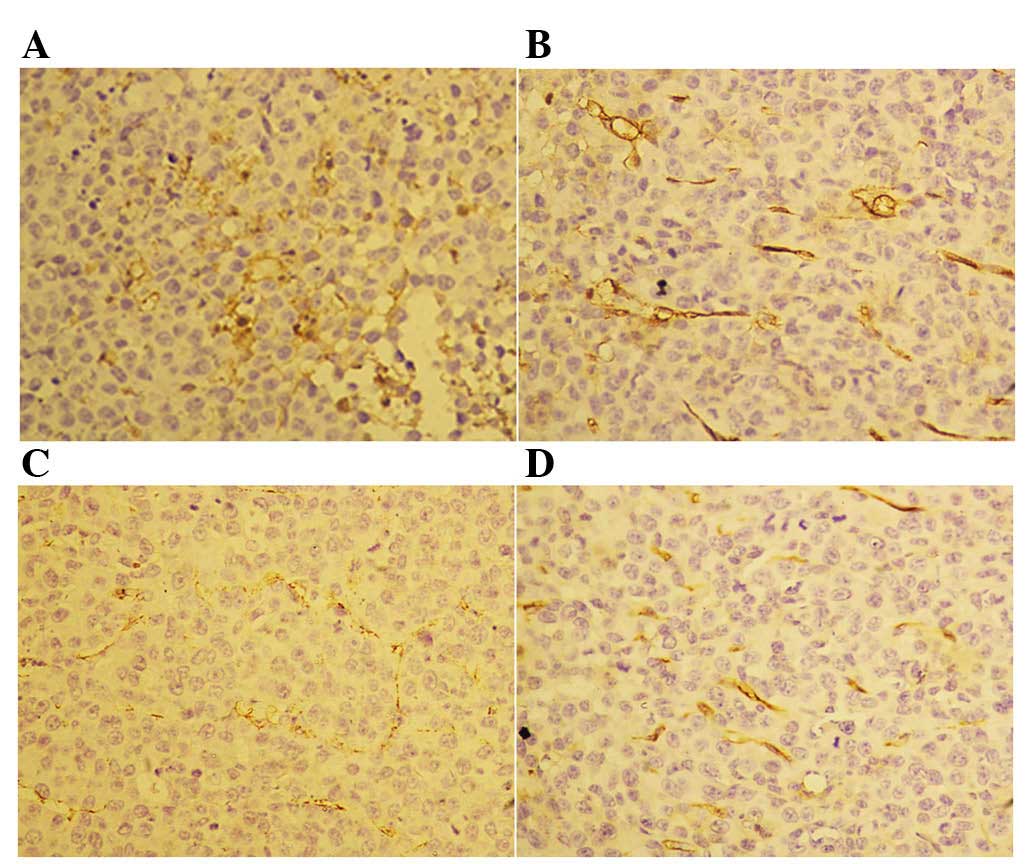
We examined the effect of rh-endostatin on the
morphology of blood vessels in the Lewis lung cancer tumors by
confocal microscopy. Normal skeletal muscle has an organized
vasculature with a relatively smooth vascular wall and uniform
diameter (Fig. 2A). In contrast, the
Lewis lung cancer tumor in the control group had abundant tortuous
vessels with abrupt changes in vessel diameter and a number of
extremely small vessels (Fig. 2B).
However, in the tumors treated with rh-endostatin, the vessels
became less tortuous and more regular, and assumed a relatively
normal morphology as the dose increased (Fig. 2C-E). These data provide further
evidence that rh-endostatin normalizes the architecture of the
vascular network.
