Introduction
Colorectal cancer (CRC) is one of the most
frequently diagnosed cancers in the world. In the USA, CRC is the
third most common malignant tumor, and there was an estimated
103,170 new cases of CRC throughout the country in 2014 (1). Previously, CRC has become the fourth
most common malignant tumor, and the third leading cause of
cancer-associated mortality in China (2). CRC also shows a high level of
metastasis, and 25% of CRC patients that present with metastatic
disease have a 5-year survival of only 10% (3). Surgical resection plus chemotherapy
and/or radiation therapy are effective treatments for CRC in the
clinic. However, chemotherapy and radiation therapy are commonly
associated with serious side effects, including bone marrow
suppression, nausea and vomiting, hair loss and loss of appetite
(3). Enhancement of cancer cell
apoptosis is a good strategy in the clinical treatment of cancer
(4).
As classic apoptosis-associated factors, the B-cell
lymphoma 2 (Bcl-2) family plays an important role in the regulation
of cell apoptosis. The Bcl-2 family is generally divided into
anti-apoptotic factors, including Bcl-2, Bcl-extra large (Bcl-xL)
and Bcl-W, and proapoptotic factors, including Bcl-2-associated X
protein (Bax), Bcl-2 associated agonist of cell death, Bcl-2
interacting mediator of cell death (Bim), Bcl-2 antagonist/killer 1
and p53 upregulated modulator of apoptosis (5,6). In the
extrinsic apoptosis pathway, the cell death receptor Fas/Fas ligand
(FasL) system plays an important role to induce receptor-ligand
mediated cell apoptosis, and also regulates the Fas-associated
death domain (FADD) and caspase-8-mediated cell apoptosis (7). In apoptosis, the depolarization of the
inner mitochondrial membrane potential causes the release of
cytochrome c (Cyto c) into the cytosol (8). The released Cyto c also activates
caspases by formation of a complex that induces the activation of
apoptotic protease-activating factor-1 (Apaf-1) and procaspase-9.
Furthermore, activated caspase-8 and −9 cleaved the final
executioner caspase-3, and directly induced the chromatin
condensation and DNA fragmentation to promoting apoptosis in cells
(9,10).
Traditional herbal medications are commonly used to
treat colon cancer in the clinic (11–13). Lotus
is traditionally used as a Chinese folk medicine to disperse the
summer heat, and has shown numerous health benefits and
pharmacological activities, including antioxidant (14), antidiarrheal (15), antiviral (16), anti-obesity (17–20),
anti-angiogenic (21),
hepatoprotective (22),
immunomodulatory (23) and insulin
secretagogue activity (24).
Therefore, the present study focused on investigating the potential
anticancer activity of Nelumbo nucifera (Ba lotus) stamen
ethanol crude extract (BLSEE), and also to elucidate the mechanisms
underlying its anticancer effects in human colon carcinoma HCT-116
cells.
Materials and methods
Chemical reagents
TRIzol reagent, OligodT18 primer, murine
Moloney leukemia virus (MMLV) reverse transcriptase, RNase
inhibitor, ethidium bromide (EtBr), and agarose were purchased from
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA). All
other reagents were of analytical grade and purchased from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
BLSEE preparation
Fresh Ba lotus stamen purchased from Chongqing
Enterprise Engineering Research Center of Ba-lotus Breeding and
Deep Processing (Chongqing, China) were freeze-dried and then
ground into a fine powder. In total, 100 g of powdered Ba lotus
stamen was extracted twice with 1,000 ml ethanol (70%, v/v) at 50°C
for 1 h. Subsequent to filtering, the sample extraction solution
was condensed by vacuum rotary evaporator (Büchi Rotovapor RE 111;
Büchi Labortechnik AG, Flawil, Switzerland) at 37°C, freeze-dried
and stored at −80°C until further study.
Cell culture
Human colorectal HCT-116 cancer cells were purchased
from the American Type Culture Collection (Manassas, VA, USA). The
cells were routinely maintained in RPMI-1640 medium supplemented
with 10% (v/v) FBS, and 1% penicillin-streptomycin in a humidified
CO2 incubator (model 3154; Forma Scientific, Inc.,
Marietta, OH, USA) with a 5% CO2 atmosphere at 37°C.
Cell viability assay
Cell viability was measured using an MTT assay.
HCT-116 cells were seeded in 96-well plates (Nalge Nunc
International, Rochester, NY, USA) at a density of 1×104
cells/well. Following a 24-h incubation, the cells were treated
with the various concentrations (100, 200 and 400 µg/ml) of BLSEE
for 24 h. Following incubation with BLSEE 0.5 mg/ml of MTT reagent
(100 µl) was added to each well and the cells were incubated in a
humidified incubator at 37°C to allow the MTT to be metabolized. At
total of 4 h later, formazan crystals were dissolved with dimethyl
sulfoxide (100 µl in each well). Absorbance of the samples was
measured at a wavelength of 540 nm using a microplate reader
(model, 680; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Flow cytometry analysis
The BLSEE-treated HCT-116 cells were first collected
following digestion with trypsin, washed twice with cold PBS, and
resuspended in 2 ml PBS. The DNA contents of the treated cells were
stained with propidium iodide (PI) using a DNA staining kit
(CycleTEST™ PLUS DNA reagent kit; Becton Dickinson, Franklin Lakes,
NJ, USA), according to the manufacturer's protocol. Fluorescence
intensity was determined using a FACScan flow cytometer (EPICS
XL-MCL, Beckman Coulter KK, Brea, CA, USA) and analyzed using
CellQuest software (Becton Dickinson).
Reverse transcription-polymerase chain
reaction (RT-PCR) assay
Total RNA was isolated from BLSEE-treated HCT-116
cells using TRIzol reagent, according to the manufacturer's
protocol, and centrifuged at 12,000 × g for 15 min at 25°C
following the addition of chloroform (200 µl). Isopropanol was
added to the supernatant at a 1:1 ratio and the RNA was pelleted by
centrifugation at 12,000 × g for 15 min at 4°C. Subsequent to
washing with ethanol, the RNA was solubilized in diethyl
pyrocarbonate-treated RNase-free water and quantified by measuring
the absorbance at 260 nm using a UV-1750 spectrophotometer
(Shimadzu, Kyoto, Japan). Equal amounts of RNA (1 µg) were reverse
transcribed in a master mix containing 1X reverse transcriptase
buffer, dNTPs (1 mM), oligodT18 primers (500 ng), MMLV
reverse transcriptase (140 U), and RNase inhibitor (40 U) for 45
min at 42°C. PCR was then performed in an automatic thermocycler
(Bioneer, Daejeon, South Korea) for 40 cycles (94°C for 5 min, 58°C
for 30 sec, and 72°C for 90 sec) followed by a 10 min extension at
95°C. The primer sequences as presented at Table I. The PCR products were separated in
2% agarose gels and visualized by EtBr staining. GAPDH was used for
normalization of the results. Gene expression was quantified using
ImageJ software (version 1.44; National Institutes of Health,
Bethesda, MD, USA) and results presented as fold change compared to
the control group.
 | Table I.Sequences of reverse
transcription-polymerase chain reaction primers. |
Table I.
