Introduction
Cluster of differentiation (CD) 44 transmembrane
glycoproteins are cell-adhesion molecules that are associated with
cancer cell aggressiveness and metastasis (1,2). In
numerous types of cancer, including gastric cancer, CD44 has been
associated with increased invasion, metastasis and poor prognosis
(3,4).
This molecule has also been identified as a marker of stem-like
gastric cancer cells (5,6); however, its role in this phenotype
remains to be defined.
Previous studies have suggested that hypoxia
provides a suitable niche for stem cells to maintain their
precursor status (7,8). Hypoxic tumor microenvironments induce
phenotypic changes that make cancer cells aggressive (9,10),
refractory to treatment (11) and
likely to metastasize (12). These
phenotypic changes are mediated by hypoxia-inducible factors (HIFs)
(12). HIF is a heterodimer
consisting of an oxygen-dependent α subunit and a constitutively
expressed β subunit (9). Previous
studies have demonstrated that HIF-1α is overexpressed in gastric
cancer (13–15); furthermore, HIF-1α is associated with
metastatic potential in gastric cancer cells via undefined
underlying mechanisms (10).
HIF-1α is a regulator of CD44 in breast cancer cells
under hypoxic conditions; HIF-1α increases CD44 expression levels
and the number of CD44-positive cells (16). In gastric cancer cells, a significant
correlation between HIF-1α expression levels and the
immunohistochemical staining pattern of CD133 has been observed
(17). However, whether HIF-1α
regulates CD44 expression levels in gastric cancer cells remains to
be established. The present study examined the effects of hypoxia
on HIF-1α and CD44 expression levels in the moderately
differentiated gastric cancer cell line SGC-7901 and in the poorly
differentiated gastric cancer cell line BGC-823. In addition, the
effects of HIF-1α downregulation on CD44 expression levels were
evaluated in these gastric cancer cell lines.
Materials and methods
Cell culture and hypoxia
treatment
The human gastric cancer cell lines SGC-7901 and
BGC-823 were obtained from the China Center for Type Culture
Collection (Wuhan, China). All cell lines were maintained in RPMI
1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal calf serum (Gibco; Thermo Fisher
Scientific, Inc.) under normoxic or hypoxic conditions for 7 days.
Subsequently, the cells were treated with 20 nM rapamycin
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) for 72 h at
37°C in a humidified atmosphere of 5% CO2 and 95% air.
Rapamycin was used to downregulate the expression levels of HIF-1α
(18). For hypoxic exposure, tumor
cells were incubated in an hypoxic incubator (BINDER GmbH,
Tuttlingen, Germany) containing 1% O2, which was
balanced by CO2 and nitrogen.
Cell proliferation assay
The Cell Counting kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) assay was used to evaluate
cell viability, according to the manufacturer's protocol. Cells
were seeded onto 96-well plates at a density of 5×103
cells/well and incubated for 24 h, and subsequently incubated in
culture medium containing 20 nM rapamycin at 37°C in a humidified
atmosphere of 5% CO2 and 95% air. CCK-8 solution (10 µl)
was added to each well at 24, 48 and 72 h. The color intensity was
evaluated using a microplate reader (Beijing Liuyi Biotechnology
Co., Ltd., Beijing, China) at an absorbance wavelength of 450 nm.
All experiments were performed in triplicate and repeated
independently three times.
Cell migration and invasion
assays
These assays were performed according to a
previously described protocol (19).
Briefly, Transwell units with 8.0-µm-pore polycarbonate filters
(Corning Incorporated, Corning, NY, USA) were precoated with 50 µl
of 1:5 diluted Matrigel (BD Biosciences, Franklin Lakes, NJ, USA).
A total volume of 200 µl of gastric cancer cell suspension, which
contained ~1×105 rapamycin-treated cells, was added to
the upper compartment of the precoated units. The units were then
transferred to the wells of the culture plate and incubated for 24
h at 37°C. Subsequently, the cells and Matrigel on the upper
surface of the membrane were removed. Cells that had migrated to
the underside were stained with 0.1% crystal violet for 15 min at
room temperature, and cell numbers were determined using light
microscopy. Five randomly selected fields were counted per
insert.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The gastric cancer cell lines were maintained with
medium containing 20 nM rapamycin under normoxic or hypoxic
conditions for 72 h. Total RNA was then isolated using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. The corresponding
complementary DNA (cDNA) was synthesized using the PrimeScript™ RT
Reagent kit (Takara Biotechnology Co., Ltd., Dalian, China).
RT-qPCR was performed using SYBR® premix Ex Taq™ (Takara
Biotechnology Co., Ltd.) and gene-specific primers in the
Rotor-Gene 3000 system (Qiagen, Inc., Valencia, CA, USA). The PCR
reaction mixtures contained 12.5 µl Premix Ex Taq™, 0.2 µM PCR
primers, 0.4 µM SYBR Green I and 0.08 µM cDNA. All primers were
designed using Primer Premier 5.0 (Premier Biosoft International,
Palo Alto, CA, USA), and their sequences and annealing temperatures
are presented in Table I. GAPDH was
used as the housekeeping gene for normalization of the mRNA
expression levels. Fold changes in the expression levels for each
mRNA were calculated using the 2−∆∆Cq method (20).
 | Table I.Oligonucleotide primer sequences and
reverse transcription-quantitative polymerase chain reaction
thermocycling conditions. |
Table I.