Rh-endostatin affects differentiated
and undifferentiated blood vessels differently
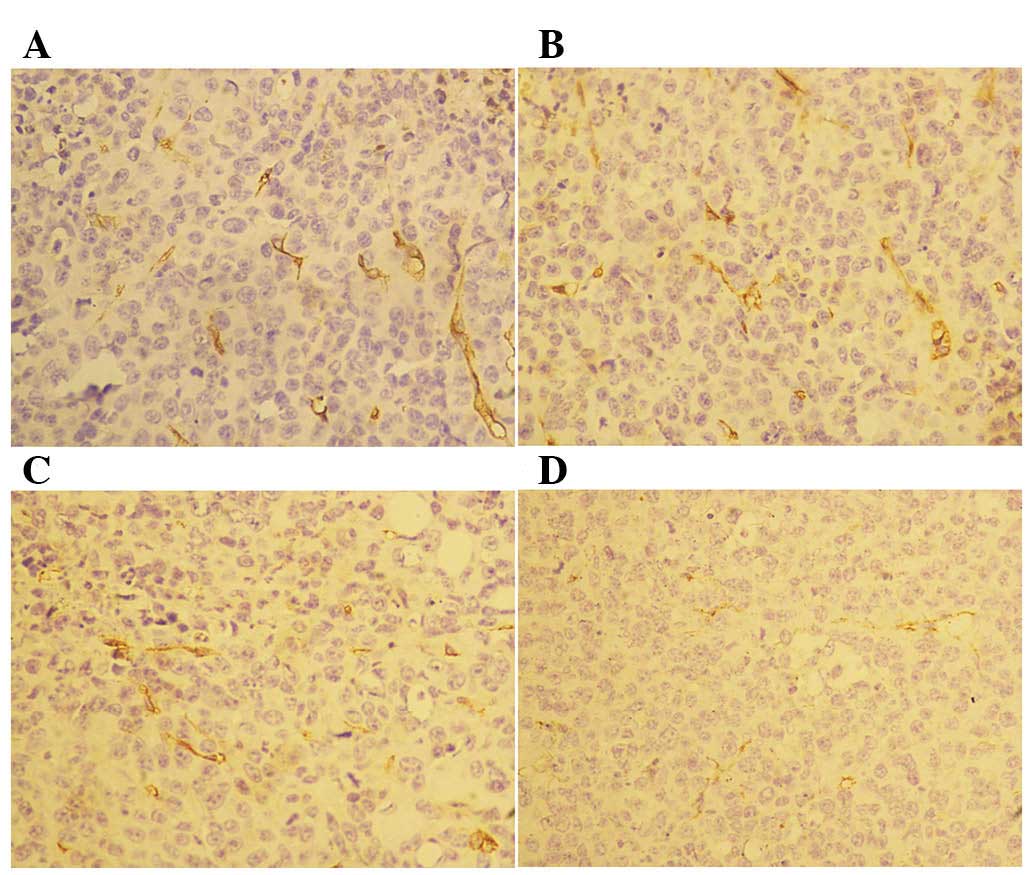
Following the 14-day treatment with rh-endostatin,
we observed a decreased number of CD31+ cells in the
tumor blood vessels (Fig. 3).
