Introduction
Breast cancer is the most common malignancy among
women and the leading cause of cancer-associated mortality,
accounting for 14% of global cases (1,2). In the
United States, there were ~234,190 recorded new cases and 40,730
mortalities due to breast cancer in 2015 (3). The etiology of breast cancer remains
unclear, although genetic and epigenetic alterations are considered
to contribute to tumorigenesis and progression (4). Despite advances in modern therapies for
patients with breast cancer, including surgery, radiotherapy,
hormonal therapy and various types of chemotherapeutic approaches
using targeted and non-targeted drugs, numerous patients with
breast cancer respond only transiently to conventional chemotherapy
(5,6).
A high proportion of these patients eventually exhibit tumor
metastasis, which is a major contributor to cancer mortality
(5,6).
Therefore, it is necessary to explore the molecular mechanisms
underlying the tumorigenesis and progression of breast cancer in
order to develop more effective treatments.
Numerous previous studies have demonstrated that
microRNAs (miRNAs) are involved in the tumorigenesis and
progression of breast cancers (7–9). miRNAs
are a novel group of non-protein-coding, single-stranded, short
(generally 18–24 nucleotides in length) proteins, which regulate
the translational or post-transcriptional levels of target mRNAs,
through binding to the 3′-untranslated region (UTR) of those target
mRNAs (10). These miRNAs are located
in the introns of non-coding genes, the introns of protein-coding
genes or the exons of non-coding genes (11). miRNAs have crucial functions in
various physiological and pathological processes, including cell
proliferation, survival, migration, invasion and the cell cycle
(12). Previous studies have
suggested that specific miRNAs may be downregulated or upregulated
in certain types of tumors (13–15).
Downregulated miRNAs may normally function as tumor suppressor
genes, whereas upregulated miRNAs may normally function as
oncogenes (16). Dysregulated miRNA
expression has been observed in various types of human
malignancies, including breast cancers (17). These previous studies indicated that
specific dysregulated miRNAs may serve as useful biomarkers for
breast cancer tumorigenesis, progression and clinical prognosis, as
well as potential targets for breast cancer therapy (16,17).
The expression patterns and underlying mechanisms of
miR-134 have been previously studied in various types of cancer.
However, to the best of our knowledge, this is the first study of
miR-134 in human breast cancer (18–20). In
our current study, we examined miR-134 expression in breast cancer
tissues and cell lines. The association between miR-134 expression
and clinicopathological features was also analyzed. In addition,
the effects of miR-134 on breast cancer cell proliferation,
migration and invasion were evaluated. Furthermore, the molecular
mechanism underlying the biological roles of miR-134 on breast
cancer cells was explored. The results of the current study have
potential therapeutic applications and may be used to develop
current and novel treatments for breast cancers.
Materials and methods
Ethics statement and clinical
specimens
The Ethics Committee of the Chengdu Military General
Hospital approved the present study. At initial diagnosis, written
informed consent was obtained from all patients. A total of 85
pairs of breast cancer tissue and normal adjacent tissue (NAT)
samples were obtained from patients (age range, 23–82 years) who
had undergone breast surgery at the Chengdu Military General
Hospital (Chengdu, China). In the current study, the patients
involved had not received chemotherapy or radiotherapy prior to
breast surgery. Clinicopathological data for these patients,
including age, tumor diameter, lymph node metastasis, TNM stage and
pathological differentiation, were also collected. The tissue
samples were snap frozen in liquid nitrogen immediately following
surgery and stored at −80°C until use in the present study.
Cell culture and transfection
The MCF-7 and MDA-MB-231 breast cancer cell lines,
and the MCF-10A normal mammary epithelial cell line, were acquired
from the American Type Culture Collection (Manassas, VA, USA). All
cell lines were grown in Dulbecco's modified Eagle's medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) or RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.)
and 1% penicillin/streptomycin at 37°C in an atmosphere containing
5% CO2 and 100% humidity. miR-134 mimics, negative
control (NC) and luciferase reporter plasmids were synthesized by
GenePharma Co. Ltd. (Shanghai, China). Cells were seeded in a
six-well plate at 40–50% confluence. Following overnight
incubation, the cells were transfected with miR-134 mimics or NC
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at a final concentration of 50 nmol/l.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the tissue samples and
cells using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. RNA
concentration was determined with NanoDrop ND-1000
Spectrophotometer. Equal amounts of RNA were subjected to cDNA
synthesis using a PrimeScript RT Regent kit (Takara, Bio, Inc.,
Otsu, Japan). RT-qPCR was subsequently performed using a SYBR green
kit (Takara Bio, Inc.) with U6 as an internal control, according to
the manufacturer's protocol. The reaction system contained 10 µl
SYBR Green I mix, 2 µl cDNA, 2 µl forward primer, 2 µl reverse
primer and 4 µl ddH2O. The thermal cycling conditions of
the reaction were as follows: 95°C for 10 min; and 40 cycles of
95°C for 15 sec and 60°C for 1 min. U6 RNA was used as an internal
control. Primers were purchased from Guangzhou RiboBio Co., Ltd.
(Guangzhou, China). All RT-qPCR was performed in ABI 7500 RT-qPCR
detection system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). All assays were performed in triplicate.
MTT assay
An MTT assay was used to investigate the effect of
miR-134 on breast cancer cell growth. Transfected cells (miR-134
and NC) were seeded into 96-well plates at a density of
3×103 cells/well. The cells were incubated with 20 µl
MTT (5 mg/ml; Sigma-Aldrich, St. Louis, MO, USA). Following
incubation for 4 h at 37°C, the formazan precipitates were
dissolved in 200 µl dimethyl sulfoxide. The absorbance at 490 nm
was evaluated using an ELISA reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). All experiments were repeated in
triplicate.