Sequences of reverse
transcription-polymerase chain reaction primers.
| Gene name | Sequence |
|---|
| Caspase-3 |
|
|
Forward |
5′-CAAACTTTTTCAGAGGGGATCG-3′ |
|
Reverse |
5′-GCATACTGTTTCAGCATGGCA-3′ |
| Caspase-8 |
|
|
Forward |
5′-CCCCACCCTCACTTTGCT-3′ |
|
Reverse |
5′-GGAGGACCAGGCTCACTTA-3′ |
| Caspase-9 |
|
|
Forward |
5′-GGCCCTTCCTCGCTTCATCTC-3′ |
|
Reverse |
5′-GGTCCTTGGGCCTTCCTGGTAT-3′ |
| Bax |
|
|
Forward |
5′-AAGCTGAGCGAGTGTCTCCGGCG-3′ |
|
Reverse |
5′-CAGATGCCGGTTCAGGTACTCAGTC-3′ |
| Bcl-2 |
|
|
Forward |
5′-CTCGTCGCTACCGTCGTGACTTGG-3′ |
|
Reverse |
5′-CAGATGCCGGTTCAGGTACTCAGTC-3′ |
| Bcl-xL |
|
|
Forward |
5′-CCCAGAAAGGATACAGCTGG-3′ |
|
Reverse |
5′-GCGATCCGACTCACCAATAC-3′ |
| TRAIL |
|
|
Forward |
5′-GGAACCCAAGGTGGGTAGAT-3′ |
|
Reverse |
5′-TCTCACCACACTGCAACCTC-3′ |
| DR4 |
|
|
Forward |
5′-AAGTCCCTGCACCACGAC-3′ |
|
Reverse |
5′-CCACAACCTGAGCCGATG-3′ |
| DR5 |
|
|
Forward |
5′-TGAGATAAAGGTGGCTAAA-3′ |
|
Reverse |
5′-AAAGGTAAACCAGGGAAG-3′ |
| NF-κB |
|
|
Forward |
5′-CACTTATGGACAACTATGAGGTCTCT |
|
| GG-3′ |
|
|
Reverse |
5′-CTGTCTTGTGGACAACGCAGTGGAATTTTAGG-3′ |
| IκBα |
|
|
Forward |
5′-GCTGAAGAAGGAGCGGCTACT-3′ |
|
Reverse |
5′-TCGTACTCCTCGTCTTTCATGGA-3′ |
| iNOS |
|
|
Forward |
5′-AGAGAGATCGGGTTCACA-3′ |
|
Reverse |
5′-CACAGAACTGAGGGTACA-3′ |
| COX-2 |
|
|
Forward |
5′-TTAAAATGAGATTGTCCGAA-3′ |
|
Reverse |
5′-AGATCACCTCTGCCTGAGTA-3′ |
| MMP-2 |
|
|
Forward |
5′-CTTCTTCAAGGACCGGTTCA-3′ |
|
Reverse |
5′-GCTGGCTGAGTACCAGTA-3′ |
| MMP-9 |
|
|
Forward |
5′-TGGGCTACGTGACCTATGAC-3′ |
|
Reverse |
5′-GCCCAGCCCACCTCCACTCC-3′ |
| TIMP-1 |
|
|
Forward |
5′-GTCAGTGAGAAGCAAGTCGA-3′ |
|
Reverse |
5′-ATGTTCTTCTCTGTGACCCA-3′ |
| TIMP-2 |
|
|
Forward |
5′-TGGGGACACCAGAAGTCAAC-3′ |
|
Reverse |
5′-TTTTCAGAGCCTTGGAGGAG-3′ |
| Fas |
|
|
Forward |
5′-GAAATGAAATCCAAAGCT-3′ |
|
Reverse |
5′-TAATTTAGAGGCAAAGTGGC-3′ |
| FasL |
|
|
Forward |
5′-GGATTGGGCCTGGGGATGTTTCA-3′ |
|
Reverse |
5′-TTGTGGCTCAGGGGCAGGTTGTTG-3′ |
| GAPDH |
|
|
Forward |
5′-CGGAGTCAACGGATTTGGTC-3′ |
|
Reverse |
5′-AGCCTTCTCCATGGTCGTGA-3′ |
Statistical analysis
Data are presented as the mean ± standard deviation.
Differences between the mean values for individual groups were
assessed by a one-way analysis of variance with Duncan's multiple
range tests. P<0.05 was considered to indicate a statistically
significant difference. The SAS v9.1 statistical software package
(SAS Institute Inc., Cary, NC, USA) was used for the statistical
analysis.
Results
BLSEE reduced HCT-116 cancer cell
growth
As shown in Table II,
BLSEE significantly inhibited the colon cancer HCT-116 cell growth
in vitro in a dose-dependent manner. At a high dose of 400
µg/ml, BLSEE showed the highest inhibition activity of HCT-116
cells (86.3%; P=0.0002), compared with doses of 100 (P=0.0006) and
200 µg/ml BLSEE (P=0.0017).
 | Table II.Growth inhibition of human HCT-116
colon cancer cells by Ba lotus stamen extract as evaluated by MTT
assay. |
Table II.
Growth inhibition of human HCT-116
colon cancer cells by Ba lotus stamen extract as evaluated by MTT
assay.
| Treatment
(µg/ml) |
OD540 | Inhibitory rate,
% | P-value |
|---|
| Control | 0.488±0.003 |
|
|
| 100 µg/ml
BLSEE | 0.373±0.008 | 23.6±0.4 | 0.0017a |
| 200 µg/ml
BLSEE | 0.238±0.010 | 51.2±0.5 | 0.0006a |
| 400 µg/ml
BLSEE | 0.067±0.002 | 86.3±0.4 | 0.0002a |
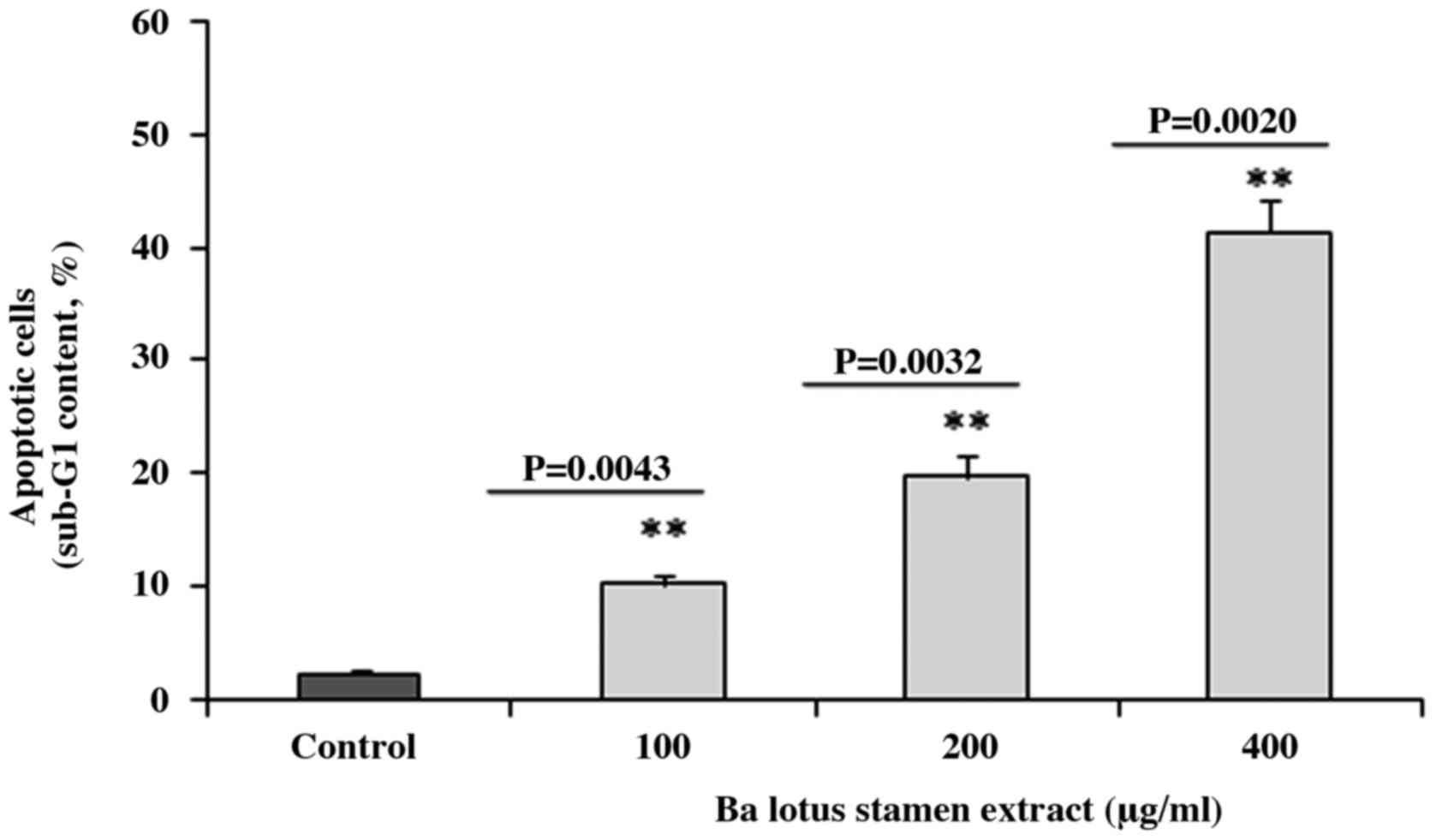
BLSEE induced the apoptosis in HCT-116
cancer cells
Flow cytometry analysis revealed that BLSEE
treatment was able to promote apoptosis in HCT-116 cells. As shown
in Fig. 1, the BLSEE treatment
significantly increased the proportion of apoptotic cells to 10.2
(P=0.0043), 19.7 (P=0.0032) and 41.3% (P=0.0020) compared with the
non-treated cells (2.3%).
