Introduction
Sarcomatoid urothelial carcinoma is an uncommon
histological variant of urothelial carcinoma. The majority of
sarcomatoid urothelial carcinomas are found in the urinary bladder
(1). Generally, >90% of ureteral
tumors are urothelial carcinomas; other types of ureteral tumors,
including squamous cell carcinoma, adenocarcinoma and small cell
carcinoma, are less common (2). To
the best of our knowledge, only 24 cases of the ureteral
sarcomatoid urothelial carcinoma have been reported in the PubMed
database (http://www.ncbi.nlm.nih.gov/pubmed) (Table I) (3–24), the
majority of which were accompanied by a malignant heterologous
element (chondrosarcoma). Sarcomatoid carcinoma of the urinary
bladder has been associated with a poor prognosis (25). Due to the rarity of this tumor, there
is limited information regarding its pathological features,
clinical behavior and prognosis.
 | Table I.Reported cases of sarcomatoid
urothelial carcinoma of the ureter. |
Table I.
Reported cases of sarcomatoid
urothelial carcinoma of the ureter.
| Author (year) | Age
(years)/gender | Symptoms/clinical
findings | Tumor histology | Outcome | Reference |
|---|
| Renner (1931) | 71/M | Unknown | Papillary carcinoma,
SCT, chondrosarcoma | Unknown | (3) |
| McDade et al
(1974) | 66/M | Hematuria, colic-like
abdominal pain | Malignant epithelial
and stromal elements | Local recurrence 4
months later; treatment with diathermy; patient was symptom-free at
time of publication | (12) |
| Yano et al
(1984) | 66/M | Flank pain,
intermittent fever | SQCCA, SCT,
chondrosarcoma | Succumbed to disease
2 years later; autopsy showed recurrence of sarcomatous component
(chondrosarcoma) | (4) |
| Bryard et al
(1987) | 75/F | 3-week history of
urinary frequency, left flank pain and fever | Urothelial carcinoma,
SQCCA, adenocarcinoma, chondrosarcoma | Local recurrence 6
months later with left iliac paraaortic lymph node metastasis;
succumbed to disease 2.5 years later | (5) |
| Fleming et al
(1987) | 68/M | 6-year history of
mild difficulty initiating micturition and urinary frequency; loin
pain, single episode of hematuria | Pleomorphic
carcinoma, SCT | Pedunculated mass in
the bladder measuring 6 cm arising from right ureteral stump 6
months later; succumbed to widespread metastasis | (24) |
| Fukuda et al
(1991) | 69/M | Hematuria and
hydronephrosis | Urothelial carcinoma,
SQCCA, SCT, chondrosarcoma | Patient alive at time
of publication | (6) |
| Tsutsumi et al
(1993) | 60/M | Asymptomatic gross
hematuria | SCCA, urothelial
carcinoma, SQCCA, LMS, chondrosarcoma | Recurrence of
high-grade invasive transitional cell carcinoma on bladder neck 8
months later; radiation and chemotherapy treatment; back pain 4
months later due to thoracic vertebra metastasis; no evidence of
metastasis 16 months s/p primary diagnosis and subsequent
radiation | (7) |
| Ishikura et al
(1994) | 73/M | Computed tomography
revealed a solid, cystic mass in the left retroperitoneum and
multiple nodules in the liver | Undifferentiated
carcinoma, blastomatous cells, chondrosarcoma | Large recurrent tumor
filling the abdomen s/p surgery and chemotherapy; succumbed to
disease 3 months later | (8) |
| Murata et al
(1994) | 80/M | Edema in right lower
extremity | SQCCA, MFH-like
SCT | Succumbed to disease
3 months after diagnosis | (13) |
| Murata et al
(1994) | 62/M | Gross hematuria | Urothelial carcinoma,
SQCCA, MFH-like sarcoma | No evidence of
disease, patient alive 23 months later | (13) |
| Burt et al