Cell migration and invasion assay
To investigate the effect of miR-134 on cell
migration and invasion, Transwell chambers with an 8-µm pore
polycarbonate membrane (Costar; Corning Incorporated, Corning, NY,
USA) were used. Diluted Matrigel (50 µl; 2 mg/ml; BD Biosciences,
San Jose, CA, USA) was placed on the inner chamber membrane surface
for the invasion assay. Transfected cells were collected, counted
and resuspended in single cell suspension (FBS-free culture
medium). Subsequently, 1×105 cells were added to the
upper chamber and 500 µl culture medium supplemented with 20% FBS,
was added into the lower chamber as a chemoattractant. Following a
24 h incubation, any non-migrated cells were carefully removed from
the top of the chamber using a cotton swab. Subsequently, the
chambers were fixed with 100% methanol, stained with 0.5% crystal
violet (Beyotime Institute of Biotechnology, Haimen, China) for 10
min and washed with PBS (Gibco; Thermo Fisher Scientific, Inc.) 3
times. The chambers were photographed and counted in five random
fields under a light microscope with ×200 magnification using
Photoshop (Adobe, San Jose, CA, USA). All experiments were repeated
in triplicate.
miR-134 target prediction
The target genes of miR-134 were predicted by using
TargetScan (version 7.0; http://www.targetscan.org/index.html) (21).
Western blotting
A western blot analysis was used to quantify the
changes in Kirsten rat sarcoma viral oncogene homolog (KRAS)
protein expression levels. Cells transfected with miR-134 and NC
were lysed with a radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology). The protein concentration
was determined by using the bicinchoninic acid assay (Thermo Fisher
Scientific, Inc., Rockford, IL, USA). An equal amount of protein
(20 µg) from each cell line was subjected to 10% sodium dodecyl
sulfate polyacrylamide gel electrophoresis and the proteins were
subsequently transferred to polyvinylidene difluoride membranes