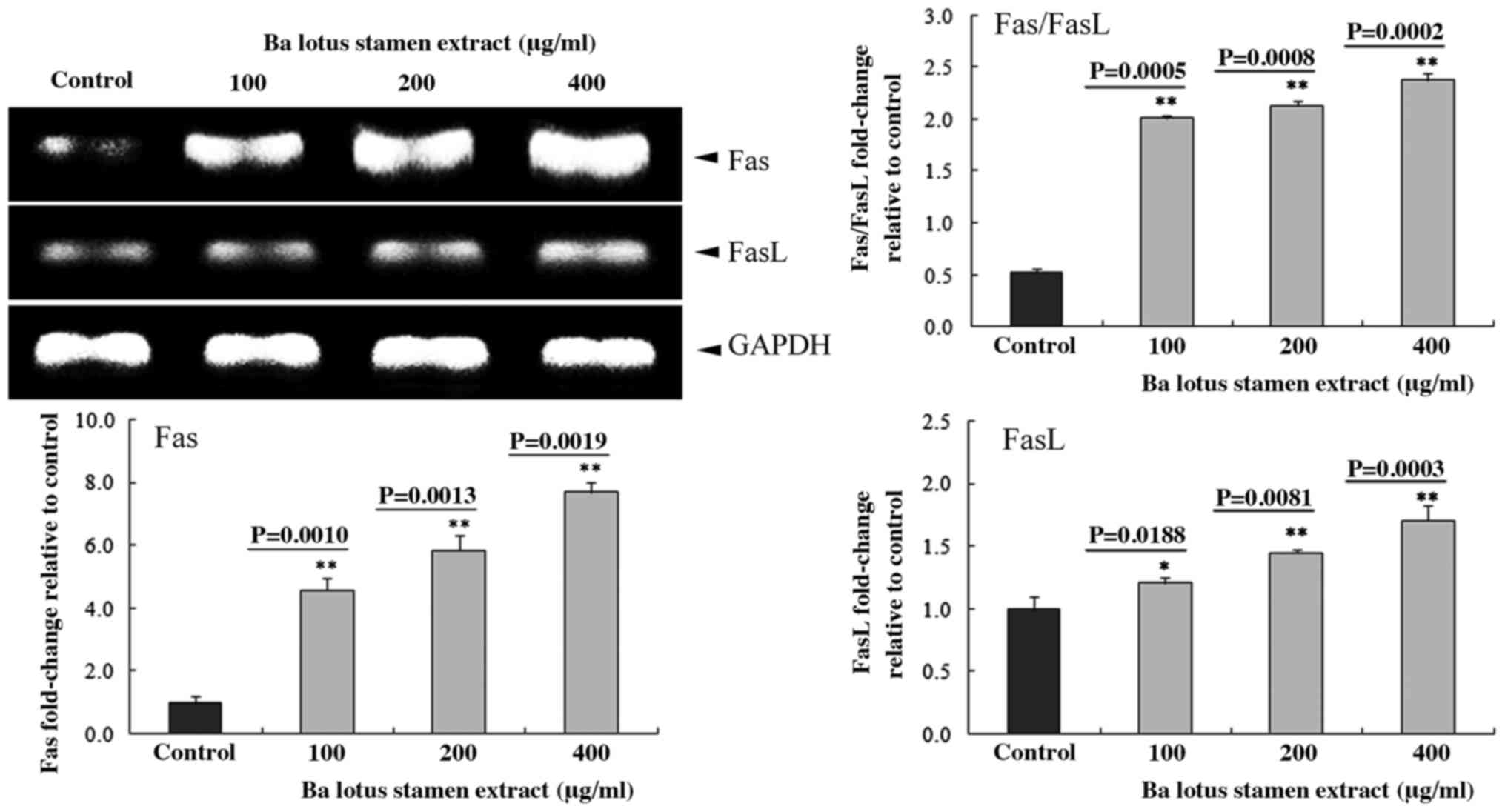
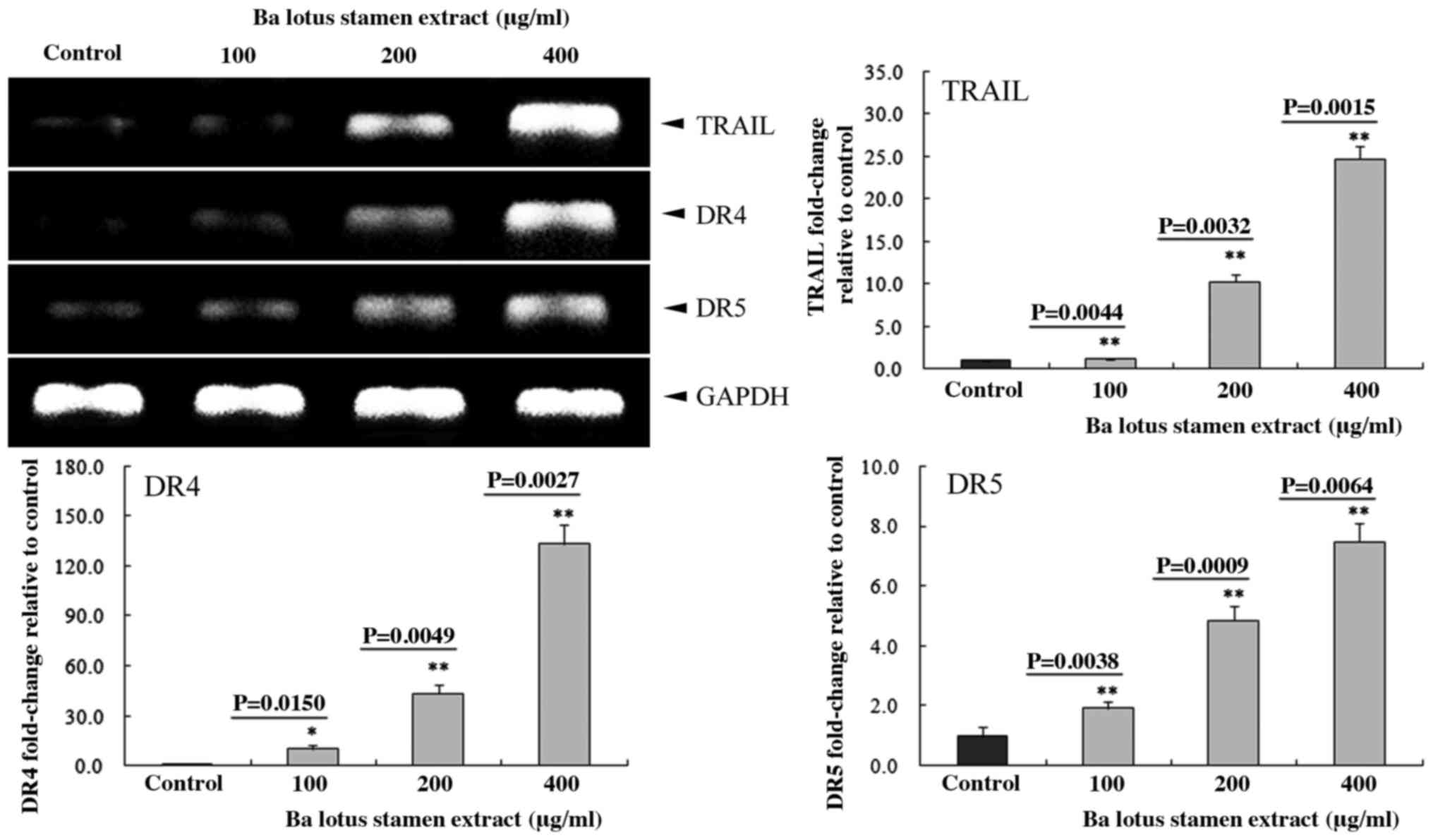
Effect of BLSEE on the expression of
Fas, FasL, tumor necrosis factor (TNF)-related apoptosis-inducing
ligand (TRAIL), death receptor 4 (DR4) and death receptor 5 (DR5)
in HCT-116 cells
Following treatment with various doses of BLSEE, the
mRNA levels of Fas (P=0.0019 at 400 µg/ml) and FasL (P=0.0003 at
400 µg/ml) were upregulated in HCT-116 cells (Fig. 2). In addition, 400 µg/ml of BLSEE
significantly upregulated the mRNA levels of TRAIL (24.7-fold;
P=0.0015), DR4 (133.2-fold; P=0.0027) and DR5 (7.5-fold; P=0.0064)
