Introduction
Invasion and metastasis is a complicated progress
that is accomplished with the aid of numerous bioactive enzymes,
such as plasmin, cathepsin and matrix metallopeptidases (MMPs)
(1). MMPs are known to be
Zn2+ dependent proteinases that have also been reported
to promote the invasion and metastasis of cancer cells by
degradating the extracellular matix components to damage the
histological barrier around the extracellular matrix (ECM)
(2). MMP-9 is produced by
mesenchymal, epithelial and hematopoietic cells, and also by
distinct tumor cell types, such as those of gastric, lung and colon
cancer. MMP-9 expression is induced by cytokines, growth factors
and cell/stroma interactions, and has been associated with numerous
biological processes, including bone resorption, inflammation and
arthritis. Numerous studies have found that the invasion of breast
cancer MCF-7 cells is hindered by the inhibition of MMP-9, which is
expressed significantly more in carcinoma compared with normal
tissues (3,4). MMP-9 activity has also been linked with
the process of tumor cell intravasation (5). These studies indicated that MMP-9 may be
crucial in cancer invasion.
Melittin is a polypeptide containing 26 amino acid
residues, which may inhibit the inflammatory stimulus of
phospholipase A2 activity, and repress interleukin (IL)-1, tumor
necrosis factor (TNF)-α, cyclooxygenase, and reactive oxygen
species in numerous inflammatory diseases, including rheumatoid
arthritis in humans and experimental animals (6,7).
Additionally, it has been reported that melittin could exert
anti-cancer effects by suppressing the production of matrix
metalloproteinase (MMPs), and inhibiting the activity of caspase
(8–10). The caspase molecules are a class of
highly-conserved protein molecules that are evolutionarily
conserved and can be used in the detection of tumor cells (8–10).
CD147, a transmembrane glycoprotein, induces ECM
metalloproteinase, which is widely expressed in numerous cells,
particularly in malignant breast tumor, bladder tumor and catuneum
carcinoma cells (11,12). It has been known that CD147 promotes
the expression of numerous members of the MMP family (12–15).
Additionally, CD147 is also known as the receptor of cyclophilin A
(CypA), which can induce numerous signal transductions and perform
chemotactic activity (15). CypA is a
cytosol protein in a number of cells that may be secreted in
vesicles when stimulated by inflammatory factors or oxidative
stress (16–19). Therefore, it is crucial to block
carcinoma cell invasion through inhibiting CypA and CD147-induced
MMP expression.
The previous study indicated that melittin may
inhibit CypA expression in macrophage cells. The present study aims
to investigate CypA-induced CD147 expression and MMP-9 secretion to
identify notable targets of melittin involved in the invasion of
mammary carcinoma MCF-7 cells. The invasion level of MCF-7 cells
was found to be downregulated by melittin in a dose-dependent
manner. And the results also showed that melittin could decrease
the expression of CD147 and MMP-9, whereas CypA could upregulate
the expression of CD147 and MMP-9 by using flow cytometry and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR). In summary, the present study demonstrated that melittin
decreases the invasion level of MCF-7 cells by downregulating CD147
and MMP-9 through inhibition of CypA expression. The results of the
present study provide evidence for melittin in anticancer therapy
and mechanisms.
Materials and methods
Melittin and MCF-7 cell line
Synthetic high-purity melittin (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) was dissolved in sterile water, with
the highest final concentration at 2.5 µg/ml (0, 0.5, 1.5 and 2.5
µg/ml) and stored at −20°C. The human breast carcinoma MCF-7 cell
line (Beijing Dingguo Biological Technology Co., Ltd., Beijing,
China) was cultured in Dulbecco's modified Eagle's medium
(DMEM)-low glucose (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), containing 10% fetal bovine serum (Beijing Sijiqing
Buiological Science and Technology Co., Ltd., Beijing, China) and
1% antibiotics (penicillin and streptomycin; Beijing Dingguo
Biological Technology Co., Ltd.), and subcultured at 37°C in a 5%
CO2 incubator.