(EMD Millipore, Billerica, MA, USA). The membranes were blocked
with 5% non-fat dry milk (Beyotime Institute of Biotechnology) in
Tris-buffered saline. The membranes were then incubated with a
mouse anti-human monoclonal KRAS primary antibody at a 1:500
dilution (cat. no. ab157255; Abcam, Cambridge, MA, USA) overnight
at 4°C. After washing with Tris-buffered saline with 0.5% Tween 20
(Beyotime Institute of Biotechnology) 3 times, the membranes were
incubated with a goat anti-mouse horseradish peroxidase-conjugated
secondary antibody (1:1,000 dilution; cat. no. ab97023; Abcam) at
room temperature for 1 h. The protein bands were visualized using
an enhanced chemiluminescence solution (Pierce Biotechnology, Inc.,
Rockford, IL, USA). Glyceraldehyde 3-phosphate dehydrogenase was
used as an internal control. The protein blots were quantified
using AlphaEase FC software (Cell Biosciences, Inc., San Jose, CA,
USA).
Luciferase assay
To determine whether KRAS was a direct target of
miR-134, a luciferase assay was used. Cells were seeded in 12-well
plates at 30–40% confluence, and then were transfected with miR-134
mimics or NC, following which the cells were co-transfected with a
reporter plasmid containing the wild-type (Wt) and mutant 3′-UTR of
KRAS; Lipofectamine® 2000 was used as a
transfection reagent. Following a 48 h transfection, a luciferase
assay was performed using a dual-luciferase reporter assay system
(Promega Corporation, Madison, WI, USA). The firefly luciferase
activity was normalized to the corresponding Renilla
luciferase activity. All Luciferase assays were repeated in 3
independent experiments.
Statistical analysis
Data were presented as the mean ± standard
deviation. Data were compared with SPSS 17 software (SPSS, Inc.,
Chicago, IL, USA) using a Student's t-test. A 2-tailed value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-134 is downregulated in breast
cancer tissue samples and cell lines
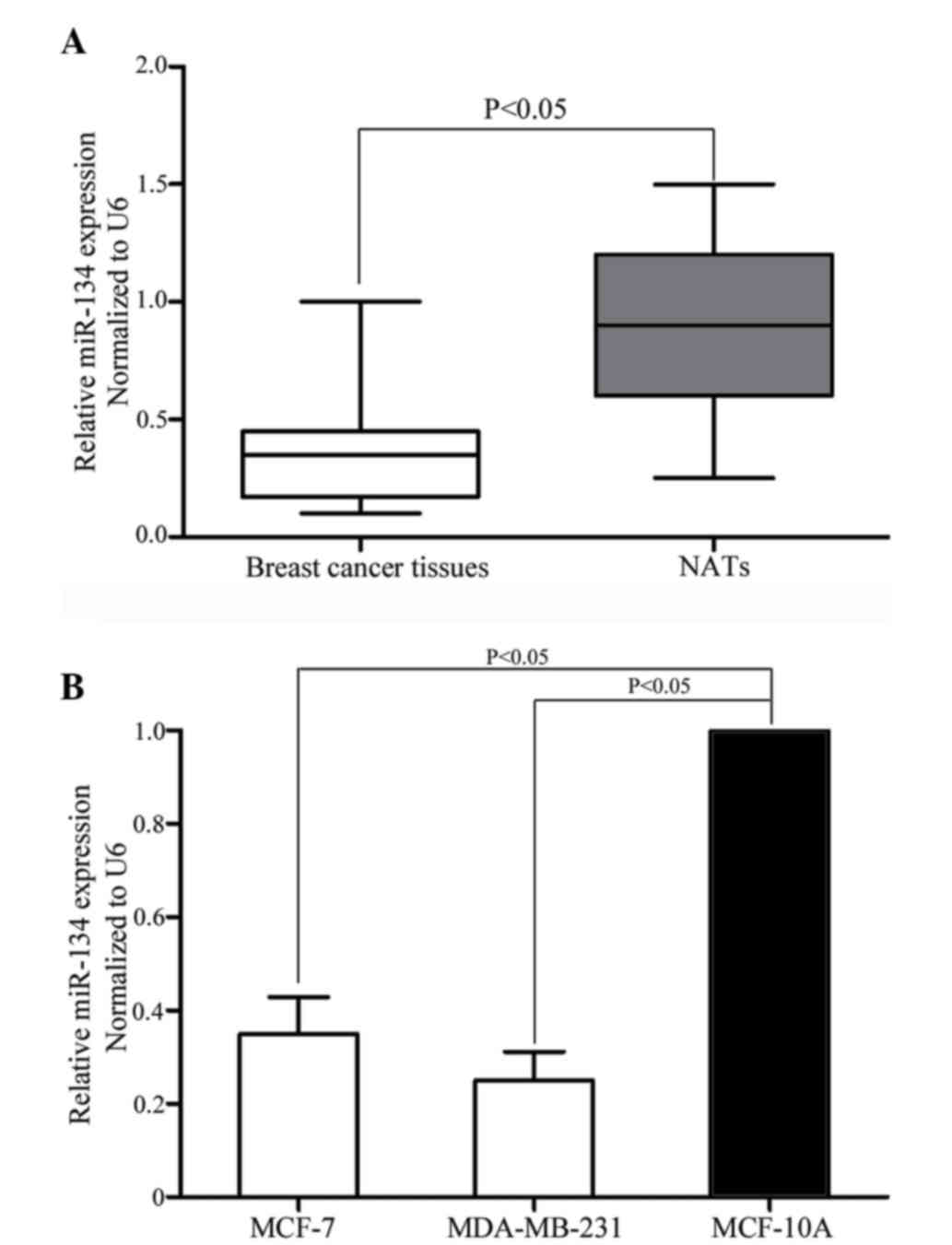