compared with non-treated HCT-116 cells (Fig. 3).
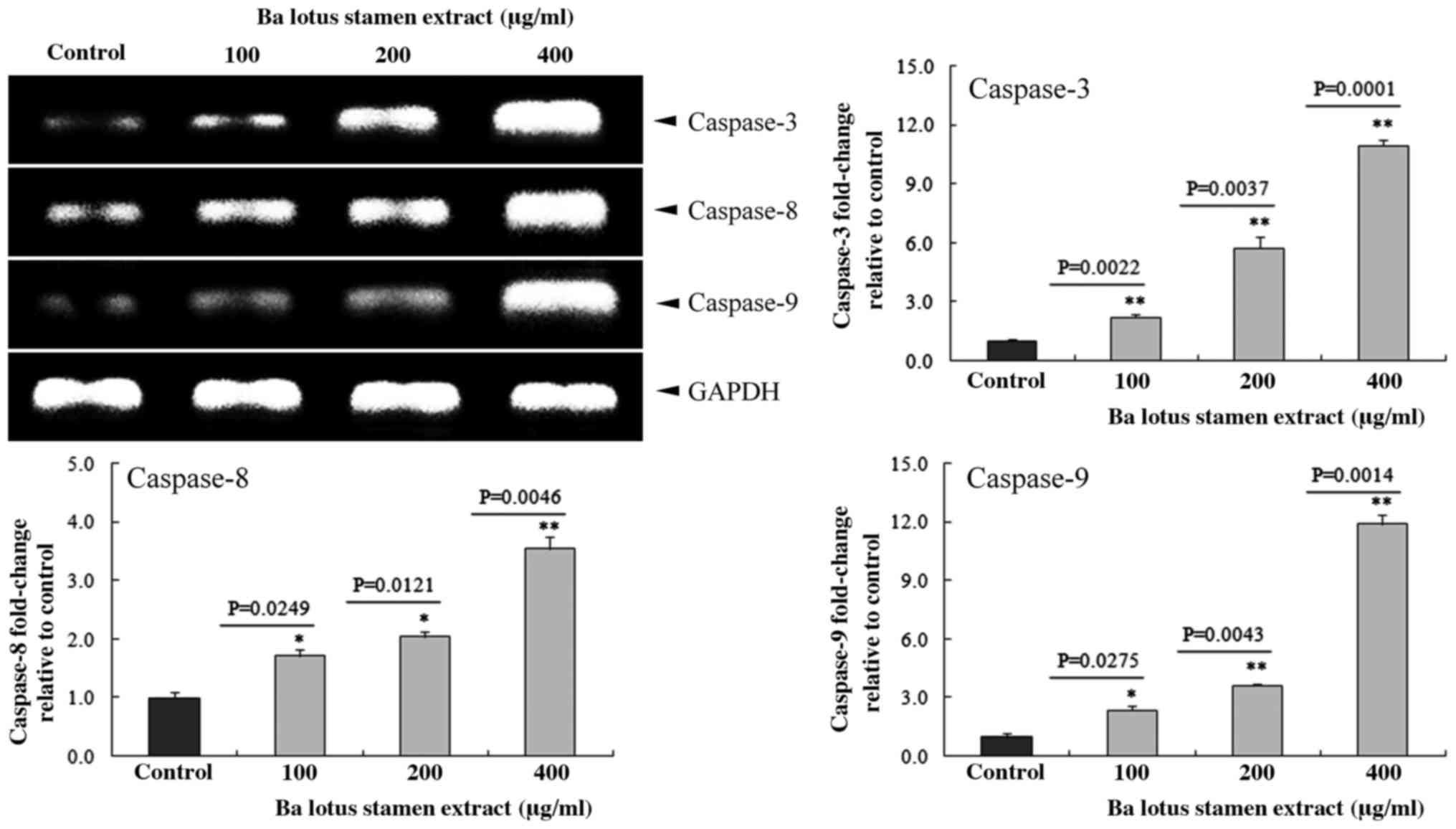
Effect of BLSEE on the expression of
caspases 3, 8 and 9 in HCT-116 cells
The effect of BLSEE on the mRNA caspase family,
including caspases 3, 8 and 9 in HCT-116 colon cancer cells was
determined by RT-PCR assay. BLSEE treatment significantly increased
the mRNA expressions of these caspases in HCT-116 cells in a
dose-dependent manner (Fig. 4). The
highest dose (400 µg/ml) of BLSEE also increased the mRNA levels of
caspase-3 (10.9-fold; P=0.0001), caspase-8 (3.5-fold; P=0.0046) and
caspase-9 (11.9-fold; P=0.0014) compared to the non-treated HCT-116
cells.