Oligonucleotide primer sequences and
reverse transcription-quantitative polymerase chain reaction
thermocycling conditions.
|
| Cycling
conditions |
|---|
|
|
|
|---|
| Target gene primer
sequence, 5′-3′ | Number of cycles | Annealing
temperature, °C (time) |
|---|
| Cluster of
differentiation 44 |
|
|
| F:
CAAGCAATAGGAATGATGTC | 45 | 60 (15 sec) |
| R:
GGTCACTGGGATGAAGGT |
|
|
| Hypoxia-inducible
factor-1α |
|
|
| F:
GGGAGAAAATCAAGTCGTGC | 45 | 60 (20 sec) |
| R:
AGCAAGGAGGGCCTCTGATG |
|
|
| GAPDH |
|
|
| F:
GCACCGTCAAGGCTGAGAAC | 45 | 60 (15 sec) |
| R:
TGGTGAAGACGCCAGTGGA |
|
|
Western blot analysis
Using the TRIzol® reagent, total protein
was extracted from the cells. The protein concentration was
evaluated using a Protein Assay kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The proteins (50 mg/well) were separated by 12%
SDS-PAGE and then electrophoretically transferred onto
nitrocellulose membranes. The membranes were blocked with 5% bovine
serum albumin (Gibco; Thermo Fisher Scientific, Inc.) at room
temperature and then probed overnight at 4°C using polyclonal
antibodies against CD44 (ab51037; Abcam, Cambridge, UK) and HIF-1α
(sc-10790; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at a
final dilution of 1:200 (w/v). The anti-GAPDH antibody (sc-25778,
Santa Cruz Biotechnology, Inc.) was used overnight at 4°C at a
final dilution of 1:600 (w/v). The membranes were then washed in
PBS with Tween-20 and incubated with peroxidase-conjugated
anti-rabbit immunoglobulin G [dilution, 1:3,000 (w/v); BA1054;
Wuhan Boster Biological Technology, Ltd., Wuhan, China) (21) for 30 min at room temperature. The
bands were visualized using an enhanced chemiluminescence kit