The expression levels of miR-134 in breast cancer
tissue samples, matched NATs, breast cancer cell lines and a normal
mammary epithelial cell line were quantified using RT-qPCR. As
indicated in Fig. 1A, miR-134
expression levels were significantly downregulated in breast cancer
tissue samples compared with matched NATs (P=0.001). As indicated
in Fig. 1B, downregulation of miR-134
was also observed in MCF-7 (P=0.010) and MDA-MB-231 (P=0.005) cells
compared with the MCF-10A normal mammary epithelial cell line. The
results indicate that miR-134 may have an important role in breast
cancer.
The association between miR-134
expression levels and the clinicopathological features of patients
with breast cancer
The present study examined whether the expression
levels of miR-134 were associated with the clinicopathological
features of breast cancer. Statistical analysis revealed that low
expression levels of miR-134 were significantly associated with
lymph node metastasis (P=0.021), TNM stage (P=0.037) and reduced
cell differentiation (P=0.01; Table
I). However, there was no significant correlation between
miR-134 expression levels and other clinicopathological factors,
including patient age and tumor diameter.
 | Table I.Correlation between miR-134
expression and the clinicopathological features of patients with
breast cancer. |
Table I.
Correlation between miR-134
expression and the clinicopathological features of patients with
breast cancer.
|
|
| miR-134
expression |
|
|---|
|
|
|
|
|
|---|
| Clinical
feature | Case number | Low | High | P-value |
|---|
| Age |
|
|
|
|
| <50
years | 34 | 23 | 11 | 0.819 |
| ≥50
years | 51 | 33 | 18 |
|
| Tumor diameter |
|
|
|
|
| <2.5
cm | 45 | 29 | 16 | 0.821 |
| ≥2.5
cm | 40 | 27 | 13 |
|
| Lymph node
metastasis |
|
|
|
|
|
Positive | 30 | 35 | 10 | 0.021 |
|
Negative | 55 | 21 | 19 |
|
| TNM stage |
|
|
|
|
|
I–II | 47 | 26 | 21 | 0.037 |
|
III | 38 | 30 | 8 |
|
| Pathological
differentiation |
|
|
|
|
|
Moderately and highly