(1995) | 74/M | Severe right-sided
abdominal pain, vomiting and fever for 24 h | Urothelial carcinoma,
osteosarcoma, SCT, myxoid areas | No evidence of
disease, patient alive 9 months later | (14) |
| Nagayoshi et
al (1997) | 60/F | History of right
flank pain s/p oophorectomy and radiation therapy for ovarian
cancer at the age of 40 years | Urothelial carcinoma,
SCT | No evidence of
disease, patient alive 5 months later | (15) |
| Ichiniyagi et
al (1998) | 67/F | Hematuria | Urothelial carcinoma,
SQCCA, chondrosarcoma | Succumbed to
disease at 10 months s/p adjuvant radiotherapy | (9) |
| Kakoi et al
(2002) | 89/M | Gross hematuria,
left hydronephrosis caused by ureteral tumor | Carcinosarcoma of
renal pelvis and ureter | Recurrent gross
hematuria s/p radiation and chemotherapy; cancer-free for 1.5 years
s/p left nephroureterectomy | (20) |
| Perimenis et
al (2003) | 68/F | Hematuria, low back
pain | PD carcinoma,
SCT | Pelvic recurrence
at 18 months with liver metastasis 2 months later; succumbed to
disease 2 years later | (16) |
| Johnin et al
(2003) | 58/F | Painless
hematuria | Urothelial
carcinoma, osteosarcoma and chondrosarcoma | Tumor recurrence in
bladder 2 months later; succumbed 6 months after presentation, due
to local recurrence despite anterior exenteration | (23) |
| Lee et al
(2004) | 90/F | Left iliac fossa
pain | Malignant SCT | The patient refused
treatment and succumbed to the disease 3 months after
diagnosis | (17) |
| Petsch et al
(2004) | 82/F | Left flank
pain | SC | Not stated | (19) |
| Darko et al
(2006) | 81/F | Gross
hematuria | Urothelial
carcinoma, SQCCA, mesenchymal chondrosarcoma | Positron emission
tomography identified new nodules in the lungs and an enlarged
paraaortic lymph node compressing the vena cava 4 months later;
chemotherapy administered | (22) |
| Busby et al
(2006) | Not stated | Unknown | SC | Recurrence in tumor
bed 3 months later; adjuvant chemotherapy treatment; succumbed to
disease 3 months later | (18) |
| Maeda et al
(2007) | 63/M | Asymptomatic gross
hematuria | Basaloid SQCCA,
SCT, chondrosarcoma | Uneventful
postoperative course; no tumor recurrence at 10 months | (10) |
| Völker et al
(2008) | 83/M | Painless
macrohematuria and hydronephrosis | Urothelial
carcinoma, SCT, poorly differentiated transitional cell carcinoma,
chondrosarcoma | No evidence of the
disease; patient alive 36 months later | (11) |
| Völker et al
(2008) | 67/F | Hydronephrosis | Urothelial
carcinoma, SCT, RMS, undifferentiated sarcoma | No evidence of the
disease; patient alive 18 months later | (11) |
| Nicolas et
al (2014) | 63/M | Difficulty of
urination for several months | Urothelial
carcinoma, PD carcinoma, adenocarcinoma, SCT, chondrosarcoma | Recurrent lesions
progressed despite treatment and patient succumbed to disease 16
months after initial diagnosis | (21) |
| Lu et
al | 72/M | Gross hematuria,
urinary irritation, pain in left flank and groin | Urothelial
carcinoma, SCT, chondrosarcoma | Patient refused
treatment and succumbed to bone and omentum metastases 6 months
later | Present case |
The present study reports a rare case of sarcomatoid
urothelial carcinoma with chondrosarcomatous differentiation of the
ureter. In addition, the clinical and pathological features of
ureteral sarcomatoid carcinomas, with particular emphasis on the
differential diagnosis and prognosis of the disease, are discussed
based on a review of the existing literature. Written informed
consent was obtained from the patient.