However, we did not observe any differences in CD34 staining
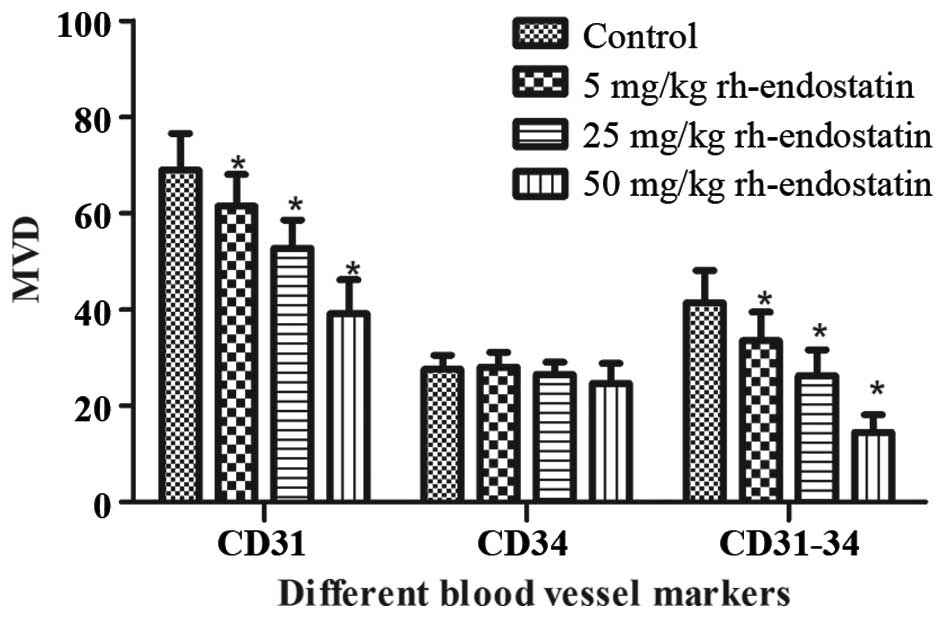
between the control and rh-endostatin-treated groups (Fig. 4). The MVD of CD31+ cells
significantly decreased following treatment with rh-endostatin
(61.6±6.53, 52.8±5.8 and 39.2±6.94, respectively, in the 5, 25 and
50 mg/kg rh-endostatin groups vs. 69.0±7.62 in the control group;
P<0.05) (Fig. 5). This decrease
occurred in a dose-dependent manner. Furthermore, the MVD of
CD34+ cells was similar in the treatment and control
groups (28.0±3.15, 26.5±2.54 and 24.7±4.13, respectively, in the 5,
25 and 50 mg/kg rh-endostatin groups vs. 27.5±2.98 in the control
group; P>0.05) (Fig. 5).
According to previous studies, undifferentiated
blood vessels may be quantified by CD31+CD34−
cells or by subtracting the CD34+ MVD value from the
CD31+ MVD value (4,18). We
observed that the number of undifferentiated blood vessels
(CD31-CD34) was significantly decreased following rh-endostatin
treatment (Fig. 5). Accordingly, the
decrease occurred in a dose-dependent manner (33.5±5.97, 26.3±5.33
and 14.5±3.61, respectively, in the 5, 25 and 50 mg/kg
rh-endostatin groups vs. 41.44±6.62 in the control group;
P<0.01).
Discussion
In the present study, we evaluated the effects of
recombinant human (rh-)endostatin treatment on differentiated and
undifferentiated tumor vasculature in Lewis lung cancer for the
first time. We detected a change in tumor microvessel density, the
‘gold standard’ of assessing tumor angiogenesis, following
treatment with varying doses of rh-endostatin. The tumor blood
vessel cells with positive CD31 staining represent both
differentiated and undifferentiated endothelial cells, whereas
cells with positive CD34 staining represent only differentiated
endothelial cells (4,18). Following rh-endostatin treatment,
CD31+ cells significantly decreased in a dose-dependent
manner. However, no significant reduction in differentiated
neovascularization (CD34+ cells) was observed. These
data led us to conclude that rh-endostatin primarily inhibited the
undifferentiated (CD31+CD34−)
neovascularization. This is the first study to observe the
differential effects of rh-endostatin on differentiated and
undifferentiated neovascularization.
The role played by endostatin, an efficient
anti-angiogenic and antitumor molecule, in angiogenesis inhibition
is still unclear. It may directly affect cells undergoing
angiogenesis or regulate the secretion of various growth factors
(9,19–21).
Endostatin was first isolated from the supernatant of a murine
hemangio-endothelioma, and it almost completely suppressed the
formation of new blood vessels (9).
Since then, numerous studies have investigated the effect of
endostatin on angiogenesis (19–21) and on
tumor vessel normalization (12,22).