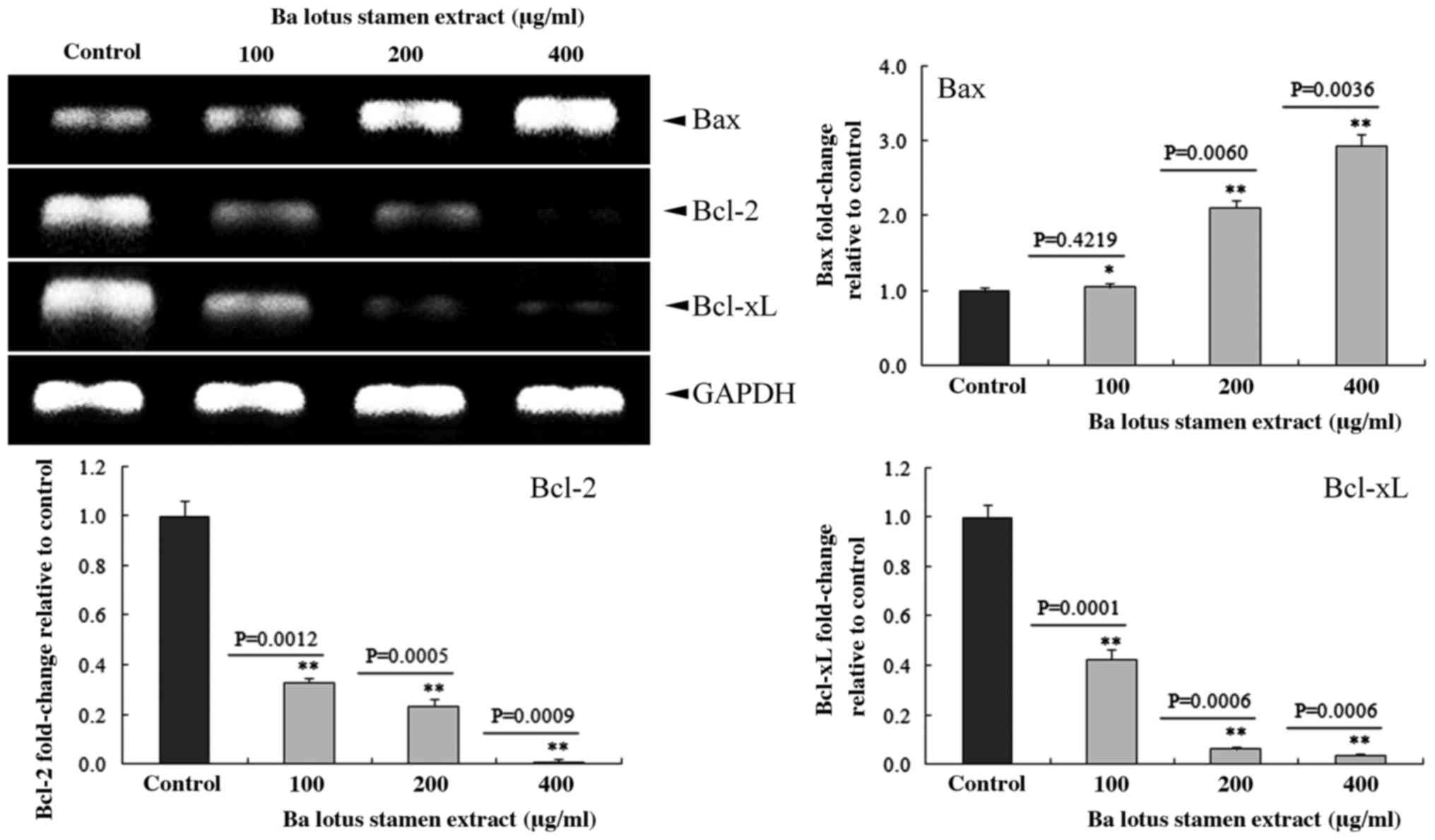
BLSEE modulated the expressions of
Bcl-2, Bcl-xL and Bax in HCT-116 cells
As shown in Fig. 5,
BLSEE treatment dose-dependently reduced the expression of Bcl-2
and Bcl-xL in HCT-116 cells. At the highest dose of 400 µg/ml,
BLSEE significantly reduced the mRNA levels of Bcl-2 (98.9%;
P=0.0009) and Bcl-xL (96.3%; P=0.0006) compared to the non-treated
HCT-116 cells, respectively. By contrast, 400 µg/ml of BLSEE also
enhanced the Bax levels of mRNA (2.9-fold; P=0.0036) compared to
non-treated HCT-116 cells.
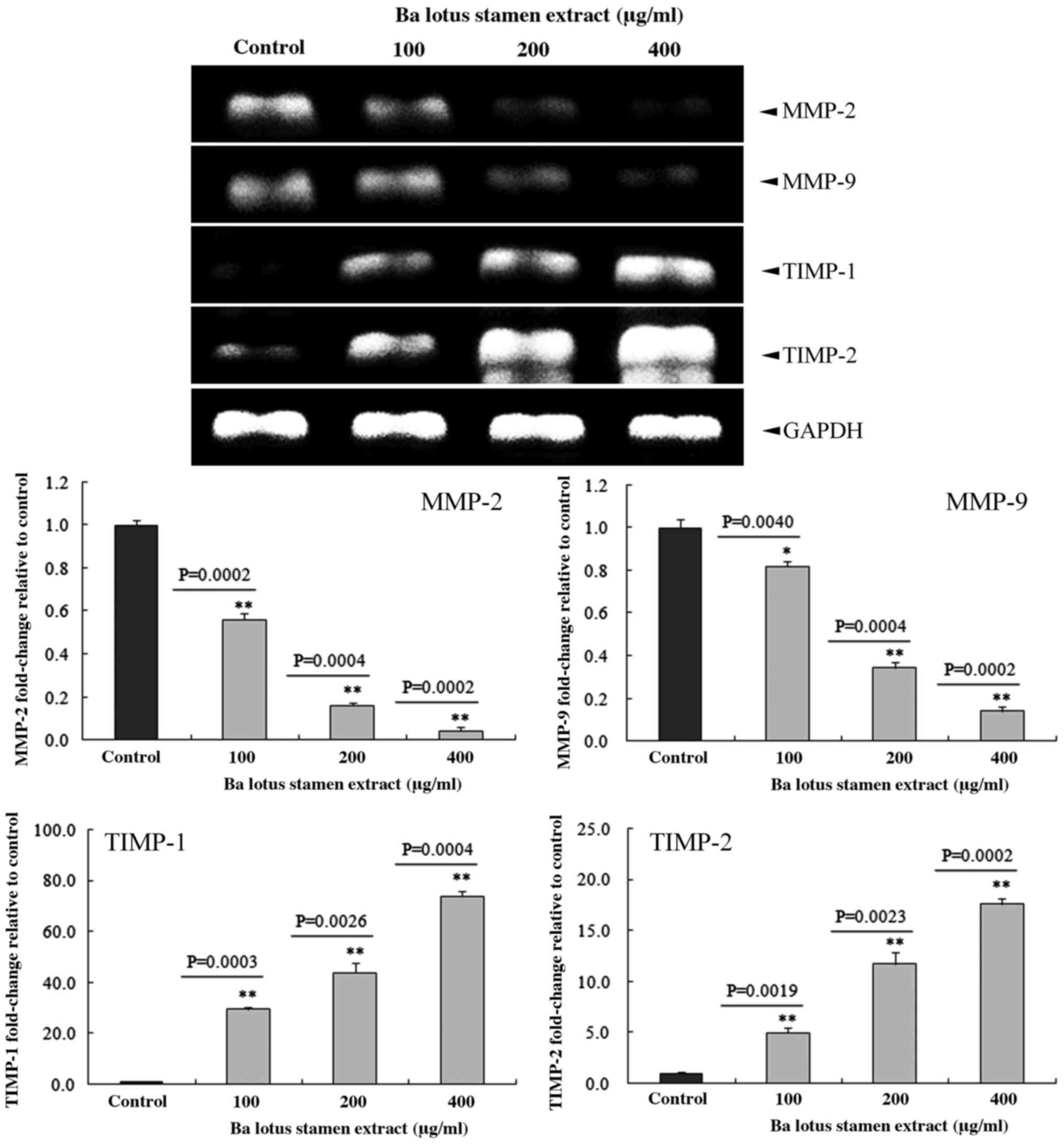
BLSEE modulated the expression of
matrix metalloproteinase (MMP)-2, MMP-9, TIMP metallopeptidase
inhibitor 1 (TIMP-1) and TIMP metallopeptidase inhibitor 2 (TIMP-2)
in HCT-116 cells
As shown in Fig. 6,
BLSEE treatment dose-dependently reduced the expression of MMP-2
and MMP-9 in HCT-116 cells. At the highest dose of 400 µg/ml, BLSEE
significantly reduced the mRNA levels of MMP-2 (95.8%; P=0.0002)
and MMP-9 (85.9%; P=0.0002) compared with non-treated HCT-116
cells. By contrast, 400 µg/ml of BLSEE was also able to enhance the
mRNA levels of TIMP-1 (17.6-fold; P=0.0004) and TIMP-2 (73.6-fold;
P=0.0002) compared with non-treated HCT-116 cells.