(P0018; Beyotime Institute of Biotechnology, Haimen, China), and
their optical densities were evaluated using a photo documentation
and imaging system (BIO-ID VL, Conn, France).
Statistical analysis
All data were expressed as the mean ± standard
deviation. Using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA),
one-way analysis of variance and the Student's t-test were employed
to analyze the data. P<0.05 was considered to indicate a
statistically significant difference.
Results
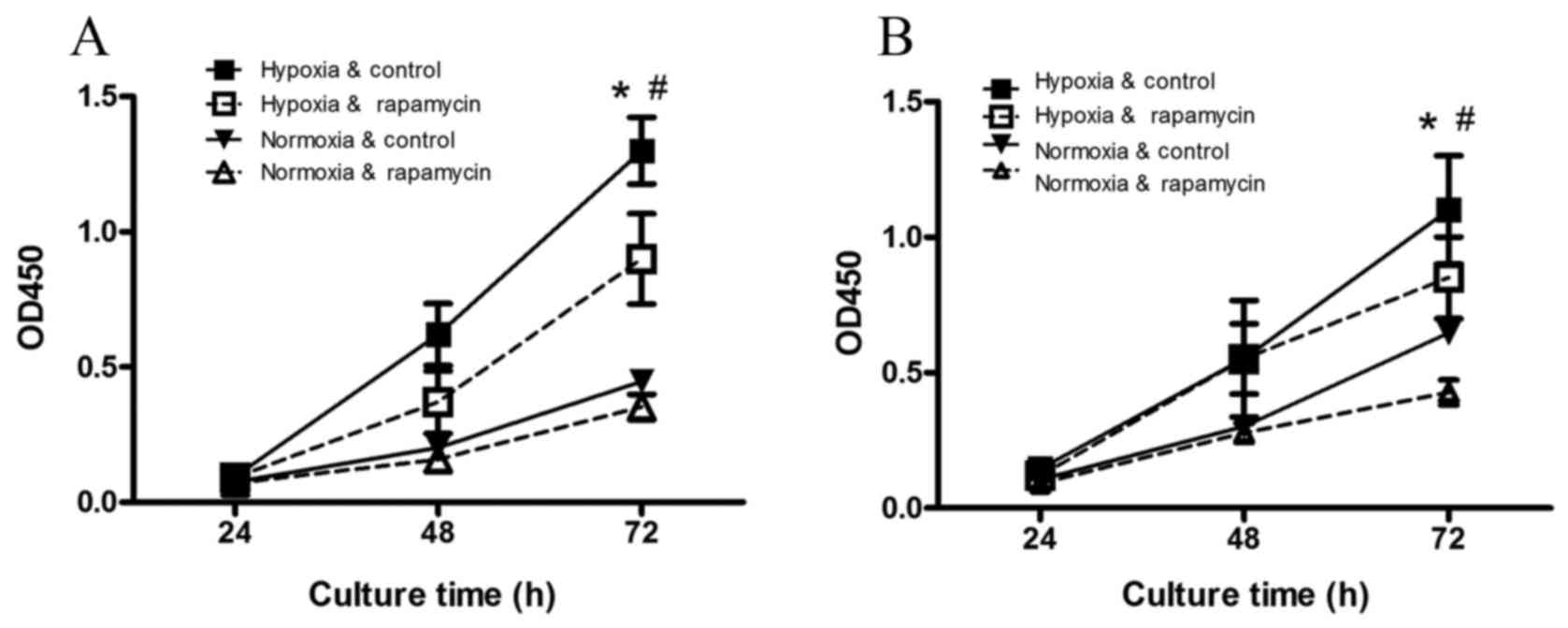
The viability of the gastric cancer
cell lines is promoted by hypoxia and inhibited by rapamycin
Compared with the normoxia control group (Fig. 1), hypoxia significantly increased the
viability of SGC-7901 and BGC-823 cells after 72 h of incubation
(P=0.001 and P=0.009, respectively). Hypoxia-induced increases in
cell viability were inhibited by rapamycin treatment (P=0.001 and
P=0.01, respectively).
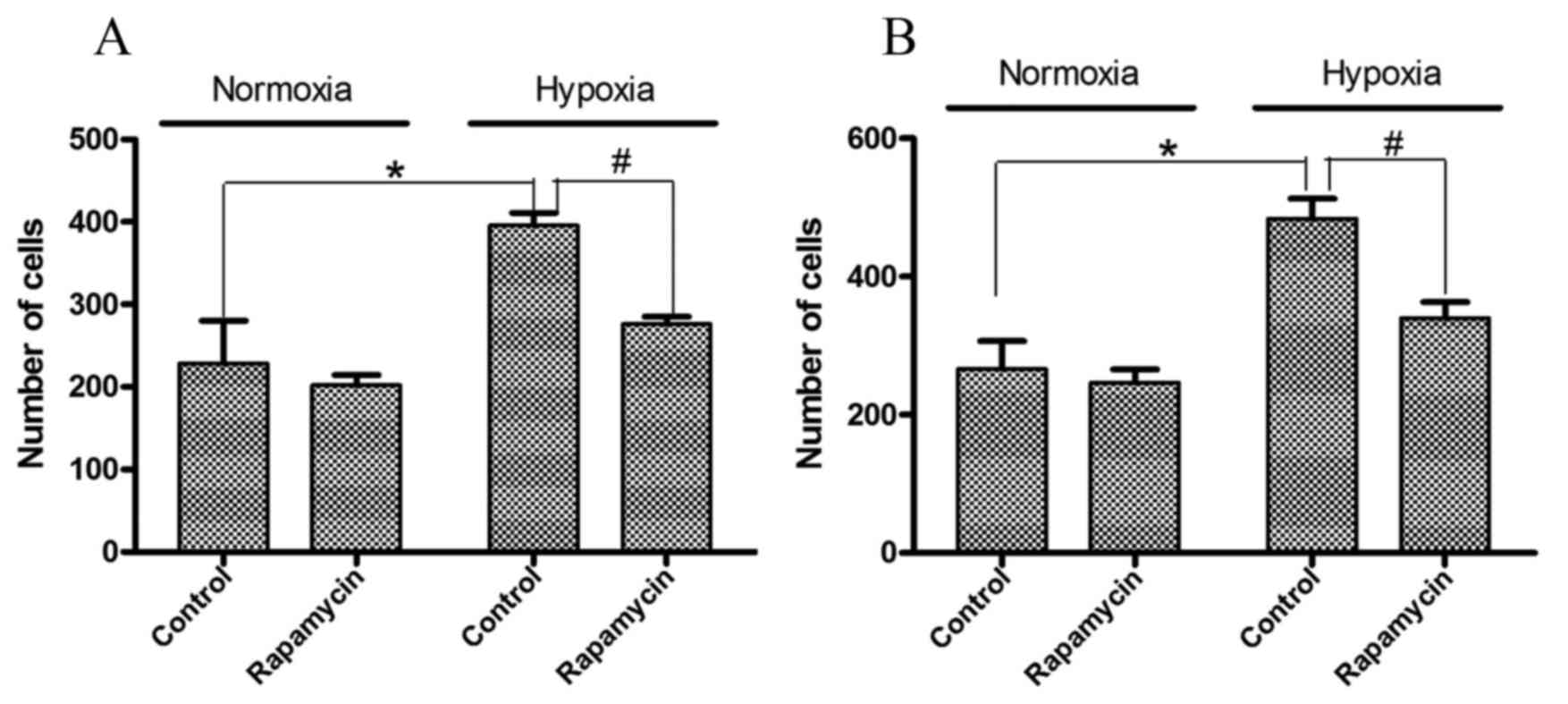
Cell migration and invasion in gastric
cancer cell lines is induced by hypoxia and inhibited by
rapamycin
To examine the effects of hypoxia and rapamycin
pretreatment on the biological behavior of the cells, migration and
invasion assays were performed. As presented in Fig. 2, significantly higher numbers of
invasive cells were observed following hypoxia treatment. Hypoxia
induced 1.3- and 1.9-fold increases in the number of invasive
SGC-7901 and BGC-823 cells, respectively (P=0.001 and P=0.005,
respectively). However, rapamycin pretreatment significantly
decreased these invasive cell numbers by 29 and 24%, respectively
(P=0.001 and P=0.008, respectively).