However, none of these studies focused on the differential effects
of rh-endostatin on differentiated and undifferentiated blood
vessels. Our research suggests that rh-endostatin has a stronger
effect on undifferentiated blood vessels than on differentiated
blood vessels. This result provided further insight into the
mechanism of tumor vessel normalization by endostatin.
In addition, we directly observed the effect of
rh-endostatin on tumor vasculature in Lewis lung cancer using
intravital two-photon confocal microscopy. Imaging revealed that
following rh-endostatin treatment, the tumor vessels were similar
to normal vessels. Tumor microvessels may be observed directly in
real time and in vivo by two-photon microscopy (17). Several studies have demonstrated tumor
vessel normalization by anti-angiogenic drugs using multi-photon
confocal microscopy. For example, Tong et al (23) demonstrated the normalization process
in four tumor types following DC101 treatment. In addition, von
Baumgarten et al (24)
observed the normalization of glioma blood vessels following
bevacizumab treatment. However, the present study is the first to
report the use of intravital confocal microscopy to visualize tumor
vessel normalization in Lewis lung cancer following endostatin
treatment.
In conclusion, we observed that rh-endostatin
significantly inhibited the formation of undifferentiated
vasculature (CD31+/CD34−) but did not inhibit
the formation of differentiated vasculature
(CD31+/CD34+). Normalization of the tumor
blood vessels was also observed. Taken together, these results
suggest that normalization of the tumor vasculature by endostatin
may be related to the differential effects of endostatin on
differentiated and undifferentiated blood vessels. Further study
into the mechanism of tumor vessel normalization by endostatin is
required in order to apply these findings to the clinic.
Acknowledgements
This study was supported by the Shandong Province
Science and Technology Development Plan [2014GGE27076]. The authors
wish to thank Dr. Edward C. Mignot from Shandong University for his
linguistic advice.
References
|
1
|
Gacche RN and Meshram RJ: Targeting tumor
micro-environment for design and development of novel
anti-angiogenic agents arresting tumor growth. Prog Biophys Mol
Biol. 113:333–354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wietecha MS, Cerny WL and DiPietro LA:
Mechanisms of vessel regression: toward an understanding of the
resolution of angiogenesis. Curr Top Microbiol Immunol. 367:3–32.
2013.PubMed/NCBI
|
|
3
|
Zhang Y, Yu LK, Lu GJ, Xia N, Xie HY, Hu
W, Hao KK, Xu CH and Qian Q: Prognostic values of VEGF and
endostatin with malignant pleural effusions in patients with lung
cancer. Asian Pac J Cancer Prev. 15:8435–8440. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yao X, Qian CN, Zhang ZF, Tan MH, Kort EJ,
Yang XJ, Resau JH and Teh BT: Two distinct types of blood vessels
in clear cell renal cell carcinoma have contrasting prognostic
implications. Clin Cancer Res. 13:161–169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qi L, Du J, Zhang Z, Diao L, Chen X and
Yao X: Low differentiated microvascular density and low expression
of platelet-derived growth factor-BB (PDGF-BB) predict distant
metastasis and poor prognosis in clear cell renal cell carcinoma.
BJU Int. 112:E415–E423. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fontanella C, Ongaro E, Bolzonello S,
Guardascione M, Fasola G and Aprile G: Clinical advances in the
development of novel VEGFR2 inhibitors. Ann Transl Med.