Transwell assay
For the Transwell Matrigel invasion assay,
2×105 cells in 200 µl of serum-free medium were plated
onto the upper chamber of 24-well Transwell inserts (8-µm pores; BD
Biosciences, Franklin Lakes, NJ, USA) coated with or without
Matrigel. Cells were incubated for 1 h for adherence and melittin
was then added in the upper chamber for another 24 h at 37°C in a
5% CO2 atmosphere. The lower chamber was filled with 600
µl DMEM containing 10% fetal bovine serum. After 24 h, the
non-invaded cells were gently scraped off from the upper chamber by
cotton swab. The cells were fixed with 10% methanol solution, which
penetrated through the polyvinylidene difluoride membrane into the
lower chamber, and then the cells were stained with 0.1% crystal
violet (Beijing Dingguo Biological Technology Co., Ltd.) for 20 min
at room temperature. Images of random fields were captured using a
light inversion microscope system. The absorbance was determined
using a microplate reader at a wavelength of 570 nm subsequent to
destaining with 33% acetic acid.
ELISA
The expression of MMP-9 in MCF-7 was determined by
ELISA using the human MMP-9 ELISA kit (Beijing Dingguo Biological
Technology Co., Ltd.). The culture supernatants were harvested,
centrifuged to remove cellular debris, and stored at −80°C prior to
ELISA. Each experiment was performed in triplicate and repeated
three times. The MMP-9 concentration in the culture supernatant was
quantified using ELISA kits (Beijing Dingguo Biological Technology
Co., Ltd.), according to the manufacturer's instructions. The MMP-9
ELISA kit includes a set of calibration standards, which may
produce a standard curve of optical density vs. MMP-9
concentration. The concentration of MMP-9 in the samples was
determined by comparing the optical density (OD) of the samples at
450 nm to the standard curve.
RT-qPCR
Total RNA was extracted using TRIzol reagent (Takara
Biotechnology Co., Ltd., Dalian, China). The quantity and purity of
the isolated RNA were measured using OD at 260 nm and 280 nm. Total
RNA was reverse transcribed into cDNA, and then amplified by PCR
using primer for CD147, MMP-9 according to the manufacturer's
protocol (Takara Biotechnology Co., Ltd.). The primers used were as
follows: CD147 forward, 5′-TCGCGCTGCTGGGCACC-3′ and reverse,
5′-TGGCGCTGTCATTCAAGGA-3′ MMP-9 forward, 5′-CACTGTCCACCCCTCAGAGC-3′
and reverse, 5′-GCCACTTGTCGGCGATAAGG-3′ and GADPH forward,
5′-TCGGAGTCAACGGATTTGGTCGTA-3′ and reverse,
5′-TGGCATGGACTGTGGTCATGAGTC-3′. Cycling conditions were as follows:
64°C for 30 sec for 35 cycles, 60°C for 30 sec for 40 cycles and
64°C for 30 sec for 30 cycles, respectively.
The primers were all purchased from Takara
Biotechnology, Co., Ltd. The analysis of relative gene expression
data was performed using RT-qPCR and the 2−ΔΔCq method
(20). PCR products were then run on
a 2% agarose gel in order to confirm the presence of a single band
with the expected size using marker2000 (TianGen Biochemical
Science and Technology, Co., Ltd. Beijing, China).
Flow cytometry analysis
MCF-7 cells were treated with trypsin, and then
digestion was stopped using staining medium (phosphate-buffered
saline containing 10% serum protein was used after the filter).
Centrifugation was performed at 4°C and 300 × g for 5 min.
Cells were incubated with phycoerythrin-labeled anti-CD147
monoclonal antibodies (1:100 dilution; ab91150; Abcam, Cambridge,
UK) for 30 min. The cells were mixed in ice-cold staining medium
and centrifuged at 300 × g for 5 min at 4°C three times. The
cells were resuspended using staining medium and the supernatants
were discarded. Positive cell count and mean fluorescence intensity
were determined by flow cytometry.