Case report
A 72-year-old male patient presenting with
hematuria, urinary irritation and pain in the left flank and groin
was referred to Sun Yat-Sen University Cancer Center (Guangzhou,
China) on March 5, 2013. The patient had undergone a
cystoureteroscopy at Jieyang People's Hospital (Jieyang, China) on
February 27, 2013, and poorly differentiated urothelial carcinoma
had been detected on the ureteral biopsy. He subsequently presented
to our hospital. The patient had a history of kidney calculi for
~10 years, tobacco smoking for ~40 years and unknown drug
allergies. His family had no notable medical history. Laboratory
examinations showed the following: White blood cell count,
6.7×109/l (normal range, 3.5–9.5×109/l);
neutrophils, 56.5% (normal range, 0.40–0.75%); red blood cell
count, 4.34×1012/l (normal range,
3.8–5.8×1012/l); hemoglobin level, 140 g/l (normal
range, 110–160 g/l); platelet count, 158×109/l (normal
range, 100–300×109/l); red blood cells in urine, 37/µl;
occult blood, 2+. The left and right renal glomerular filtration
rates were 7.16 ml/min and 51 ml/min (normal range, 80–125 ml/min),
respectively, and the levels of the tumor markers serum creatinine,
urea nitrogen and cystatin C were found to be within the normal
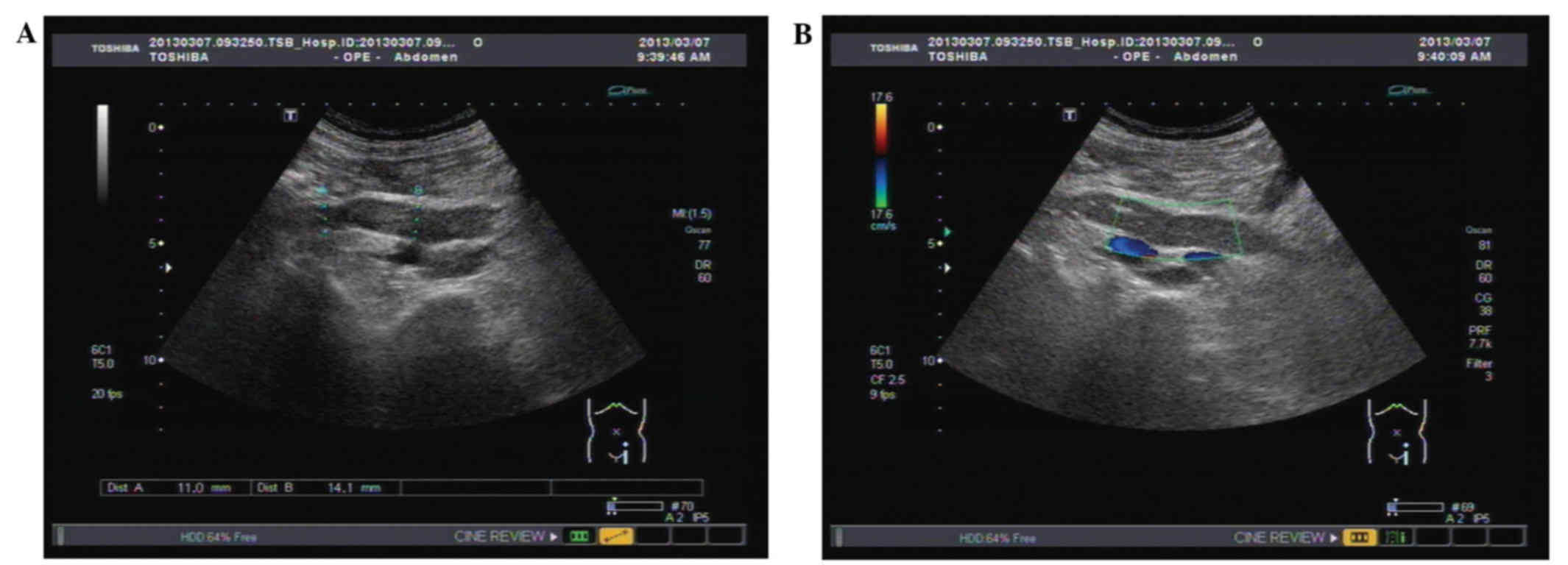
ranges. Ultrasonography of the urinary tract detected that the
tumor was located in the middle-lower ureter, and that the lumen
was obstructed by 70×15×15 mm mass, which led to the dilatation of
the proximal ureter and renal pelvis (Fig. 1A and B). Sonography led to suspicion
of upper tract urothelial carcinoma. Chest X-ray and
ultrasonography of the urinary tract indicated no signs of
metastasis.