2:1232014.PubMed/NCBI
|
|
7
|
Welti J, Loges S, Dimmeler S and Carmeliet
P: Recent molecular discoveries in angiogenesis and antiangiogenic
therapies in cancer. J Clin Invest. 123:3190–3200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jain RK: Antiangiogenesis strategies
revisited: from starving tumors to alleviating hypoxia. Cancer
Cell. 26:605–622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Reilly MS, Boehm T, Shing Y, Fukai N,
Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR and Folkman J:
Endostatin: an endogenous inhibitor of angiogenesis and tumor
growth. Cell. 88:277–285. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jain RK: Normalization of tumor
vasculature: an emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang Y, Yuan J, Righi E, Kamoun WS,
Ancukiewicz M, Nezivar J, Santosuosso M, Martin JD, Martin MR,
Vianello F, et al: Vascular normalizing doses of antiangiogenic
treatment reprogram the immunosuppressive tumor microenvironment
and enhance immunotherapy. Proc Natl Acad Sci USA. 109:17561–17566.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang G and Chen L: Recombinant human
endostatin improves anti-tumor efficacy of paclitaxel by
normalizing tumor vasculature in Lewis lung carcinoma. J Cancer Res
Clin Oncol. 136:1201–1211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giatromanolaki A, Koukourakis MI,
Theodossiou D, Barbatis K, O'Byrne K, Harris AL and Gatter KC:
Comparative evaluation of angiogenesis assessment with
anti-factor-VIII and anti-CD31 immunostaining in non-small cell
lung cancer. Clin Cancer Res. 3:2485–2492. 1997.PubMed/NCBI
|
|
14
|
Caraffi S, Corradi D, Campanini N, Govoni
P, Rocchi L, Perris R and Mangieri D: Microcirculation density and
maturity in uterine and soft tissue leiomyosarcomas: an
immunohistochemical study. Histol Histopathol. 30:69–76.
2015.PubMed/NCBI
|
|
15
|
Suster S and Wong TY: On the
discriminatory value of anti-HPCA-1 (CD-34) in the differential
diagnosis of benign and malignant cutaneous vascular
proliferations. Am J Dermatopathol. 16:355–363. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miyata Y, Mitsunari K, Asai A, Takehara K,
Mochizuki Y and Sakai H: Pathological significance and prognostic
role of microvessel density, evaluated using CD31, CD34, and CD105
in prostate cancer patients after radical prostatectomy with
neoadjuvant therapy. Prostate. 75:84–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fukumura D, Duda DG, Munn LL and Jain RK:
Tumor microvasculature and microenvironment: novel insights through
intravital imaging in pre-clinical models. Microcirculation.
17:206–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Poblet E, Gonzalez-Palacios F and Jimenez
FJ: Different immunoreactivity of endothelial markers in well and
poorly differentiated areas of angiosarcomas. Virchows Arch.
428:217–221. 1996.PubMed/NCBI
|
|
19
|
Barczyk M, Carracedo S and Gullberg D:
Integrins. Cell. 339:269–280. 2010.
|
|
20
|
Dong XP, Xiao TH, Dong H, Jiang N and Zhao
XG: Endostar combined with cisplatin inhibits tumor growth and
lymphatic metastasis of lewis lung carcinoma xenografts in mice.
Asian Pac J Cancer Prev. 14:3079–3083. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiao L, Yang S, Hao J, Yuan X, Luo W,
Jiang L, Hu Y, Fu Z, Zhang Y and Zou C: Endostar attenuates
melanoma tumor growth via its interruption of b-FGF mediated
angiogenesis. Cancer Lett. 359:148–154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng F, Xu Z, Wang J, Chen Y, Li Q, Zuo Y,
Chen J, Hu X, Zhou Q, Wang Y, et al: Recombinant human endostatin
normalizes tumor vasculature and enhances radiation response in
xenografted human nasopharyngeal carcinoma models. PLoS One.
7:e346462012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tong RT, Boucher Y, Kozin SV, Winkler F,
Hicklin DJ and Jain RK: Vascular normalization by vascular
endothelial growth factor receptor 2 blockade induces a pressure
gradient across the vasculature and improves drug penetration in
tumors. Cancer Res. 64:3731–3736. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
von Baumgarten L, Brucker D, Tirniceru A,
Kienast Y, Grau S, Burgold S, Herms J and Winkler F: Bevacizumab
has differential and dose-dependent effects on glioma blood vessels
and tumor cells. Clin Cancer Res. 17:6192–6205. 2011. View Article : Google Scholar : PubMed/NCBI
|