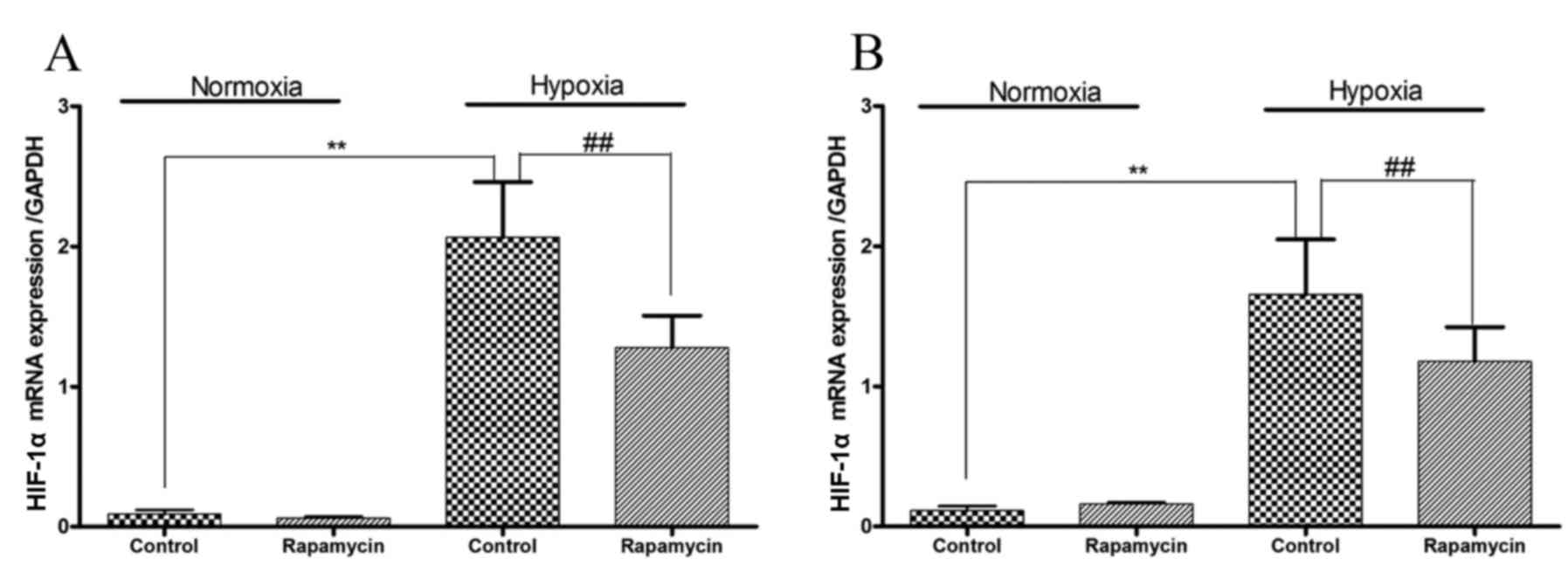
HIF-1α mRNA expression is induced by
hypoxia and inhibited by rapamycin in gastric cancer cell
lines
Hypoxia increased the mRNA expression levels of
HIF-1α in SGC-7901 and BGC-823 cells (P=0.001 and P=0.001,
respectively; Fig. 3). To
downregulate the mRNA expression of HIF-1α, the two gastric cancer
cell lines were treated with rapamycin. Under hypoxic conditions,
treatment with rapamycin significantly reduced the mRNA expression
levels of HIF-1α by 40 and 30% in SGC-7901 and BGC-823 cells,
respectively (P=0.001 and P=0.001, respectively).