differentiated | 50 | 27 | 23 | 0.010 |
| Poorly
differentiated | 35 | 29 | 6 |
|
miR-134 suppresses the proliferation
of certain breast cancer cells
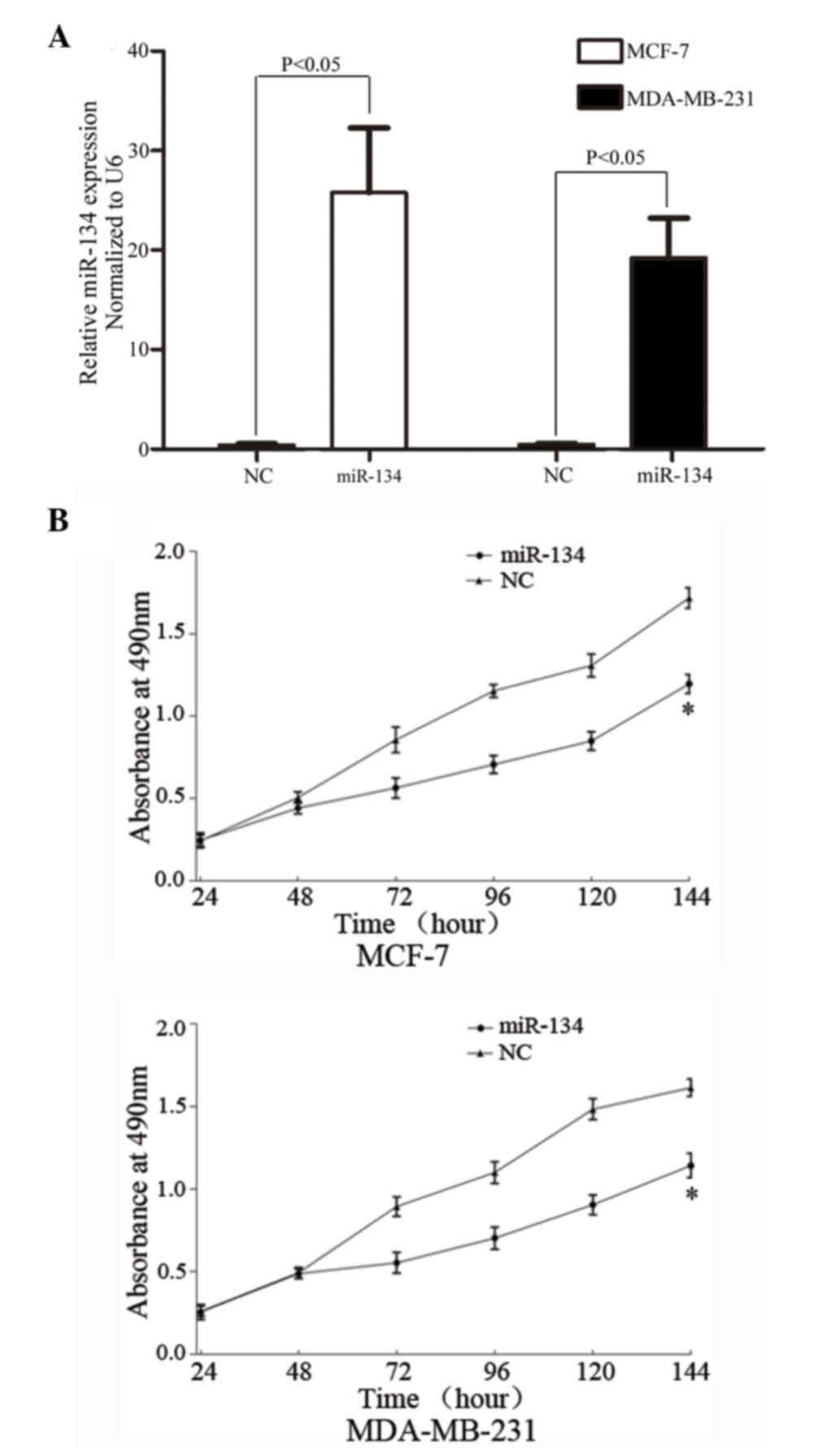
In order to assess the effect of miR-134 on breast
cancer cell proliferation, miR-134 mimics were transfected into the
MCF-7 and MDA-MB-231 cells. Following transfection (48 h incubation
period), miR-134 was significantly upregulated in MCF-7 (P=0.001)
and MDA-MB-231 (P=0.005) cells (Fig.
2A).
MTT assays were used to assess the effects of
miR-134 on cell proliferation. It was observed that miR-134
significantly inhibited cell proliferation in MCF-7 (P=0.015) and
MDA-MB-231 (P=0.012) cells (Fig. 2B),
demonstrating that miR-134 may function as a tumor growth
suppressor in human breast cancers.
miR-134 decreases the migration and
invasion of breast cancer cells
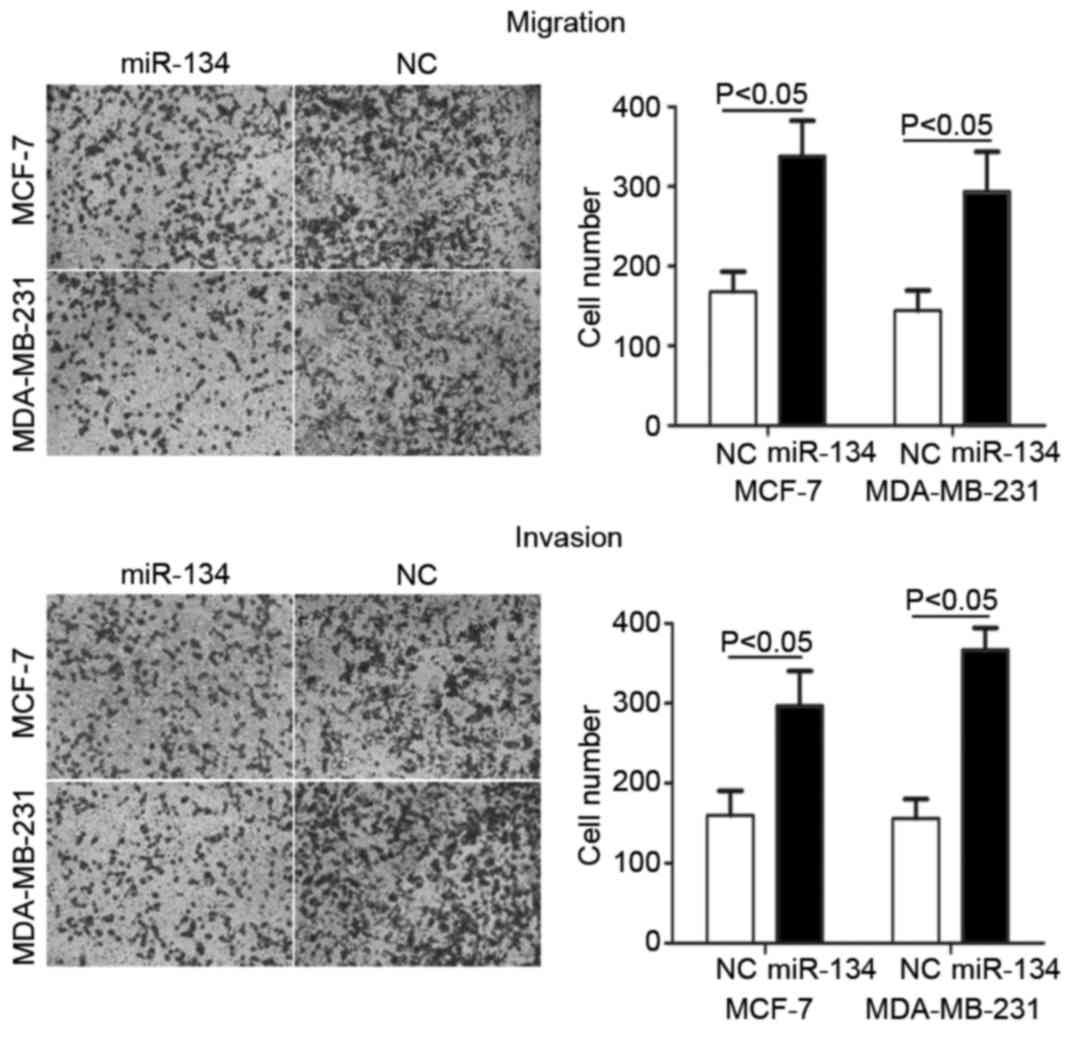
Cell migration and invasion assays were performed to
investigate the effects of miR-134 on cell motility. As indicated
in Fig. 3, the migration and invasion
of MCF-7 (P=0.024 for migration; P=0.030 for invasion) and
MDA-MB-231 cells (P=0.032 for migration; P=0.018 for invasion),
transfected with miR-134, was significantly decreased compared with
the NC (P<0.05). These results demonstrated that miR-134 may
inhibit cell metastasis in breast cancer.
KRAS is a direct target gene of
miR-134
To identify the targets of miR-134, TargetScan
version 7.0 was used. As indicated in Fig. 4A, KRAS was identified as a target of
miR-134. Subsequently, western blot analysis was used to determine
whether the protein expression levels of KRAS were downregulated
following the transfection of MCF-7 and MDA-MB-231 cells with
miR-134. As indicated in Fig. 4B,
KRAS expression levels were significantly downregulated in MCF-7
(P=0.015) and MDA-MB-231 (P=0.008) cells following their
transfection with miR-134 (P<0.05).
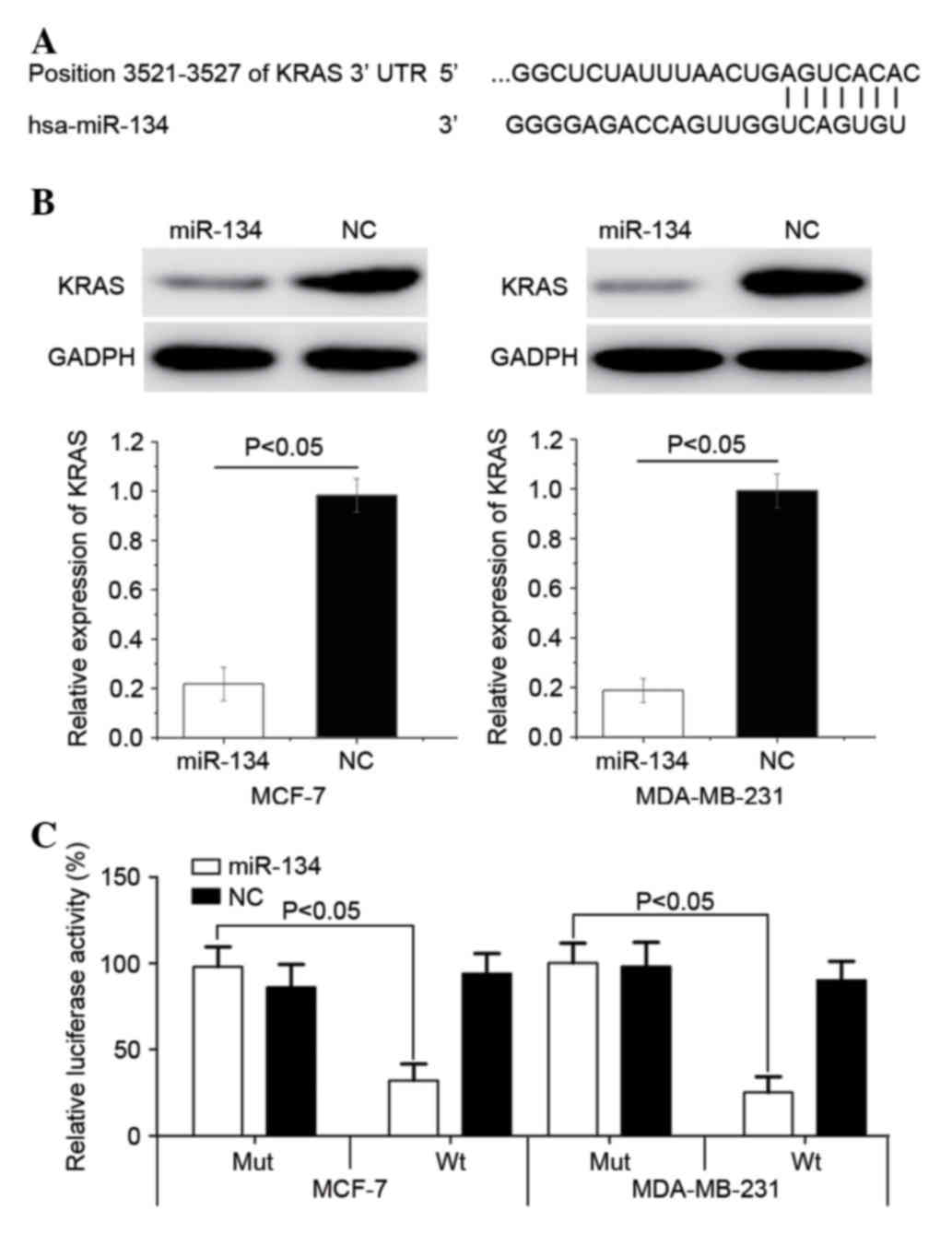 | Figure 4.KRAS is directly targeted by miR-134.