Statistical analysis
All data were expressed as the mean ± standard
deviation. Statistical analyses were performed with Student's
t-test using SigmaPlot 10.0 software (http://www.126xiazai.com/fileview_1619971.html).
P<0.05 was considered to indicate a statistically significant
difference.
Results
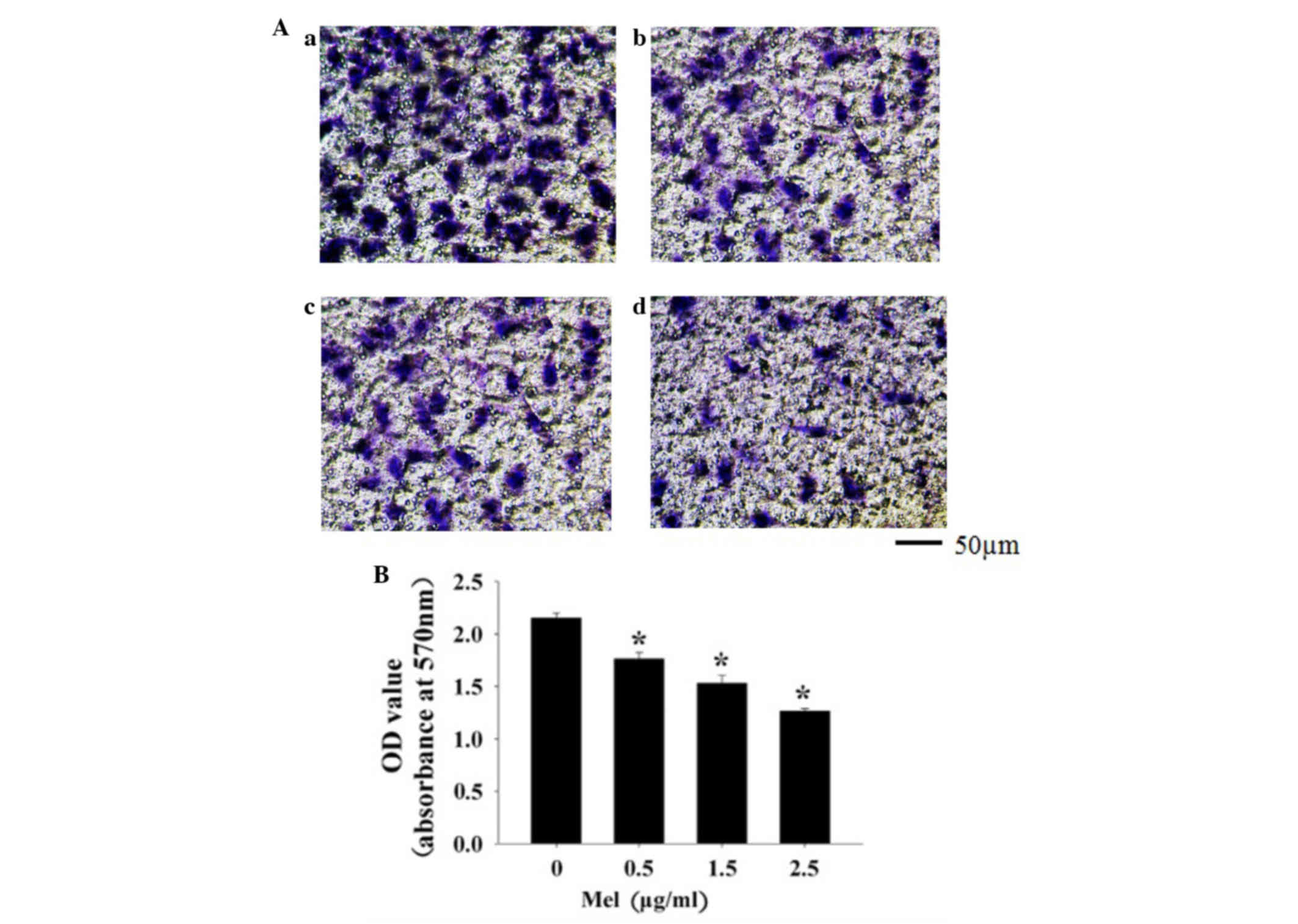
Melittin inhibits MCF-7 cell
invasion
Invasion is critical step in the initial progression
of cancer and facilitates metastasis. Transwell assay was used to
determine the effects of melittin on MCF-7 cell invasion. MCF-7
cells were treated with increasing doses of melittin (0.5–2.5
µg/ml), and invasion was assessed using the Transwell assay. It was
found that treatment with melittin significantly decreased the
invasion of MCF-7 cells in a dose-dependent manner (P=0.009,
P=0.007 and P=0.006, respectively) (Fig.