The patient underwent left nephroureterectomy and
lymphadenectomy. Macroscopically, the surgical specimen revealed a
tumor located in the distal ureter. In addition, no obvious
abnormalities in the renal pelvis were observed. On the cut section
of the tumor, fragile areas were found on the grayish-yellow cut
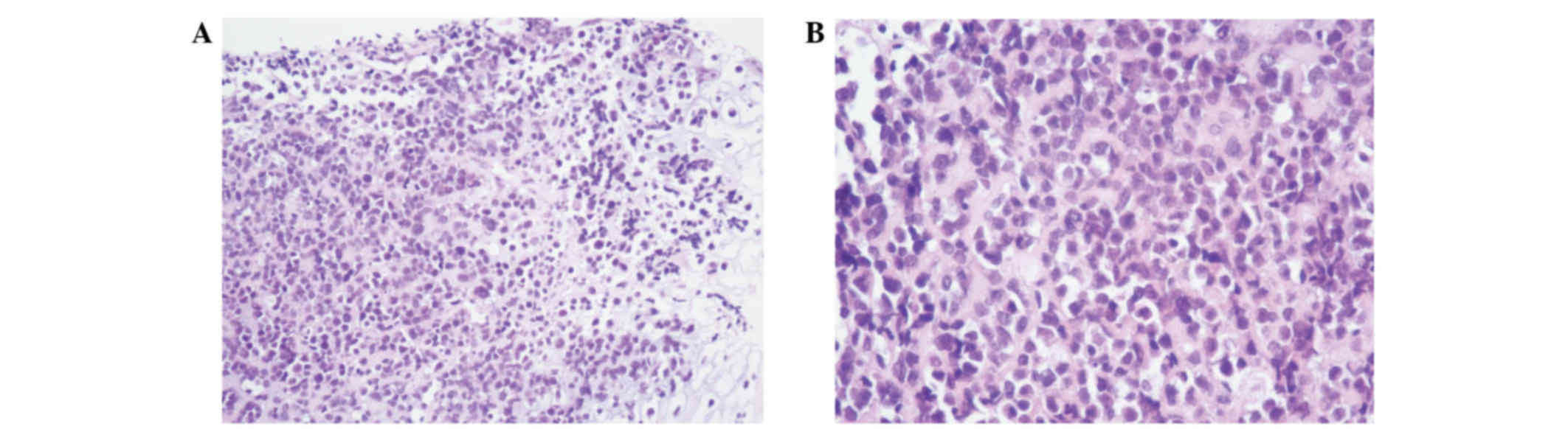
surface. Microscopic examination revealed that the tumor cells were
arranged in sheets or tubes, or had a ‘cracked’ appearance
(Fig. 2A and B). Atypia and
pathological mitosis of cancer cells was distinct.
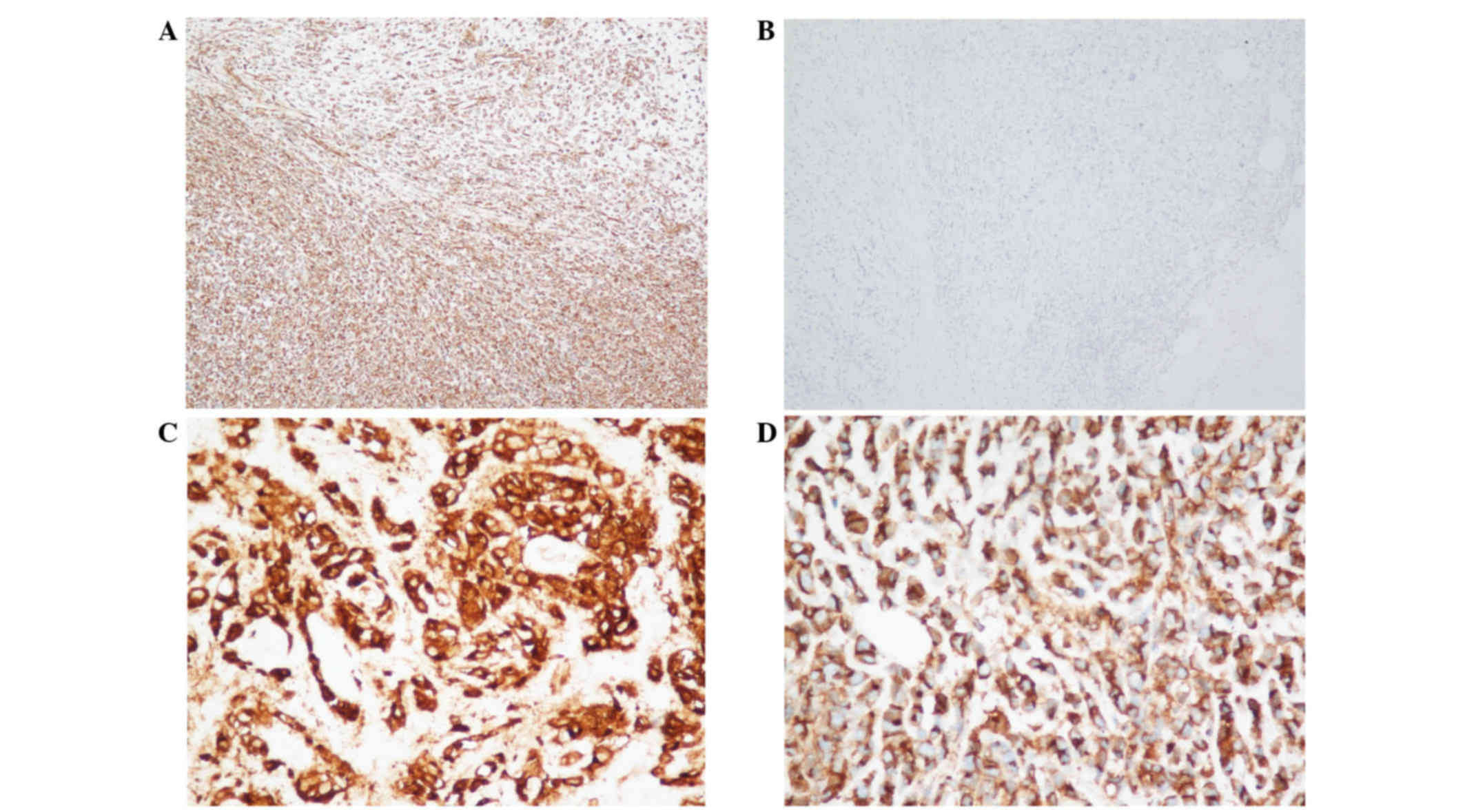
Immunohistochemical analysis indicated positivity of the tumor
cells for vimentin (monoclonal mouse antibody; cat. no. ZM-0260;
ZSGB-BIO, Beijing, China; Fig. 3A),
epithelial membrane antigen (monoclonal mouse antibody; cat. no.
ZM-0095; ZSGB-BIO; Fig. 3B), CD10
(monoclonal rabbit antibody; cat. no. NCL-CD10-270; Quanhui Imp
& Exp Int'l Co., Ltd., Macao, China; Fig. 3C), CD99 (monoclonal rabbit antibody;
cat. no. ZA-0577; ZSGB-BIO; Fig. 3D)
and CD56 (monoclonal mouse antibody; cat. no. ZM-0057; ZSGB-BIO),
and negativity for low molecular weight cytokeratin (CK)
(monoclonal mouse antibody; cat. no. Z2061; ZSGB-BIO), CK-7
(monoclonal mouse antibody; cat. no. 180234; ZSGB-BIO),
carcinoembryonic antigen (monoclonal mouse antibody; cat. no.
ZM-0061; ZSGB-BIO), CK5/6 (monoclonal mouse antibody; cat. no.
ZM-0313; ZSGB-BIO), leukocyte common antigen (monoclonal mouse
antibody; cat. no. ZM-0183; ZSGB-BIO), S100 (monoclonal rabbit