(A) TargetScan determined that KRAS mRNA contained an miR-134 seed
match at position 3521–3527 of the KRAS 3′-UTR. (B) The western
blot assay revealed that KRAS expression levels were significantly
downregulated in MCF-7 and MDA-MB-231 cells following transfection
with miR-134. (C) miR-134 significantly inhibited the KRAS Wt, but
not the KRAS Mut, luciferase activity in MCF-7 and MDA-MB-231
cells. Wt, wild type; Mut, mutant, KRAS, Kirsten rat sarcoma viral
oncogene homolog; miR-134, microRNA-134; UTR, untranslated region;
MCF-7 cells, Michigan cancer foundation-7 cells; NC, negative
control. |
Luciferase assays were also performed to determine
whether miR-134 directly targets KRAS. As indicated in Fig. 4C, miR-134 significantly inhibited the
KRAS Wt, but not the KRAS mutant luciferase activity in MCF-7
(P=0.028) and MDA-MB-231 (P=0.022) cells. Principally, KRAS was
observed to be a gene directly targeted by miR-134 in
vitro.
Discussion
Previous studies have demonstrated that the aberrant
expression of miRNAs is a characteristic of certain malignancies,
including breast cancers (22–24).
miR-134 was first identified as a brain-specific miRNA located at
14q32 (25); it was observed to be
localized in the synapto-dendritic compartment of hippocampal
neurons and involved in the regulation of the neuronal
microstructure (26). Previous
studies have indicated that miR-134 was involved in several
physiological and pathological processes; Han et al
(27) identified that miR-134 has a
crucial role in the translation-dependent guidance of nerve growth
cones. miR-134 was also identified as a potential plasma biomarker
in the diagnosis of acute pulmonary embolism (18). In the present study, the results
demonstrated that miR-134 is downregulated in breast cancer tissues
and cell lines. The expression levels of miR-134 were significantly
associated with lymph node metastasis, TNM stage and reduced cell
differentiation in patients with breast cancer. Furthermore, the
upregulation of miR-134 expression inhibits breast cancer cell
proliferation, migration and invasion. This study improved our
current understanding of the expression pattern and function of
miR-134 in breast cancer. It was also observed that the restoration
of normal miR-134 expression may serve as a novel therapeutic
approach for breast cancer treatment.
miR-134 has been previously demonstrated to function
as a tumor suppressor in several types of tumors (18–20). In
hepatocellular carcinoma, miR-134 suppressed cell metastasis by
regulating the expression of integrin β1 (19). In glioma, the downregulation of
miR-134 expression is typical, and cell growth, migration and
invasion was inhibited by the upregulation of miR-134; miR-134
overexpression also enhanced cell apoptosis in human glioma cells
(20). Li et al (28) demonstrated that the expression levels
of miR-134 were associated with the invasive potential and
epithelial-mesenchymal transition (EMT) phenotype of non-small-cell
lung cancer (NSCLC) cells. Functional assays determined that
miR-134 inhibited EMT by targeting Forkhead Box M1 in NSCLC cells
(28). Furthermore, miR-134 was
demonstrated to be associated with drug resistance by targeting the
multidrug resistance-associated protein 1/ATP binding cassette
subfamily C member 1 in H69AR lung cancer cells (29). However, miR-134 has also been
demonstrated to function as an oncogene (30). Liu et al (30) observed that miR-134 expression was
upregulated in head and neck squamous cell carcinoma (HNSCC).
miR-134 overexpression promoted the oncogenicity, carcinogenesis
and metastasis of HNSCC cell lines (30). These contradictory studies suggested
that the role of miR-134 in cancers may be tissue-type
dependent.
Identification of the miR-134 target genes is
important for understanding the role of miR-134 in breast cancer
tumorigenesis and development. It is also essential for the
development of novel targeted therapies for patients with breast
cancer. In the present study, an important molecular link between
miR-134 and KRAS was observed in breast cancer. Firstly, TargetScan
predicted that KRAS mRNA was a direct target of miR-134. Secondly,
western blotting revealed that miR-134 suppressed the expression of
KRAS in breast cancer cells. Finally, a luciferase assay
demonstrated that miR-134 directly targeted the KRAS 3′-UTR. These
results suggested that miR-134 may have a tumor suppressor role in
breast cancer tumorigenesis and development by targeting KRAS.
The rat sarcoma (RAS) genes encode a family of
homologous 21 kDa GTP-binding proteins, including Harvey RAS,
neuroblastoma RAS and KRAS (31).
Among the RAS proteins, KRAS was first identified as the
transforming factor in the Harvey and Kirsten strains of the
rat/mouse sarcoma virus (32). KRAS
primarily functions as a critical ‘on-off’ switch in cell signaling
networks that relay extracellular signals to the nucleus and
connect multiple upstream signals to various types of downstream
signaling pathways (33). These
signaling pathways are involved in cell differentiation,
proliferation, survival rate, migration, invasion, cytoskeletal
changes and the cell cycle (34,35). KRAS
is a member of the epidermal growth factor receptor
(EGFR)/RAS/mitogen activated protein kinases (MAPK) cell signaling
pathway and has previously been demonstrated to be associated with
physiological and pathological processes (36).