1A and B).
Melittin suppresses CypA-induced MMP-9
secretion
It has been reported that inhibition of MMP-9, which
is significantly stimulated by CypA, hinders MCF-7 invasion. To
evaluate whether MMP-9 stimulated by CypA may be repressed by
melittin, ELISA and RT-PCR were performed to detect the expression
of MMP-9 in MCF-7. ELISA and RT-PCR results showed that
extracellular CypA increased MMP-9 expression and secretion, while
melittin suppressed MMP-9 expression and secretion induced by CypA
(P=0.042 and P=0.039) (Fig. 2A and
B). The present results indicate that melittin could decrease
MMP-9 expression and secretion, which may be associated with
extracellular CypA.
Melittin suppressed CypA-stimulated
CD147 expression
It is well known that CD147, a matrix
metalloproteinase inducer factor, promotes MMP family expression
(12–15). Additionally, it has also been reported
that CD147 contributes to the signal transduction pathway induced
by CypA (21). In the present study,
in order to investigate whether melittin antagonizes CD147
expression that is induced by CypA, flow cytometry and RT-PCR were
performed to assess the expression of CD147 in MCF-7 cells. The
flow cytometry assay showed that CypA can stimulate CD147
expression, while melittin decreases the expression of CD147 in a
dose-dependent manner at concentrations between 0.5 and 2.5 µg/ml
(Fig. 3A). In addition, the RT-PCR
results also indicated that CD147 mRNA expression stimulated by
CypA was antagonized by melittin at a concentration of 2.5 µg/ml
(Fig. 3B).
Discussion
At present, several studies have reported that the
antineoplastic effect of melittin is involved in liver, lung,
prostate and breast cancers, and other malignant tumors (22). Park et al found that melittin
suppressed breast cancer cell invasion and tumor growth (23). Additionally, in the present study, it
was found that melittin inhibited breast cancer MCF-7 cell invasion
in a dose-dependent manner.
MMPs are a family of secreted or transmembrane
enzymes that can collectively digest almost all ECM and basement
membrane components. Thus, MMPs are largely implicated in promoting
angiogenesis and tumor metastasis (24,25).
Numerous studies have found that the breast cancer MCF-7 cell
invasion can be hindered by the inhibition of MMP-9, which is
overexpressed in carcinoma compared with normal tissue (9,10). MMP-9
activity has also been linked with the process of tumor cell
intravasation. These studies indicated that MMP-9 may play a
crucial role in cancer invasion. In dendritic cells, increased
expression of cell migration was shown to be significantly enhanced
(26). Therefore, regulation of MMP-9
expression and secretion has become an important focus of studies
investigating anticancer drugs. The present study indicates that
melittin inhibits MCF-7 invasion by repressing MMP-9 expression.