antibody; cat. no. ZA-0225; ZSGB-BIO), CD34 (monoclonal mouse
antibody; cat. no. ZM-0046; ZSGB-BIO), CD31 (monoclonal rabbit
antibody; cat. no. ZA-0568; ZSGB-BIO), synaptophysin (monoclonal
rabbit antibody; cat. no. ZA-0506; ZSGB-BIO), chromogranin A
(polyclonal rabbit antibody; cat. no. ZA-0066; ZSGB-BIO) and low
molecular weight CK (monoclonal mouse antibody; cat. no. ZM-0329;
ZSGB-BIO). A high cell proliferation rate (>60% immunoreactive
cells) was revealed by Ki-67 staining, indicating the malignant
nature of the lesion. Reticular fiber staining showed that the
cells had a nest-like distribution; therefore, a diagnosis of
invasive sarcomatoid urothelial carcinoma interspersed with
chondrosarcomatous differentiation of the ureter was proposed. The
retroperitoneal lymph nodes on the left side of the renal hilum and
paraaortic nodes were also involved. The patient refused to undergo
chemotherapy and succumbed to bone and omentum metastases 6 months
later.
Discussion
Urothelial carcinoma is the most common histological
subtype of urothelial cancer, followed by squamous cell carcinoma,
adenocarcinoma and small cell carcinoma. Over 90% of urothelial
carcinomas derive from the urinary bladder, 8% from the renal
pelvis, and the remaining 2% from the ureter and urethra (26). Urothelial carcinoma has an uncommon
sarcomatoid variant with a distinctive histological appearance.
‘Invasive urothelial carcinoma, sarcomatoid variant’, was the term
preferred by the 2004 World Health Organization Classification
Tumors of the Urinary System (27).