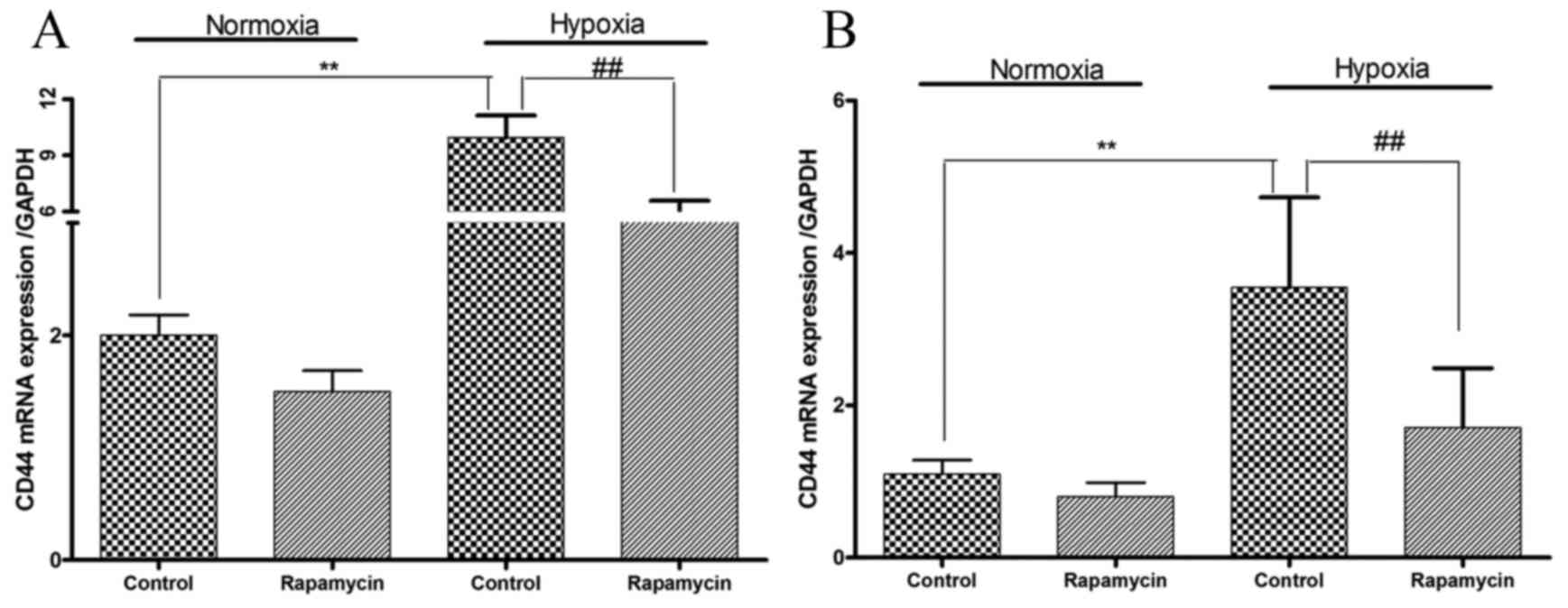
CD44 mRNA expression levels are
increased by hypoxia and decreased by rapamycin in gastric cancer
cell lines
Whether hypoxia is able to modulate the expression
levels of CD44 mRNA was subsequently examined. Hypoxia induced 3.0-
and 2.2-fold increases in CD44 mRNA expression levels in SGC-7901
and BGC-823 cells, respectively (P=0.01 and P=0.001, respectively;
Fig. 4). Treatment with rapamycin
under hypoxic conditions decreased CD44 mRNA expression levels by
45 and 52% in SGC-7901 and BGC-823 cells, respectively (P=0.007 and
P=0.03, respectively; Fig. 4).
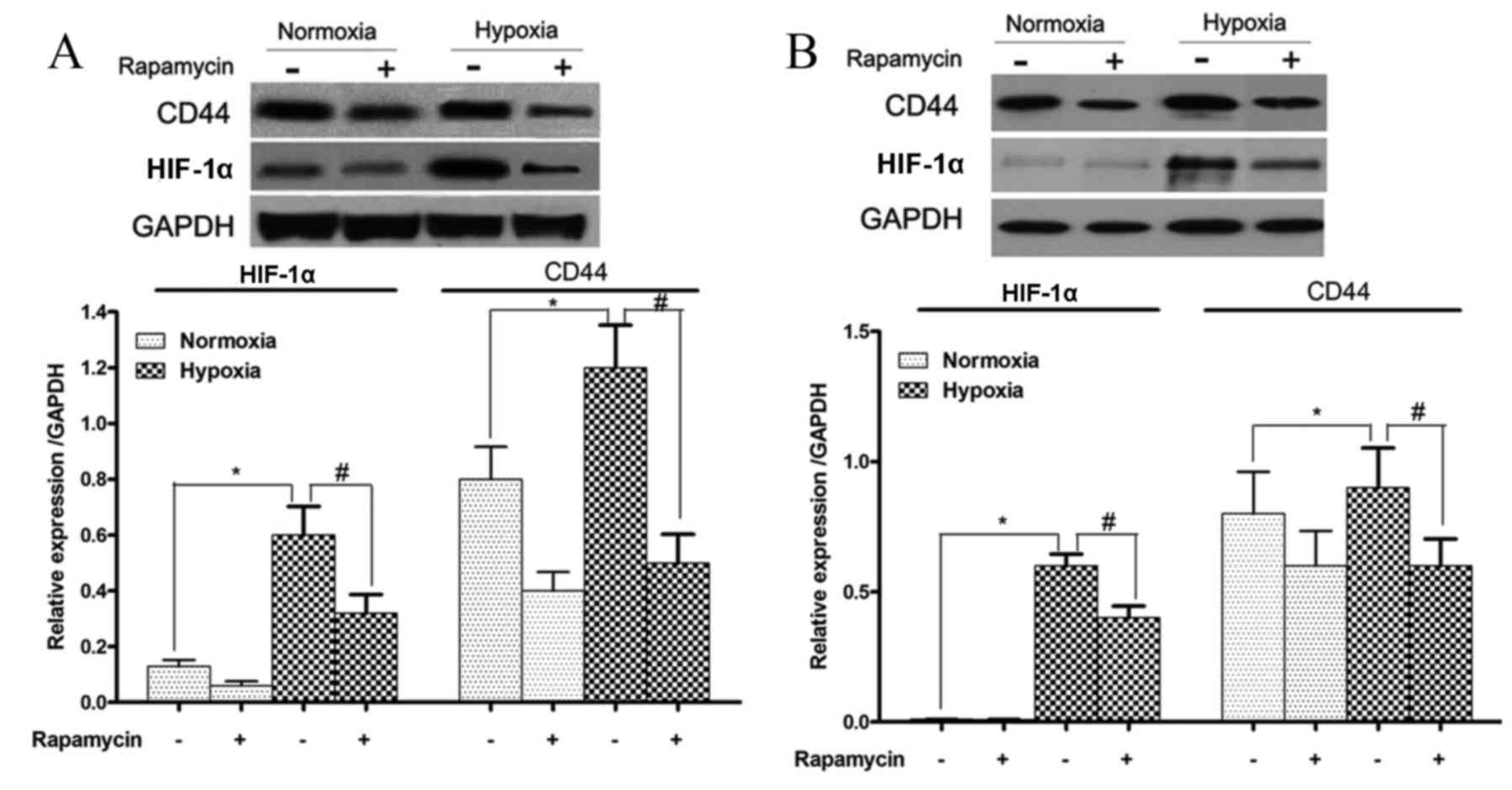
HIF-1α and CD44 protein expression
levels are increased during hypoxia and reduced by rapamycin in
gastric cancer cell lines
The protein expression levels of HIF-1α and CD44 in
SGC-7901 and BGC-823 cells that were treated with or without
rapamycin under hypoxic conditions were also examined. HIF-1α and
CD44 protein expression levels were significantly induced by
hypoxia in these two cell lines (SGC-7901 cells, P=0.001 and
P=0.03, respectively; and BGC-823 cells, P=0.029 and P=0.032,
respectively; Fig. 5). However, these
increases in HIF-1α and CD44 protein expression levels were
significantly reduced upon rapamycin treatment (SGC-7901 cells,
P=0.009 and P=0.01, respectively; and BGC-823 cells, P=0.02 and
P=0.037, respectively; Fig. 5).
Discussion
Gastric cancer is a major global malignancy and the