Additionally, Cho et al have confirmed the effect of
melittin on MMP-9 expression induced by
phorbol-12-myristate-13-acetate (PMA) (3). Although melittin has been reported to
inhibit the invasion of breast cancer cells, the effect of melittin
on normal cells is not clear.
CD147, namely matrix metalloproteinase induced
factor, can promote the expression and secretion of MMPs, and
widely expressed in numerous types of cells, particularly malignant
tumors, including mammary carcinoma and bladder cancer (27). Seizer et al (28) found that CD147 was upregulated during
the differentiation process between CD34(+) progenitor cells and
foam cells, and the CypA/CD147 activation pathway may be involved
in promoting the vulnerability of atherosclerotic plaques. In
addition, Gou et al reported that high expression of CD147,
MMP-2 and MMP-9 were associated with laryngeal carcinoma invasion
and metastasis (24). Additionally,
previous studies have identified that CD147 inhibition and
subsequent MMP-9 deletion may have anti-tumor effects by inhibiting
the invasiveness of human cervical squamous carcinoma cells
(29–31). The aforementioned studies indicated
that high expression of CD147 may be another crucial factor in the
invasion and metastasis of malignant tumor cells. In the present
study, it was found that CD147 mRNA expression stimulated by CypA
was antagonized by melittin, which indicated that melittin
inhibited MCF-7 invasion through antagonizing CD147 mRNA expression
stimulated by CypA.
In the present study, CypA stimulated MCF-7 cell
invasion by inducing CD147 expression and MMP-9 secretion, whereas
the invasion of MCF-7 cells was inhibited by melittin. Our previous
study demonstrated that melittin suppresses CypA secretion in mouse
macrophage Raw264.7 cells (31).
Thus, it has been indicated that melittin decreases the invasion of
MCF-7 cells in numerous ways. CypA is a widely expressed cytosol
protein that can be secreted to extracellular space as a cytokine
when stimulated by inflammatory factors or oxidative stress
(32–34). CypA can not only increase the
expression of CD147 and the expression of downstream factors, but
also take part in the feedback regulation of CD147 (18). Thus, there is a potential pathway that
melittin may suppress CypA secretion through inhibiting CD147 and
MMP-9. This remains to be further studied in depth.
In summary, the present study indicated that
melittin inhibits breast carcinoma MCF-7 cell invasion by
interacting with numerous targets and provided experimented basis
for melittin used for anti-cancer treatment.
Acknowledgements
This study was supported by Natural Science Fund of
Jilin (grant no., 201215001) and The National Natural Science
Foundation of China (grant no., 51478096).
Glossary
Abbreviations
Abbreviations:
|
MMP-9
|
matrix metallopeptidase 9
|
|
CD147
|
cluster of differentiation 147
|
|
CypA
|
cyclophilin A
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
References
|
1
|
Aimes RT and Quigley JP: Matrix
metalloproteinase-2 is an interstitial collagenase. Inhibitor-free
enzyme catalyzes the cleavage of collagen fibrils and soluble
native type I collagen generating the specific 3/4- and 1/4-length
fragments. J Biol Chem. 270:5872–5876. 1995.PubMed/NCBI
|
|
2
|
Folgueras AR, Pendás AM, Sánchez LM and
López-Otin C: Matrix metalloproteinases in cancer: From new
functions to improved inhibition strategies. Int J Dev Biol.