In breast cancer, KRAS/MAPK signaling has an
important role in transfer growth signaling from the extracellular
environment (37,38). The activation of the KRAS/MAPK
signaling pathway induces numerous responses in breast cancer
cells, resulting in the regulation of cell proliferation,
differentiation, migration and invasion (39). In breast cancer tumorigenesis and
progression, numerous facets may induce the upregulation of
KRAS/MAPK signaling; this has been demonstrated to be regulated in
breast cancer tissues by increased expression levels of EGFR, human
epidermal growth factor receptor 2/erythroblastic leukemia viral
oncogene homolog 2 and insulin-like growth factor receptor
(40–42). Previous studies identified that KRAS
was regulated by numerous miRNAs in breast cancer. Johnson et
al revealed that the lethal-7 miRNA family regulates KRAS and
cytoplasmic-myelocytomatosis (43).
Kent et al (44) also
demonstrated that miR-134 and miR-145 enhanced RAS signaling by
downregulating KRAS and the RAS-responsive element-binding protein.
Through overexpressing miR-134 in breast cancer cell lines, the
present study demonstrated that miR-134 decreases cell
proliferation, migration and invasion by downregulating KRAS
expression levels. Therefore, miR-134 may act as a regulator of the
KRAS oncogene, which may have certain clinical applications.
To the best of our knowledge, this study is the
first to demonstrate that miR-134 is downregulated in breast cancer
and is significantly associated with lymph node metastasis, TNM
stage and reduced cell differentiation. It was observed that
miR-134 inhibits cell proliferation, migration and invasion in
breast cancer. The identification of the candidate target genes of
miR-134 may provide an insight into the potential mechanisms
underlying the function of miR-134 in breast cancer. miR-134 may
contribute to breast cancer tumorigenesis and development through
the downregulation of KRAS, indicating that miR-134 may function as
a tumor suppressor in breast cancer.
References
|
1
|
Wang S, Li H, Wang J, Wang D, Yao A and Li
Q: Prognostic and biological significance of microRNA-127
expression in human breast cancer. Dis Markers. 2014:4019862014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma S, Kelly TK and Jones PA:
Epigenetics in cancer. Carcinogenesis. 31:27–36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu Y, Xu K and Yagüe E: miR-218 targets
survivin and regulates resistance to chemotherapeutics in breast
cancer. Breast Cancer Res Treat. 151:269–280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Su CM, Wang MY, Hong CC, Chen HA, Su YH,
Wu CH, Huang MT, Chang YW, Jiang SS, Sung SY, et al: miR-520 h is
crucial for DAPK2 regulation and breast cancer progression.
Oncogene. 35:1134–1142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Negrini M and Calin GA: Breast cancer
metastasis: A microRNA story. Breast Cancer Res. 10:2032008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang Q, Gumireddy K, Schrier M, le Sage
C, Nagel R, Nair S, Egan DA, Li A, Huang G, Klein-Szanto AJ, et al:
The microRNAs miR-373 and miR-520c promote tumour invasion and
metastasis. Nat Cell Biol. 10:202–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moreno-Moya JM, Vilella F and Simón C:
MicroRNA: Key gene expression regulators. Fertil Steril.
101:1516–1523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu X, Tang H, Chen J, Song C, Yang L, Liu
P, Wang N and Xie X, Lin X and Xie X: MicroRNA-101 inhibits cell
progression and increases paclitaxel sensitivity by suppressing
MCL-1 expression in human triple-negative breast cancer.
Oncotarget. 6:20070–20083. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu D, Zhou Y, Pan H, Zhou J, Fan Y and Qu
P: microRNA-99a inhibiting cell proliferation, migration and
invasion by targeting fibroblast growth factor receptor 3 in
bladder cancer. Oncol Lett. 7:1219–1224. 2014.PubMed/NCBI
|
|
14
|
Wang G, Zhu S, Gu Y, Chen Q, Liu X and Fu
H: MicroRNA-145 and MicroRNA-133a Inhibited Proliferation,
Migration, and Invasion, while promoted apoptosis in hepatocellular
carcinoma cells via targeting FSCN1. Dig Dis Sci. 60:3044–3052.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang W, Ren F, Wu Q, Jiang D, Li H and Shi
H: MicroRNA-497 suppresses angiogenesis by targeting vascular
endothelial growth factor A through the PI3K/AKT and MAPK/ERK
pathways in ovarian cancer. Oncol Rep. 32:2127–2133.
2014.PubMed/NCBI
|
|
16
|
Marini F, Luzi E and Brandi ML: MicroRNA
role in thyroid cancer development. J Thyroid Res. 2011:4071232011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong LL, Chen LM, Wang WM and Zhang LM:
Decreased expression of microRNA-124 is an independent unfavorable
prognostic factor for patients with breast cancer. Diagn Pathol.
10:452015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiao J, Jing ZC, Ellinor PT, Liang D,
Zhang H, Liu Y, Chen X, Pan L, Lyon R, Liu Y, et al: MicroRNA-134
as a potential plasma biomarker for the diagnosis of acute
pulmonary embolism. J Transl Med. 9:1592011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zha R, Guo W, Zhang Z, Qiu Z, Wang Q, Ding
J, Huang S, Chen T, Gu J, Yao M and He X: Genome-wide screening
identified that miR-134 acts as a metastasis suppressor by
targeting integrin β1 in hepatocellular carcinoma. PLoS One.
9:e876652014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Niu CS, Yang Y and Cheng CD: MiR-134
regulates the proliferation and invasion of glioblastoma cells by
reducing Nanog expression. Int J Oncol. 42:1533–1540.