second leading cause of cancer mortality, with estimated 951,600
new stomach cancer cases and 723,100 mortalities occurring
worldwide in 2012 (22). There is
currently no effective treatment for highly advanced gastric cancer
or recurrent gastric cancer, and the survival rates are low
(23). Treatments are frequently
ineffective due to high levels of heterogeneity between cases
(24,25). The cancer stem cell (CSC) hypothesis
may provide novel approaches for eradicating the cause of cell
heterogeneity, which is associated with therapeutic resistance,
relapse and distant metastasis (26,27).
Therefore, the establishment of a treatment that targets CSCs to
radically cure cancer is an important goal, and the identification
of gastric CSCs may aid the development of gastric cancer therapies
in the future.
CD44 is a transmembrane glycoprotein and a
cell-adhesion molecule. It has been reported to serve important
roles in extracellular matrix adhesion, motility, matrix
degradation, proliferation and cell survival (28–30). These
functions are involved in cancer pathology, including tumor
progression and metastasis. In severe combined immunodeficient
mice, CD44-positive gastric cancer cells have been demonstrated to
exhibit high tumorigenic ability and the stem cell properties of
self-renewal and differentiation (6).
This suggests that CD44 may be a potential biomarker of gastric
CSCs.
In the current study, hypoxia was able to promote
the proliferation, migration and invasion of gastric cancer cells.