48:411–424. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cho HJ, Jeong YJ, Park KK, Park YY, Chung
IK, Lee KG, Yeo JH, Han SM, Bae YS and Chang YC: Bee venom
suppresses PMA-mediated MMP-9 gene activation via JNK/p38 and
NF-kappaB-dependent mechanisms. J Ethnopharmacol. 127:662–668.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Decock J, Hendrickx W, Vanleeuw U, Van
Belle V, Van Huffel S, Christiaens MR, Ye S and Paridaens R: Plasma
MMP1 and MMP8 expression in breast cancer: Protective role of MMP8
against lymph node metastasis. BMC Cancer. 8:772008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ramos-DeSimone N, HahnDantona E, Sipley J,
Nagase H, French DL and Quigley JP: Activation of matrix
metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1
cascade enhances tumor cell invasion. J Biol Chem. 274:13066–13076.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho HJ, Jeong YJ, Park KK, Park YY, Chung
IK, Lee KG, Yeo JH, Han SM, Bae YS and Chang YC: Bee venom
suppresses PMA-mediated MMP-9 gene activation via JNK/p38 and
NF-kappaB-dependent mechanisms. J Ethnopharmacol. 127:662–668.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Somerfield SD, Stach JL, Mraz C, Gervais F
and Skamene E: Bee venom melittin blocks neutrophil
O2− production. Inflammation. 10:175–182.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Russell PJ, Hewish D, Carter T,
SterlingLevis K, Ow K, Hattarki M, Doughty L, Guthrie R, Shapira D,
Molloy PL, et al: Cytotoxic properties of immunoconjugates
containing melittin-like peptide 101 against prostate cancer: In
vitro and in vivo studies. Cancer Immunol Immunother. 53:411–421.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li B, Gu W, Zhang C, Huang XQ, Han KQ and
Ling CQ: Growth arrest and apoptosis of the human hepatocellular
carcinoma cell line BEL-7402 induced by melittin. Onkologie.
29:367–371. 2006.PubMed/NCBI
|
|
10
|
Hu H, Chen D, Li Y and Zhang X: Effect of
polypeptides in bee venom on growth inhibition and apoptosis
induction of the human hepatoma cell line SMMC-7721 in-vitro and
Balb/c nude mice in-vivo. J Pharm Pharmacol. 58:83–89. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bordador LC, Li X, Toole B, Chen B, Regezi
J, Zardi L, Hu Y and Ramos DM: Expression of emmprin by oral
squamous cell carcinoma. Int J Cancer. 85:347–352. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taylor PM, Woodfield RJ, Hodgkin MN,
Pettitt TR, Martin A, Kerr DJ and Wakelam MJ: Breast cancer
cell-derived EMMPRIN stimulates fibroblast MMP2 release through a
phospholipase A (2) and 5-lipoxygenase catalyzed pathway. Oncogene.
21:5765–5772. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Caudroy S, Polette M, NawrockiRaby B, Cao
J, Toole BP, Zucker S and Birembaut P: EMMPRIN-mediated MMP
regulation in tumor and endothelial cells. Clin Exp Metastasis.
19:697–702. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Biswas C: Collagenase stimulation in
cocultures of human fibroblasts and human tumor cells. Cancer Lett.
24:201–207. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gabison EE, HoangXuan T, Mauviel A and
Menashi S: EMMPRIN/CD147, an MMP modulator in cancer, development
and tissue repair. Biochimie. 87:361–368. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin ZG, Melaragno MG, Liao DF, Yan C,
Haendeler J, Suh YA, Lambeth JD and Berk BC: Cyclophilin A is a
secreted growth factor induced by oxidative stress. Circ Res.
87:789–796. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suzuki J, Jin ZG, Meoli DF, Matoba T and
Berk BC: Cyclophilin A is secreted by a vesicular pathway in
vascular smooth muscle cells. Circ Res. 98:811–817. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nishioku T, Dohgu S, Koga M, Machida T,
Watanabe T, Miura T, Tsumagari K, Terasawa M, Yamauchi A and
Kataoka Y: Cyclophilin A secreted from fibroblast-like synoviocytes
is involved in the induction of CD147 expression in macrophages of
mice with collagen-induced arthritis. J Inflamm (Lond). 9:442012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pap T: Cyclophilins in rheumatoid
arthritis-stepping into an undiscovered country? Clin Immunol.