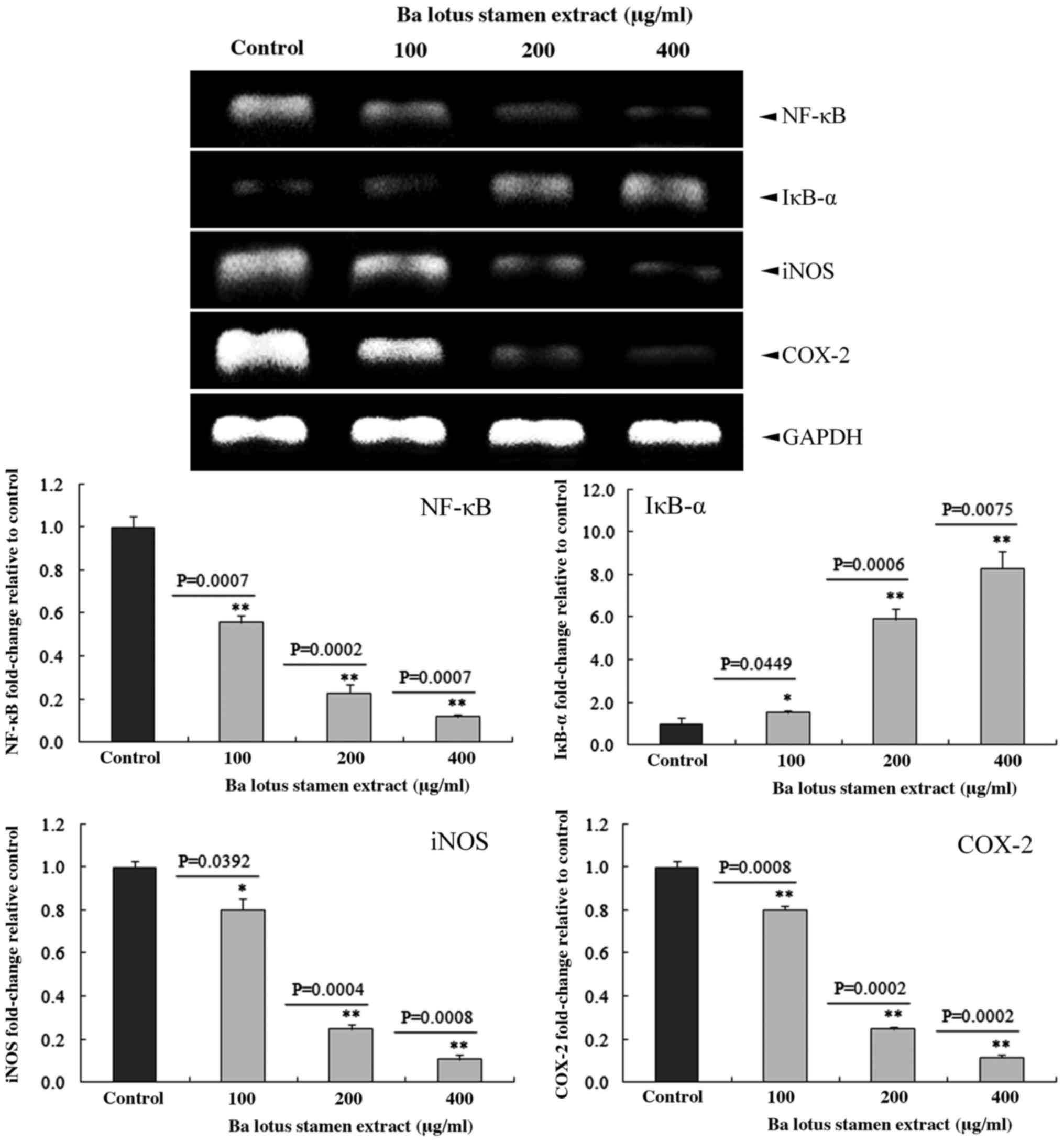
BLSEE modulated the expression of
inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX-2),
nuclear factor (NF)-κB and inhibitory κBα (IκBα) in HCT-116
cells
BLSEE treatment significantly decreased the mRNA
levels of iNOS, COX-2 and NF-κB, and increased the IκBα levels in
HCT-116 cells (Fig. 7). Subsequent to
treatment with a dose of 400 µg/ml BLSEE, the mRNA levels of iNOS,
COX-2 and NF-κB were decreased by 89.2 (P=0.0008), 88.3 (P=0.0002)
and 87.9% (P=0.0007) compared with the non-treated HCT-116 cells.
In addition, BLSEE also increased the mRNA levels of IκBα
(8.3-fold; P=0.0075) compared with non-treated HCT-116 cells.
Discussion
In the present study, BLSEE treatment was found to
significantly inhibit the cell proliferation, and also increase the
proportion of cells in the sub-G1 phase in HCT-116 cancer cells.
These results suggested that BLSEE may induce apoptosis in HCT-116
cells.
Based on these observations, the mRNA expression of
apoptosis-associated death receptors, including Fas and FasL, was
analyzed in HCT-116 cells using an RT-PCR assay. Fas and FasL are
apoptosis inducers, and play an important role during death
receptor-induced extrinsic apoptosis in cells (25). The present study found that following
incubation with 24 h of BLSEE, the mRNA levels of Fas and FasL were
each significantly increased in HCT-116 cells. Activation of
Fas/FasL was able to recruit FADD and death domain, and
subsequently induce the activation of caspases 8, 9 and 10 to
promote cellular apoptosis (26). In
addition, the present study also observed that mRNA levels of
TRAIL, DR4 and DR5 were increased by BLSEE treatment for 24 h.
TRAIL binds to TRAIL receptors, such as DR4 and DR5, to form a
trimeric complex, which leads to the recruitment of FADD, an
adaptor molecule that recruits and activates caspase-8 (27,28).