It is generally believed that sarcomatoid carcinoma is a rare type
of cancer. Furthermore, the sarcomatoid component may be a
metaplastic part of the cancer, and the heterologous sarcomatoid
component of the tumor (such as chondrosarcoma and osteosarcoma)
derives from a special type of mesenchyma (22).
With regard to the overlapping histology and
immunophenotype, as well as the aggressive biological behavior of
these tumors, a hypothesis was presented by the researchers that
both carcinomatous and sarcomatous elements have a common cell of
origin (27). This hypothesis was
validated by a series of studies. Sung et al (28) performed a study on the loss of
heterozygosity and X-chromosome inactivation, the results of which
demonstrated a considerable overlapping loss of heterozygosity
between the sarcomatoid and carcinomatous components, and the
uniform, non-random X-chromosome inactivation is consistent with
the hypothesis that sarcomatoid urothelial carcinoma of the urinary
bladder is monoclonal in origin. Subsequently, Völker et al
(11) further demonstrated
considerable, but not complete, overlapping of the genetic
alterations by comparative genomic hybridization of the two
sarcomatoid carcinomas in their unusual location, the ureter. It
was also demonstrated that the epithelial and mesenchymal
components shared similar chromosomal gains and losses. These
findings are consistent with the hypothesis that sarcomatoid
carcinoma is developed from a common pluripotent progenitor cell,
which has a potential for epithelial and mesenchymal
differentiation.
Due to the aggressive nature of this neoplasm,
sarcomatoid urothelial carcinoma has a considerably poor prognosis
compared with the other types of ureteral cancer (21). The presence of a sarcomatoid component
has been associated with a dismal prognosis and an increased risk
of metastasis (21,29,30).
Previously reported cases of urothelial carcinoma with sarcomatoid
differentiation exhibited systemic metastasis to sites including
the bone, liver, lung and lymph nodes (23,24,30). The
majority of these tumors are high-grade in histology (16). Other more common and less aggressive
types of tumors that occur in the ureter and differ from
carcinosarcoma include carcinomas with osseous or chondroid
metaplasia, carcinoma with pseudosarcomatous stroma, and
sarcomatoid carcinoma (21). The
histological features of the metaplastic part differ from those of
the primary tumor. The epithelial component consists of
transitional cell carcinoma, carcinoma in situ, small cell
carcinoma, adenocarcinoma and squamous cell carcinoma, while the
stromal component consists of chondrosarcoma, osteosarcoma and
leiomyosarcoma (27). Furthermore,
the spindle cells of sarcomatoid carcinoma are demonstrated by
immunohistochemistry. Pseudosarcomatous stromal reactions may be
distinguished from carcinosarcoma by their pathological features,
including lack of malignant characteristics, and display of minimal
atypia and mitotic activity (31).
Lichtenstein and Bernstein first described extraskeletal
mesenchymal chondrosarcoma as an occurrence of the bone in 1959
(32); it was defined as a subtype of
chondrosarcoma believed to arise from remnants of the metaplasia of
meningeal fibroblasts or embryonic cartilage (33). Mesenchymal chondrosarcoma accounts for
<1% of all sarcomas, and predominantly affects children and
young adults aged 15–35 years (34).
One third of cases occur outside the bone; other common sites of
mesenchymal chondrosarcoma include the central nervous system,
maxilla, sinuses, meninges, eyelids and thyroid (35).
Primary chondrosarcoma of the urothelial carcinoma
presents a diagnostic challenge, due to its rarity, unusual
location and nonspecific symptoms. Highly malignant,
radiation-resistant tumors with a dismal prognosis (36) also include carcinosarcomas of the
ureter, for which the optimal treatment option upon diagnosis is
surgical resection; no significant improvement on the prognosis for
this tumor type has been achieved by adjuvant radiotherapy or
chemotherapy (16). In the present
case, the patient underwent radical nephroureterectomy; however, he
and his family refused to receive chemotherapy and radiotherapy to
improve his general condition. Five months after surgery and 6
months after initial diagnosis, the patient succumbed to extensive
bone and omentum metastases.
In conclusion, the pathological features, prognosis
and treatment options for the histological variants of urothelial
carcinoma differ from those of traditional urothelial carcinoma. It
is important for urologists and pathologists to fully understand
the features of each variant in order to improve the process of
clinical diagnosis, assessment of prognosis, and, most importantly,
treatment of these types of tumors.