2013.PubMed/NCBI
|
|
21
|
Wang J, Li J, Guo F and Yan Y:
MicroRNA-133a inhibits the malignant behavior of glioma via
downregulation of matrix metallopeptidase 9. Mol Med Rep.
13:3220–3226. 2016.PubMed/NCBI
|
|
22
|
Yang Z, Han Y, Cheng K, Zhang G and Wang
X: miR-99a directly targets the mTOR signalling pathway in breast
cancer side population cells. Cell Prolif. 47:587–595. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu ZS, Wang CQ, Xiang R, Liu X, Ye S, Yang
XQ, Zhang GH, Xu XC, Zhu T and Wu Q: Loss of miR-133a expression
associated with poor survival of breast cancer and restoration of
miR-133a expression inhibited breast cancer cell growth and
invasion. BMC Cancer. 12:512012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen L, Li J, Xu L, Ma J, Li H, Xiao X,
Zhao J and Fang L: miR-497 induces apoptosis of breast cancer cells
by targeting Bcl-w. Exp Ther Med. 3:475–480. 2012.PubMed/NCBI
|
|
25
|
Schratt GM, Tuebing F, Nigh EA, Kane CG,
Sabatini ME, Kiebler M and Greenberg ME: A brain-specific microRNA
regulates dendritic spine development. Nature. 439:283–289. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yin C, Wang PQ, Xu WP, Yang Y, Zhang Q,
Ning BF, Zhang PP, Zhou WP, Xie WF, Chen WS and Zhang X: Hepatocyte
nuclear factor-4α reverses malignancy of hepatocellular carcinoma
through regulating miR-134 in the DLK1-DIO3 region. Hepatology.
58:1964–1976. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han L, Wen Z, Lynn RC, Baudet ML, Holt CE,
Sasaki Y, Bassell GJ and Zheng JQ: Regulation of chemotropic
guidance of nerve growth cones by microRNA. Mol Brain. 4:402011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J, Wang Y, Luo J, Fu Z, Ying J, Yu Y
and Yu W: miR-134 inhibits epithelial to mesenchymal transition by
targeting FOXM1 in non-small cell lung cancer cells. FEBS Lett.
586:3761–3765. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo L, Liu Y, Bai Y, Sun Y, Xiao F and Guo
Y: Gene expression profiling of drug-resistant small cell lung
cancer cells by combining microRNA and cDNA expression analysis.
Eur J Cancer. 46:1692–1702. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu CJ, Shen WG, Peng SY, Cheng HW, Kao
SY, Lin SC and Chang KW: miR-134 induces oncogenicity and
metastasis in head and neck carcinoma through targeting WWOX gene.
Int J Cancer. 134:811–821. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tao S, Wang S, Moghaddam SJ, Ooi A,
Chapman E, Wong PK and Zhang DD: Oncogenic KRAS confers
chemoresistance by upregulating NRF2. Cancer Res. 74:7430–7441.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Parada LF, Tabin CJ, Shih C and Weinberg
RA: Human EJ bladder carcinoma oncogene is homologue of Harvey
sarcoma virus ras gene. Nature. 297:474–478. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zuber J, Tchernitsa OI, Hinzmann B,
Schmitz AC, Grips M, Hellriegel M, Sers C, Rosenthal A and Schäfer
R: A genome-wide survey of RAS transformation targets. Nat Genet.
24:144–152. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Crespo P and León J: Ras proteins in the
control of the cell cycle and cell differentiation. Cell Mol Life
Sci. 57:1613–1636. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu Y, Zhuang Y, Han M, Xu T and Deng K:
Ras promotes cell survival by antagonizing both JNK and Hid signals
in the Drosophila eye. BMC Dev Biol. 9:532009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu B, Niu X, Zhang X, Tao J, Wu D, Wang Z,
Li P, Zhang W, Wu H, Feng N, et al: miR-143 decreases prostate
cancer cells proliferation and migration and enhances their
sensitivity to docetaxel through suppression of KRAS. Mol Cell
Biochem. 350:207–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Haagenson KK and Wu GS: The role of MAP
kinases and MAP kinase phosphatase-1 in resistance to breast cancer
treatment. Cancer Metastasis Rev. 29:143–149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dunn KL, Espino PS, Drobic B, He S and
Davie JR: The Ras-MAPK signal transduction pathway, cancer and
chromatin remodeling. Biochem Cell Biol. 83:1–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Atanaskova N, Keshamouni VG, Krueger JS,
Schwartz JA, Miller F and Reddy KB: MAP kinase/estrogen receptor
cross-talk enhances estrogen-mediated signaling and tumor growth
but does not confer tamoxifen resistance. Oncogene. 21:4000–4008.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Salh B, Marotta A, Matthewson C, Ahluwalia
M, Flint J, Owen D and Pelech S: Investigation of the Mek-MAP
kinase-Rsk pathway in human breast cancer. Anticancer Res.
19:731–740. 1999.PubMed/NCBI
|
|
41
|
Eckert LB, Repasky GA, Ulkü AS, McFall A,
Zhou H, Sartor CI and Der CJ: Involvement of Ras activation in
human breast cancer cell signaling, invasion, and anoikis. Cancer
Res. 64:4585–4592. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lo HW, Hsu SC and Hung MC: EGFR signaling
pathway in breast cancers: From traditional signal transduction to
direct nuclear translocalization. Breast Cancer Res Treat.
95:211–218. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Johnson SM, Grosshans H, Shingara J, Byrom
M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D and Slack
FJ: RAS is regulated by the let-7 microRNA family. Cell.
120:635–647. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kent OA, Fox-Talbot K and Halushka MK:
RREB1 repressed miR-143/145 modulates KRAS signaling through
downregulation of multiple targets. Oncogene. 32:2576–2585. 2013.
View Article : Google Scholar : PubMed/NCBI
|