The caspase signaling cascade is a key event in
extrinsic and intrinsic cell apoptosis, which is characterized by
the activation of caspase-8 and caspase-9, respectively (9). Caspase-8 is a main initiator caspase in
Fas signaling, and is recruited to the activated Fas receptor, as
well as inducing death receptor-induced cell apoptosis (29). In addition, caspase-9 is an apoptosis
effector molecule in the mitochondrial channel and starts
programmed cell death subsequent to activation (10). Activated caspase-8 and caspase-9 are
each able to activate caspase-3, which is an executor of apoptosis
that subsequently induces apoptosis in cells (8). In the present study, it was found that
the mRNA levels of caspases 3, 8 and 9 were significantly increased
in BLSEE-treated HCT-116 cells.
It was also found that BLSEE treatment was able to
modulate the mRNA expression of apoptosis-associated Bcl-2 family
members in HCT-116 cells. BLSEE treatment significantly reduced the
mRNA levels of anti-apoptotic Bcl-2 and Bcl-xL in HCT-116 cells.
Bcl-2 and Bcl-xL are generally known as factors that prevent the
release of Cyto c from the mitochondria into the cytosol,
inhibiting apoptosis and promoting the survival of cells (5). Bcl-xL can also block the formation of
the apoptosome, which converges with Cyto c, Apaf-1 and
procaspase-9, to reduce the activation of caspase-3 and inhibit
apoptosis (30,31). The balance between anti-apoptosis and
pro-apoptosis factors may affect the occurrence of apoptosis in
cells, and is also associated with the success or failure of
chemotherapy in cancer patients (32). By contrast, BLSEE was able to increase
the mRNA levels of Bax, which is a pro-apoptotic protein, to
promote apoptosis in cells (5,7). The
activated Bax may be directly engaged by Bim to promote apoptosis
in cells (33). In addition, Bax also
induces the activation of caspase-8 and the release of Cyto
c from the mitochondria, resulting in the cleavage of
caspase-9, and contributes to the activation of executor caspase-3
(5,34). The present results suggest that the
BLSEE may modulate the balance between anti-apoptosis and
pro-apoptosis factors, in particular enhancing the expression
levels of pro-apoptotic Bax to promote apoptosis in HCT-116
cells.
MMPs are an important family of proteolytic enzymes
that not only contribute to organ development and tissue
regeneration, but also show an extremely important role in the
regulation of tumor invasion, angiogenesis and metastasis (35,36). In
particular, MMP-2 exhibits a key role in the progression of colon
cancer and the promotion of metastasis (37). Additionally, overexpression of MMP-9
is closely associated with poor prognosis in patients with colon
cancer (38). Numerous studies have
reported that herbs extracts were able to inhibit the migration or
invasion of colorectal cancer cells by downregulating MMP-2 and
MMP-9 in vivo (39–41). In the present study, it was found that
BLSEE significantly reduced the expression of MMP-2 and MMP-9, and
also increased the activation of TIMP-1 and TIMP-2 in HCT-116 colon
cancer cells. As natural MMP inhibitors, TIMP-1 and TIMP-2 are able
to inhibit the activation of MMP-2 and MMP-9 to reduce the invasion
and metastasis in patients with colon cancer (42,43).
Subsequent to treatment with BLSEE, the expression levels of TIMP-1
and TIMP-2 were significantly increased. Furthermore, maintaining
an increased level of TIMP-1 and TIMP-2 may improve the poor
survival of CRC patients (37,44).
Over activation of NF-κB has been found in numerous
human cancers (45,46). NF-κB promotes cell proliferation,
negatively regulates cell apoptosis (47), reduces cell death in tumorigenesis
(48) and induces the inflammatory
reaction in human disease (46,49). The
activation of NF-κB is able to reduce TNF-α-induced cell apoptosis
(50). TNF-α directly activates the
apoptosis inhibitor Bcl-xL (51), and
suppresses anti-apoptosis factors, including inhibitor of
apoptosis, caspase 8-FADD-like IL-1b-converting enzyme inhibitory
protein, TNF receptor associated factor (TRAF) 1 and TRAF2 to
regulate the pro- or anti-apoptotic pathways (52). In the present study, BLSEE treatment
significantly reduced the mRNA levels of NF-κB, and also increased
the mRNA levels of IκBα in HCT-116 cells. Enhancement of the IκBα
activation is a potential strategy to reduce cancer cell growth in
clinical chemotherapy (53,54).
In conclusion, the present study has clearly
indicated that BLSEE suppresses the proliferation of human HCT-116
colon carcinoma cells in vitro. BLSEE may effectively induce
the apoptosis through reducing the ratio of anti-apoptotic Bcl-2 to
pro-apoptotic Bax, and increasing the activation of the death
receptor system, as well as promoting the cleavage of caspases-3, 8
and 9 in HCT-116 cells. These results suggest that the BLSEE is
able to induce HCT-116 cell apoptosis through activating death
receptor and mitochondrial apoptotic pathways.
Acknowledgements
The present study was supported by Chongqing
Engineering Research Center for Functional Food (grant no.,
cstc2015yfpt_gcjsyjzx0027) and the Program for Innovation and Team
Building at the Chongqing Institute of Higher Education (grant no.
CXTDX201601040).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Long N, Moore M, Chen W, Gao CM, Lai MS,
Mizoue T, Oyunchimeg D, Park S, Shin HR, Tajima K, et al: Cancer
epidemiology and control in north-East Asia-past, present and
future. Asian Pac J Cancer Prev. 11:(Suppl 2). S107–S148. 2010.
|
|
3
|
Wang ZX, Cao JX, Liu ZP, Cui YX, Li CY, Li
D, Zhang XY, Liu JL and Li JL: Combination of chemotherapy and
immunotherapy for colon cancer in China: A meta-analysis. World J
Gastroenterol. 20:1095–1060. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Volkmann N, Marassi FM, Newmeyer DD and
Hanein D: The rheostat in the membrane: BCL-2 family proteins and
apoptosis. Cell Death Differ. 206–215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peter ME and Krammer PH: The
CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 10:26–35. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cullen SP and Martin SJ: Caspase
activation pathways: Some recent progress. Cell Death Differ.
16:935–938. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Y, Lei M, Wang Z, Qiao G, Yang T and
Zhang J: TCR-induced, PKC-θ-mediated NF-κB activation is regulated
by a caspase-8-caspase-9-caspase-3 cascade. Biochem Biophys Res
Commun. 450:526–531. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsiao WL and Liu L: The role of
traditional Chinese herbal medicines in cancer therapy-from TCM
theory to mechanistic insights. Planta Med. 76:1118–1131. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qi F, Li A, Inagaki Y, Gao J, Li J, Kokudo
N, Li XK and Tang W: Chinese herbal medicines as adjuvant treatment
during chemo-or radio-therapy for cancer. Biosci Trends. 4:297–307.
2010.PubMed/NCBI
|
|
14
|
Parsons ME and Keeling DJ: Novel
approaches to the pharmacological blockade of gastric acid
secretion. Expert Opin Investig Drugs. 14:411–421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Talukder MJ and Nessa J: Effect of Nelumbo
nucifera rhizome extract on the gastrointestinal tract of rat.