References
|
1
|
Beltran A Lopez, Sauter G, Gasser T, et
al: Infiltrating urothelial carcinoma. World Health Organization
Classification of Tumors. Pathology And GeneticsTumours of the
Urinary System and Male Genital Organs. Eble JN, Sauter G, Epstein
JI and Sesterhenn IA: IARC Press; Lyon: pp. 93–109. 2004
|
|
2
|
Tanaka MF and Sonpavde G: Diagnosis and
management of urothelial carcinoma of the bladder. Postgrad Med.
123:43–55. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Renner MJ: Primary malignant tumors of the
ureter. Surg Gynecol Obstet. 52:793–803. 1931.
|
|
4
|
Yano S, Arita M, Ueno F, Yoshida M,
Ikegami K and Fukuda S: Carcinosarcoma of the ureter. Eur Urol.
10:711984.PubMed/NCBI
|
|
5
|
Byard RW, Bell ME and Alkan MK: Primary
carcinosarcoma: A rare cause of unilateral ureteral obstruction. J
Urol. 137:732–733. 1987.PubMed/NCBI
|
|
6
|
Fukuda T, Ohnishi Y, Sato K, Tachikawa S,
Tamura T, Uehara T and Emura I: Transitional cell carcinoma with
sarcomatous elements in the urinary tract. Six cases examined by
immunohistochemistry. Acta Pathol Jpn. 41:143–149. 1991.PubMed/NCBI
|
|
7
|
Tsutsumi M, Kamiya M, Sakamoto M, Tobisu K
and Kakizoe T: A ureteral small cell carcinoma mixed with malignant
mesodermal and ectodermal elements: A clinicopathological,
morphological and immunohistochemical study. Jpn J Clin Oncol.
23:325–329. 1993.PubMed/NCBI
|
|
8
|
Ishikura H, Kumagai F and Yoshiki T:
Carcinosarcoma of the ureter with unusual histologic features. Jpn
J Clin Oncol. 24:175–180. 1994.PubMed/NCBI
|
|
9
|
Ichiyanagi N, Yamada T and Sakai Y:
Carcinosarcoma of the ureter: A case report. (Abstract). Rinsho
Hinyokika. 52:965–967. 1998.
|
|
10
|
Maeda D, Fujii A, Yamaguchi K, Tominaga T,
Fukayama M and Mori M: Sarcomatoid carcinoma with a predominant
basaloid squamous carcinoma component: The first report of an
unusual biphasic tumor of the ureter. Jpn J Clin Oncol. 37:878–883.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Völker HU, Zettl A, Schön G, Heller V,
Heinrich E, Rosenwald A, Handwerker M, Müller-Hermelink HK, Marx A
and Ströbel P: Molecular genetic findings in two cases of
sarcomatoid carcinoma of the ureter: Evidence for evolution from a
common pluripotent progenitor cell? Virchows Arch. 452:457–463.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McDade HB, Armstrong EM and Graham AG:
Proceedings: A case of carcinosarcoma of ureter. J Clin Pathol.
27:5141974. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Murata T, Soga T, Tajima K, Saito K,
Komeda Y, Ioshii SO, Shiraishi T, Sakakura T and Yatani R:
Sarcomatoid carcinoma of the urinary tract. Pathol Int. 44:138–144.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burt JD, Murphy D and Heffernan EB:
Carcinosarcoma of the ureter presenting as biliary colic. Aust N Z
J Surg. 65:63–64. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagayoshi J, Kawakami T and Maruyama Y:
Sarcomatoid carcinoma of the ureter: A case report. Int J Urol.
4:618–620. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Perimenis P, Athanasopoulos A, Geragthy J
and Speakman M: Carcinosarcoma of the ureter: A rare, pleomorphic,
aggressive malignancy. Int Urol Nephrol. 35:491–493. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee G, Rankin A, Williamson M and Pope A:
Case report: Sarcomatoid carcinoma arising from the ureter: A rare
case and a treatment dilemma. Int Urol Nephrol. 36:153–154. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Busby JE, Brown GA, Tamboli P, Kamat AM,
Dinney CP, Grossman HB and Matin SF: Upper urinary tract tumors
with nontransitional histology: A single-center experience.