This result was consistent with a previous study demonstrating that
the hypoxic microenvironment upgrades the stem-like properties of
gastric cancer cells by promoting invasion and metastasis (31). HIF-1α has been revealed to regulate
specific surface markers, including CD133 and CD24, in several
cancer cell types (32–34). However, an association between HIF-1α
and CD44 in gastric cancer cells has not yet been reported. The
current study hypothesized that HIF-1α also regulated the
expression levels of CD44 in gastric cancer cells. The results
demonstrated that hypoxia increased the mRNA and protein expression
levels of HIF-1α in two gastric cancer cell lines. Furthermore, the
mRNA and protein expression levels of CD44 were also increased. As
a downstream molecule in the mammalian target of rapamycin (mTOR)
signaling pathway, the mRNA and protein expression levels of HIF-1α
were reduced upon treatment with rapamycin, which is an inhibitor
of mTOR, under hypoxic conditions (33). In addition, the downregulation of
HIF-1α expression decreased CD44 expression levels and
significantly inhibited the proliferation and invasive ability of
gastric cancer cells. This suggests that hypoxia may regulate the
expression levels of CD44 via HIF-1α and influence the
proliferation and invasion activity of gastric cancer cells.
The molecular mechanism underlying the
HIF-1α-mediated regulation of CD44 expression levels remains to be
elucidated. Certain studies have suggested that HIF-1α activation
regulates the Wnt/β-catenin signaling pathway, which activates the
expression of target genes and contributes to the enhanced invasion
of hypoxic gastric cancer cells (35–37). CD44
is a target gene of the Wnt/β-catenin signaling pathway (38); therefore, the mechanism by which
HIF-1α regulates CD44 may be associated with the Wnt/β-catenin
signaling pathway in gastric cancer. Further studies are required
to investigate these underlying mechanisms in gastric cancer.
In conclusion, the results of the present study
suggest that, in gastric cancer cells, hypoxia may potentially
regulate CD44 expression via HIF-1α in order to promote cell
proliferation and invasion, which may be a potential target for the
treatment of gastric cancer in the future.
Acknowledgements
The present study was supported by the Youth Science
and Technology Talent Project of Hubei Health Department (grant no.
QJX2012-21).
References
|
1
|
Götte M and Yip GW: Heparanase, hyaluronan
and CD44 in cancers: A breast carcinoma perspective. Cancer Res.
66:10233–10237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peng ST, Su CH, Kuo CC, Shaw CF and Wang
HS: CD44 crosslinking-mediated matrix metalloproteinase-9
relocation in breast tumor cells leads to enhanced metastasis. Int
J Oncol. 31:1119–1126. 2007.PubMed/NCBI
|
|
3
|
Go SI, Ko GH, Lee WS, Kim RB, Lee JH,
Jeong SH, Lee YJ, Hong SC and Ha WS: CD44 Variant 9 serves as a
poor prognostic marker in early gastric cancer, but not in advanced
gastric cancer. Cancer Res Treat. 48:142–152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cao X, Cao D, Jin M, Jia Z, Kong F, Ma H,
Wang Y and Jiang J: CD44 but not CD24 expression is related to poor
prognosis in non-cardia adenocarcinoma of the stomach. BMC
Gastroenterol. 14:1572014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xue Z, Yan H, Li J, Liang S, Cai X, Chen
X, Wu Q, Gao L, Wu K, Nie Y and Fan D: Identification of cancer
stem cells in vincristine preconditioned SGC7901 gastric cancer
cell line. J Cell Biochem. 113:302–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takaishi S, Okumura T, Tu S, Wang SS,
Shibata W, Vigneshwaran R, Gordon SA, Shimada Y and Wang TC:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Keith B and Simon MC: Hypoxia-inducible
factors, stem cells, and cancer. Cell. 129:465–472. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Panchision DM: The role of oxygen in
regulating neural stem cells in development and disease. J Cell
Physiol. 220:562–568. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Li Z, Zhang H, Jin H, Sun L, Dong
H, Xu M, Zhao P, Zhang B, Wang J, et al: HIF-1α and HIF-2α
correlate with migration and invasion in gastric cancer. Cancer
Biol Ther. 10:376–382. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blenner JL: The therapeutic functions of
companion animals in infertility. Holist Nurs Pract. 5:6–10. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krishnamachary B, BergDixon S, Kelly B,
Agani F, Feldser D, Ferreira G, Iyer N, LaRusch J, Pak B, Taghavi P
and Semenza GL: Regulation of colon carcinoma cell invasion by
hypoxia-inducible factor 1. Cancer Res. 63:1138–1143.
2003.PubMed/NCBI
|
|
13
|
Sumiyoshi Y, Kakeji Y, Egashira A,
Mizokami K, Orita H and Maehara Y: Overexpression of
hypoxia-inducible factor 1alpha and p53 is a marker for an
unfavorable prognosis in gastric cancer. Clin Cancer Res.
12:5112–5117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Urano N, Fujiwara Y, Doki Y, Tsujie M,
Yamamoto H, Miyata H, Takiguchi S, Yasuda T, Yano M and Monden M:
Overexpression of hypoxia-inducible factor-1 alpha in gastric
adenocarcinoma. Gastric Cancer. 9:44–49. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rohwer N, Lobitz S, Daskalow K, Jöns T,
Vieth M, Schlag PM, Kemmner W, Wiedenmann B, Cramer T and Höcker M:
HIF-1alpha determines the metastatic potential of gastric cancer
cells. Br J Cancer. 100:772–781. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krishnamachary B, Penet MF, Nimmagadda S,
Mironchik Y, Raman V, Solaiyappan M, Semenza GL, Pomper MG and
Bhujwalla ZM: Hypoxia regulates CD44 and its variant isoforms
through HIF-1α in triple negative breast cancer. PLoS One.