116:199–201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak and Schmittgen, . Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lai WC, Zhou M, Shankavaram U, Peng G and
Wahl LM: Differential regulation of lipopolysaccharide-induced
monocyte matrix metalloproteinase (MMP)-1 and MMP-9 by p38 and
extracellular signal-regulated kinase 1/2 mitogen-activated protein
kinases. J Immunol. 170:6244–6249. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jang MH, Shin MS, Lim S, Han SM, Park HJ,
Shin I, Lee JS, Kim KA, Kim EH and Kim CJ: Bee venom induces
apoptosis and inhibits expression of cyclooxygenase-2 mRNA in human
lung cancer cell line NCI-H1299. J Pharmacol Sci. 91:95–104. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park JH, Jeong YJ, Park KK, Cho HJ, Chung
IK, Min KS, Kim M, Lee KG, Yeo JH, Park KK and Chang YC: Melittin
suppresses PMA-induced tumor cell invasion by inhibiting NF-kappaB
and AP-1-dependent MMP-9 expression. Mol Cells. 29:209–215. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gou X, Chen H, Jin F, Wu W, Li Y, Long J,
Gong X, Luo M, Bi T, Li Z and He Q: Expressions of CD147, MMP-2 and
MMP-9 in laryngeal carcinoma and its correlation with poor
prognosis. Pathol Oncol Res. 20:475–481. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheung LW, Leung PC and Wong AS:
Gonadotropin-releasing hormone promotes ovarian cancer cell
invasiveness through c-jun NH2-terminal kinase-Mediated activation
of matrix metalloproteinase (MMP)-2 and MMP-9. Cancer Res.
66:10902–10910. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yen JH, Kocieda VP, Jing H and Ganea D:
Prostaglandin E2 induces matrix metalloproteinase 9 expression in
dendritic cells through two independent signaling pathways leading
to activator protein 1 (AP-1) activation. J Biol Chem.
286:38913–38923. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yurchenko V, Constant S, Eisenmesser E and
Bukrinsky M: Cyclophilin-CD147 interactions: A new target for
anti-inflammatory therapeutics. Clin Exp Immunol. 160:305–317.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Seizer P, Fuchs C, UngernSternberg SN,
Heinzmann D, Langer H, Gawaz M, May AE and Geisler T:
Platelet-bound cyclophilin A in patients with stable coronary
artery disease and acute myocardial infarction. Platelets.
27:155–158. 2016.PubMed/NCBI
|
|
29
|
Farabegoli F, Papi A and Orlandi M:
(−)-Epigallocatechin-3-gallate down-regulates EGFR, MMP-2, MMP-9
and EMMPRIN and inhibits the invasion of MCF-7 tamoxifen-resistant
cells. Biosci Rep. 31:99–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fan X, Wu W, Shi H and Han J: RNA
interference targeting CD147 inhibits the invasion of human
cervical squamous carcinoma cells by downregulating MMP-9. Cell
Biol Int. 37:737–741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dang D, Atakilit A and Ramos DM: EMMPRIN
modulates migration and deposition of TN-C in oral squamous
carcinoma. Anticancer Res. 28:2049–2054. 2008.PubMed/NCBI
|
|
32
|
Trachtenberg A, Pushkarsky T, Heine S,
Constant S, Brichacek B and Bukrinsky M: The level of CD147
expression correlates with cyclophilin-induced signalling and
chemotaxis. BMC Res Notes. 4:3962011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takahashi M, Suzuki S and Ishikawa K:
Cyclophilin A-EMMPRIN interaction induces invasion of head and neck
squamous cell carcinoma. Oncol Rep. 27:198–203. 2012.PubMed/NCBI
|
|
34
|
Yang Y, Lu N, Zhou J, Chen ZN and Zhu P:
Cyclophilin A up-regulates MMP-9 expression and adhesion of
monocytes/macrophages via CD147 signalling pathway in rheumatoid
arthritis. Rheumatology (Oxford). 47:1299–1310. 2008. View Article : Google Scholar : PubMed/NCBI
|