Bangladesh Med Res Counc Bull. 24:6–9. 1998.PubMed/NCBI
|
|
16
|
Kuo YC, Lin YL, Liu CP and Tsai WJ: Herpes
simplex virus type 1 propagation in HeLa cells interrupted by
Nelumbo nucifera. J Biomed Sci. 12:1021–1034. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ono Y, Hattori E, Fukaya Y, Imai S and
Ohizumi Y: Anti-obesity effect of Nelumbo nucifera leaves extract
in mice and rats. J Ethnopharmacol. 106:238–244. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin HY, Kuo YH, Lin YL and Chiang W:
Antioxidative effect and active components from leaves of Lotus
(Nelumbo nucifera). J Agric Food Chem. 57:6623–6629. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu CH, Yang MY, Chan KC, Chung PJ, Ou TT
and Wang CJ: Improvement in high-fat diet-induced obesity and body
fat accumulation by a Nelumbo nucifera leaf flavonoid-rich extract
in mice. J Agric Food Chem. 58:7075–7081. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Du H, You JS, Zhao X, Park JY, Kim SH and
Chang KJ: Antiobesity and hypolipidemic effects of lotus leaf hot
water extract with taurine supplementation in rats fed a high fat
diet. J Biomed Sci. 17:(Suppl 1). S422010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee JS, Shukla S, Kim JA and Kim M:
Anti-angiogenic effect of Nelumbo nucifera leaf extracts in human
umbilical vein endothelial cells with antioxidant potential. PLoS
One. 10:e01185522015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sohn DH, Kim YC, Oh SH, Park EJ, Li X and
Lee BH: Hepatoprotective and free radical scavenging effects of
Nelumbo nucifera. Phytomedicine. 10:165–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu CP, Tsai WJ, Lin YL, Liao JF, Chen CF
and Kuo YC: The extracts from Nelumbo Nucifera suppress cell cycle
progression, cytokine genes expression, and cell proliferation in
human peripheral blood mononuclear cells. Life Sci. 75:699–716.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang CF, Chen YW, Yang CY, Lin HY, Way
TD, Chiang W and Liu SH: Extract of lotus leaf (Nelumbo nucifera)
and its active constituent catechin with insulin secretagogue
activity. J Agric Food Chem. 59:1087–1094. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
O'Reilly LA, Tai L, Lee L, Kruse EA,
Grabow S, Fairlie WD, Haynes NM, Tarlinton DM, Zhang JG, Belz GT,
et al: Membrane-bound Fas ligand only is essential for Fas-induced
apoptosis. Nature. 461:659–663. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Waring P and Müllbacher A: Cell death
induced by the Fas/Fas ligand pathway and its role in pathology.
Immunol Cell Biol. 77:312–317. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang S and El-Deiry WS: TRAIL and
apoptosis induction by TNF-family death receptors. Oncogene.
22:8628–8633. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang L and Fang B: Mechanisms of
resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther.
12:228–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scaffidi C, Medema JP, Krammer PH and
Peter ME: FLICE is predominantly expressed as two functionally
active isoforms, caspase-8/a and caspase-8/b. J Biol Chem.
272:26953–26958. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pan G, O'Rourke K and Dixit VM: Caspase-9,
Bcl-XL, and Apaf-1 form a ternary complex. J Biol Chem.
273:5841–5845. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu Y, Benedict MA, Wu D, Inohara N and
Núñez G: Bcl-XL interacts with Apaf-1 and inhibits Apaf-1-dependent
caspase-9 activation. Proc Natl Acad Sci USA. 95:4386–4391. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Letai A, Bassik MC, Walensky LD,
Sorcinelli MD, Weiler S and Korsmeyer SJ: Distinct BH3 domains
either sensitize or activate mitochondrial apoptosis, serving as
prototype cancer therapeutics. Cancer Cell. 2:183–192. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Finucane DM, BossyWetzel E, Waterhouse NJ,
Cotter TG and Green DR: Bax-induced caspase activation and
apoptosis via cytochrome c release from mitochondria is inhibitable
by Bcl-xL. J Biol Chem. 274:2225–2233. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moss LA Shuman, JensenTaubman S and
Stetler-Stevenson WG: Matrix metalloproteinases: Changing roles in
tumor progression and metastasis. Am J Pathol. 181:1895–1899. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Groblewska M, Mroczko B, Gryko M,
Pryczynicz A, Guzińska-Ustymowicz K, Kędra B, Kemona A and
Szmitkowski M: Serum levels and tissue expression of matrix
metalloproteinase 2 (MMP-2) and tissue inhibitor of
metalloproteinases 2 (TIMP-2) in colorectal cancer patients. Tumour
Biol. 35:3793–3802. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang B, Tang F, Zhang B, Zhao Y, Feng J
and Rao Z: Matrix metalloproteinase-9 overexpression is closely
related to poor prognosis in patients with colon cancer. World J
Surg Oncol. 12:242014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Seo EY and Kim WK: Red ginseng extract
reduced metastasis of colon cancer cells in vitro and in vivo. J
Ginseng Res. 35:315–324. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Deng W, Sui H, Wang Q, He N, Duan C, Han
L, Li Q, Lu M and Lv S: A Chinese herbal formula, Yi-Qi-Fu-Sheng,
inhibits migration/invasion of colorectal cancer by down-regulating
MMP-2/9 via inhibiting the activation of ERK/MAPK signaling
pathways. BMC Complement Altern Med. 13:652013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao X, Sun P, Qian Y and Suo H: D.
candidum has in vitro anticancer effects in HCT-116 cancer cells
and exerts in vivo anti-metastatic effects in mice. Nutr Res Pract.
8:487–493. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li BH, Zhao P, Liu SZ, Yu YM, Han M and
Wen JK: Matrix metalloproteinase-2 and tissue inhibitor of
metallo-proteinase-2 in colorectal carcinoma invasion and
metastasis. World J Gastroenterol. 11:3046–3050. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Murnane MJ, Cai J, Shuja S, McAneny D,
Klepeis V and Willett JB: Active MMP-2 effectively identifies the
presence of colorectal cancer. Int J Cancer. 125:2893–2902. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mroczko B, Groblewska M, Okulczyk B, Kędra
B and Szmitkowski M: The diagnostic value of matrix
metalloproteinase 9 (MMP-9) and tissue inhibitor of matrix
metalloproteinases 1 (TIMP-1) determination in the sera of
colorectal adenoma and cancer patients. Int J Colorectal Dis.
25:1177–1184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rayet B and Gélinas C: Aberrant rel/nfkb
genes and activity in human cancer. Oncogene. 18:6938–6947. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-kB in development and progression of human
cancer. Virchows Arch. 446:475–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ohshima K, Sugihara M, Haraoka S, Suzumiya
J, Kanda M, Kawasaki C, Shimazaki K and Kikuchi M: Possible
immortalization of Hodgkin and Reed-Sternberg cells: Telomerase
expression, lengthening of telomere, and inhibition of apoptosis by
NF-kappaB expression. Leuk Lymphoma. 41:367–376. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ghosh S and Karin M: Missing pieces in the
NF-kappaB puzzle. Cell. 109(Suppl): S81–S96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tak PP and Firestein GS: NF-kappaB: A key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Van Antwerp DJ, Martin SJ, Verma IM and
Green DR: Inhibition of TNF-induced apoptosis by NF-kappaB. Trends
Cell Biol. 8:107–111. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen C, Edelstein LC and Gélinas C: The
Rel/NF-kappaB family directly activates expression of the apoptosis
inhibitor Bcl-x(L). Mol Cell Biol. 20:2687–2695. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lin A and Karin M: NF-kappaB in cancer: A
marked target. Semin Cancer Biol. 13:107–114. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tergaonkar V, Bottero V, Ikawa M, Li Q and
Verma IM: IkappaB kinase-independent IkappaBalpha degradation
pathway: Functional NF-kappaB activity and implications for cancer
therapy. Mol Cell Biol. 23:8070–8083. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lee CH, Jeon YT, Kim SH and Song YS:
NF-kappaB as a potential molecular target for cancer therapy.
Biofactors. 29:19–35. 2007. View Article : Google Scholar : PubMed/NCBI
|