7:e440782012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hashimoto K, Aoyagi K, Isobe T, Kouhuji K
and Shirouzu K: Expression of CD133 in the cytoplasm is associated
with cancer progression and poor prognosis in gastric cancer.
Gastric Cancer. 17:97–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alain Ravauda and Hervé Wallerand:
Molecular pathways in metastatic renal cell carcinoma: The evolving
role of mammalian target of rapamycin inhibitors. European Urology.
8:793–798. 2009. View Article : Google Scholar
|
|
19
|
Li GG, Li L, Li C, Ye LY, Li XW, Liu DR,
Bao Q, Zheng YX, Xiang DP, Chen L and Chen J: Influence of
up-regulation of Notch ligand DLL4 on biological behaviors of human
gastric cancer cells. World J Gastroenterol. 19:4486–4494. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu D, Zhang B, Liang G, Ping J, Kou H, Li
X, Xiong J, Hu D, Chen L, Magdalou J and Wang H: Caffeine-induced
activated glucocorticoid metabolism in the hippocampus causes
hypothalamic-pituitary-adrenal axis inhibition in fetal rats. PLoS
One. 7:e444972012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Torre LA, Bray F, Siegel RL, Ferlay J,
LortetTieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tan IB, Ivanova T, Lim KH, Ong CW, Deng N,
Lee J, Tan SH, Wu J, Lee MH, Ooi CH, et al: Intrinsic subtypes of
gastric cancer, based on gene expression pattern, predict survival
and respond differently to chemotherapy. Gastroenterology.
141:476–485. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim B, Bang S, Lee S, Kim S, Jung Y, Lee
C, Choi K, Lee SG, Lee K, Lee Y, et al: Expression profiling and
subtype-specific expression of stomach cancer. Cancer Res.
63:8248–8255. 2003.PubMed/NCBI
|
|
26
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells-perspectives on current status and future directions:
AACR Workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jang BI, Li Y, Graham DY and Cen P: The
Role of CD44 in the Pathogenesis, Diagnosis and Therapy of Gastric
Cancer. Gut Liver. 5:397–405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nagano O and Saya H: Mechanism and
biological significance of CD44 cleavage. Cancer Sci. 95:930–935.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ponta H, Sherman L and Herrlich PA: CD44:
From adhesion molecules to signalling regulators. Nat Rev Mol Cell
Biol. 4:33–45. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo J, Wang B, Fu Z, Wei J and Lu W:
Hypoxic Microenvironment Induces EMT and Upgrades Stem-Like
Properties of Gastric Cancer Cells. Technol Cancer Res Treat.
15:60–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Matsumoto K, Arao T, Tanaka K, Kaneda H,
Kudo K, Fujita Y, Tamura D, Aomatsu K, Tamura T, Yamada Y, et al:
mTOR signal and hypoxia-inducible factor-1 alpha regulate CD133
expression in cancer cells. Cancer Res. 69:7160–7164. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Platet N, Liu SY, Atifi ME, Oliver L,
Vallette FM, Berger F and Wion D: Influence of oxygen tension on
CD133 phenotype in human glioma cell cultures. Cancer Lett.
258:286–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fujikuni N, Yamamoto H, Tanabe K, Naito Y,
Sakamoto N, Tanaka Y, Yanagihara K, Oue N, Yasui W and Ohdan H:
Hypoxia-mediated CD24 expression is correlated with gastric cancer
aggressiveness by promoting cell migration and invasion. Cancer
Sci. 105:1411–1420. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu HL, Liu D, Ding GR, Liao PF and Zhang
JW: Hypoxia-inducible factor-1α and Wnt/β-catenin signaling
pathways promote the invasion of hypoxic gastric cancer cells. Mol
Med Rep. 12:3365–3373. 2015.PubMed/NCBI
|
|
36
|
Mazumdar J, O'Brien WT, Johnson RS,
LaManna JC, Chavez JC, Klein PS and Simon MC: O2 regulates stem
cells through Wnt/β-catenin signalling. Nat Cell Biol.
12:1007–1013. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Braunschweig L, Meyer AK, Wagenführ L and
Storch A: Oxygen regulates proliferation of neural stem cells
through Wnt/β-catenin signalling. Mol Cell Neurosci. 67:84–92.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Siapati EK, Papadaki M, Kozaou Z, Rouka E,
Michali E, Savvidou I, Gogos D, Kyriakou D, Anagnostopoulos NI and
Vassilopoulos G: Proliferation and bone marrow engraftment of AML
blasts is dependent on β-catenin signalling. Br J Haematol.
152:164–174. 2011. View Article : Google Scholar : PubMed/NCBI
|