Urology. 67:518–523. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Petsch MJ, Planz B, Tschahargane C and
Caspers HP: Primary sarcomatoid carcinoma of the ureter. Aktuelle
Urol. 35:137–139. 2004.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kakoi N, Miyajima A, Motizuku T, Mizuguchi
Y, Asano T and Hayakawa M: Carcinosarcoma of the renal pelvis and
ureter: A case report. Hinyokika Kiyo. 48:29–32. 2002.(In
Japanese). PubMed/NCBI
|
|
21
|
Nicolas MM, Nazarullah A and Guo CC:
Sarcomatoid urothelial carcinoma with chondrosarcomatous
differentiation of the ureter: A case report. Anal Quant Cytopathol
Histpathol. 36:111–116. 2014.PubMed/NCBI
|
|
22
|
Darko A, Das K, Bhalla RS and Heller D:
Carcinosarcoma of the ureter: Report of a case with unusual
histology and review of the literature. Int J Urol. 13:1528–1531.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Johnin K, Kadowaki T, Kushima M, Ushida H,
Koizumi S and Okada Y: Primary heterologous carcinosarcoma of the
ureter with necrotic malignant polyps. Report of a case and review
of the literature. Urol Int. 70:232–235. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fleming S: Carcinosarcoma (mixed
mesodermal tumor) of the ureter. J Urol. 138:1234–1235.
1987.PubMed/NCBI
|
|
25
|
Lopez-Beltran A, Pacelli A, Rothenberg HJ,
Wollan PC, Zincke H, Blute ML and Bostwick DG: Carcinosarcoma and
sarcomatoid carcinoma of the bladder: Clinicopathological study of
41 cases. J Urol. 159:1497–1503. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Clark PE, Agarwal N, Biagioli MC,
Eisenberger MA, Greenberg RE, Herr HW, Inman BA, Kuban DA, Kuzel
TM, Lele SM, et al: Bladder cancer. J Natl Compr Canc Netw.
11:446–475. 2013.PubMed/NCBI
|
|
27
|
Eble JN, Sauter G, Epstein J and
Sesterhenn I: World Health Organiztion Classification of Tumours:
Pathology and Genetics of Tumours of the Urinary System and Male
Genital Organs. 7. 3rd. IARC Press; Lyon: 2004
|
|
28
|
Sung MT, Wang M, MacLennan GT, Eble JN,
Tan PH, Lopez-Beltran A, Montironi R, Harris JJ, Kuhar M and Cheng
L: Histogenesis of sarcomatoid urothelial carcinoma of the urinary
bladder: Evidence for a common clonal origin with divergent
differentiation. J Pathol. 211:420–430. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gira FA, Barbieri A, Fellegara G,
Zompatori M and Corradi D: Dedifferentiated chromophobe renal cell
carcinoma with massive osteosarcoma-like divergent differentiation:
A singular entity in the spectrum of retroperitoneal calcifying
tumors. Int J Surg Pathol. 18:419–423. 2010.PubMed/NCBI
|
|
30
|
Cserni G, Kovács BR, Tarján M, Sápi Z,
Domján Z and Szabó Z: Sarcomatoid renal cell carcinoma with foci of
chromophobe carcinoma. Pathol Oncol Res. 8:142–144. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X, MacLennan GT, Zhang S, Montironi
R, Lopez-Beltran A, Tan PH, Foster S, Baldridge LA and Cheng L:
Sarcomatoid carcinoma of the upper urinary tract: Clinical outcome
and molecular characterization. Hum Pathol. 40:211–217. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lichtenstein L and Bernstein D: Unusual
benign and malignant chondroid tumors of bone. A survey of some
mesenchymal cartilage tumors and malignant chondroblastic tumors,
including a few multicentric ones, as well as many atypical benign
chondroblastomas and chondromyxoid fibromas. Cancer. 12:1142–1157.
1959. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bahr AL and Gayler BW: Cranial
chondrosarcomas. Report of four cases and review of the literature.
Radiology. 124:151–156. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huvos AG, Rosen G, Dabska M and Marcove
RC: Mesenchymal chondrosarcoma. A clinicopathologic analysis of 35
patients with emphasis on treatment. Cancer. 51:1230–1237. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shapeero LG, Vanel D, Couanet D, Contesso
G and Ackerman LV: Extraskeletal mesenchymal chondrosarcoma.
Radiology. 186:819–826. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yilmaz E, Birlik B, Arican Z and Guney S:
Carcinosarcoma of the renal pelvis and urinary bladder: A case
report. Korean J Radiol. 4:255–259. 2003. View Article : Google Scholar : PubMed/NCBI
|