Introduction
Epithelial-mesenchymal transition (EMT) is important
for early tumor invasion and metastases (1). During this process, high expression of
N-cadherin (cell-cell adhesion molecule) and β-catenin (important
Wnt/β-catenin cascade protein) are hallmarks of EMT (2). N-cadherin and β-catenin are expressed
normally in mesenchymal cells, but are upregulated in a various
forms of human cancer, including gastric cancer, breast tumors and
nasopharyngeal carcinoma (NPC), providing these cancer cells with
more invasive and motile phenotypes (3). N-cadherin and β-catenin inactivation
inhibits cell migration and metastatic formation (4). Furthermore, these two proteins are often
associated with unfavorable prognoses in a diverse range of human
malignancies (5,6). Notably, accumulating evidence suggests
that N-cadherin and β-catenin are molecular targets in current
cancer gene therapy and have been evaluated in preclinical and
clinical studies (7,8). Thus, N-cadherin and β-catenin may be
important for tumor development and progression.
NPC is a distinctive malignant disease that is
prevalent in southern China, and has an annual incidence rate of
~20 per 100,000 people (9). Unlike
other types of head and neck cancer, NPC exhibits features of
regional lymph node metastases, and patients with NPC often present
at an advanced clinical stage for diagnosis and/or treatment
(10). Thus, it is imperative to
understand the molecular changes in NPC that drive tumor
progression and metastasis (11,12).
Positive N-cadherin and β-catenin have been reported in multiple
forms of human carcinoma to date with the exception of NPC.
Therefore, the present study aimed to investigate the expression of
N-cadherin and β-catenin in patients with NPC and further study
their prognostic value.
Materials and methods
Tumor sample collection
A total of 128 NPC paraffin-embedded specimens were
collected between January 2007 and October 2009 from Xiangya
Hospital and Hunan Provincial Tumor Hospital, Central South
University (CSU; Changsha, China). Individuals were excluded if
they had a previous history of cancer and underwent radiotherapy or
chemotherapy at diagnosis. All patients were classified and staged
according to the sixth edition of the Union for International
Cancer Control/American Joint Committee on Cancer
Tumor-Node-Metastasis staging system (13). Follow-up information was collected
every 6 months after diagnosis or until mortality. The complete
cohort characteristics of the 91 patients with NPC are listed in
Table I.
 | Table I.Correlations between protein
expression of N-cadherin and β-catenin and clinicopathological
parameters in patients with nasopharyngeal carcinoma. |
Table I.
Correlations between protein
expression of N-cadherin and β-catenin and clinicopathological
parameters in patients with nasopharyngeal carcinoma.
|
|
| N-cadherin
expression |
| β-catenin
expression |
|---|
|
|
|
|
|
|
|---|
| Characteristics | No. | High | Low | P-value | No. | High | Low | P-value |
|---|
| Age, years | 91 | 55 | 36 |
| 91 | 59 | 32 |
|
| ≤48 | 46 | 25 | 21 |
| 46 | 32 | 14 |
|
|
>48 | 45 | 30 | 15 | 0.230 | 45 | 27 | 18 | 0.339 |
| Gender |
|
|
|
|
|
|
|
|
| Male | 54 | 29 | 25 |
| 54 | 34 | 20 |
|
| Female | 37 | 26 | 11 | 0.112 | 37 | 25 | 12 | 0.651 |
| Clinical stages |
|
|
|
|
|
|
|
|
| I and
II | 32 | 10 | 22 |
| 32 | 13 | 19 |
|
| III and
IV | 59 | 45 | 14 | <0.001 | 59 | 46 | 13 | 0.001 |
| Lymph node |
|
|
|
|
|
|
|
|
|
Metastasis | 63 | 37 | 26 |
| 63 | 50 | 13 |
|
| No
metastasis | 28 | 18 | 10 | 0.617 | 28 | 9 | 19 | <0.001 |
Additionally, 26 fresh biopsy samples from patients
with NPC and 8 nasopharyngeal epithelial (NPE) tissue samples were
collected from Xiangya Hospital, CSU between August 2013 and
October 2013. These samples were validated and diagnosed by
qualified pathologists, and were used to determine the mRNA
expression of N-cadherin and β-catenin. All tissue samples were
infiltrated in RNAlater® Stabilization Solution (Qiagen,
Inc., Valencia, CA, USA) and snap-frozen in liquid nitrogen. Prior
informed consent was obtained, and the study protocol was approved
by the Ethics Committee of Xiangya Hospital, CSU.
RNA isolation
Total RNA was isolated using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's protocol. RNA was extracted
using the RNeasy® Mini kit (Qiagen, Inc.) and treated
with DNase I according to the manufacturer's protocol. The
integrity and quality of RNA was confirmed using agarose gel
electrophoresis and absorbance at 260 nm. Then, 2 µg total RNA was
reverse transcribed to cDNA using a PrimeScript RT reagent kit with
a DNA Eraser (Takara Biotechnology Co., Ltd., Tokyo, Japan) via the
SuperScript® First-Strand Synthesis system (Thermo
Fisher Scientific, Inc.) with random hexamer primers (Promega
Corporation, Madison, WI, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed using the aforementioned cDNA.
Primers for mRNA were designed and synthesized for N-cadherin
(forward, 5′-GCGTCTGTAGAGGCTTCTGG-3′ and reverse,
5′-GCCACTTGCCACTTTTCCTG-3′) and β-catenin (forward,
5′-GGGAAGAGTCCGGAGGAGAT-3′ and reverse,
5′-GGCTGTCAGGTTTGATCCCA-3′). qPCR was performed using the Bio-Rad
iQ™5 Multicolor Real-Time PCR Detection system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The final PCR reaction
mixture (10 µl) included 1 µl cDNA product, 4 µl DEPC water and 5
µl SYBR® Premix Ex Taq™ II (Takara Biotechnology Co.,
Ltd.). Reactions were incubated at 95°C for 30 sec, followed by 40
cycles at 95°C for 5 sec and 60°C for 30 sec. All reactions were
run in triplicate with GAPDH as an internal reference (forward
primer, 5′-GAGAAGGCTGGGGCTCATTT-3′ and reverse primer,
5′-AGTGATGGCATGGACTGTGG-3′). Gene expression levels were quantified
using the 2−ΔΔCq method (14). The data were representative of the
means of three experiments.
Immunohistochemistry
Tissue samples were fixed in 4% formaldehyde for 48
h at room temperature and then dehydrated by ethanol from 75–100%
for 1 h at room temperature. The tissues were subsequently
infiltrated by xylene twice for 30 min at room temperature,
embedded in paraffin and cut into 4 µm sections. Following this,
sections were deparaffinized by washing with xylene twice and
rehydrated with ethanol from 75–100%, following antigen retrieval
by heating in a microwave for 4 min at 100% power followed by 16
min at 20% power. After blocking peroxidase activity with 3%
H2O2 for 5 min at room temperature, sections
were blocked by 5% goat serum (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) in PBS for 30 min at room temperature. Negative
control slides were probed with 5% normal goat serum in PBS under
the same experimental conditions. Primary antibodies were added in
blocking buffer (horse serum (Santa Cruz Biotechnology, Inc.) 0.2%
Triton X-100 and 0.1% bovine serum albumin in PBS) and incubated
with sections at 4°C overnight. The primary antibodies were as
follows: Rabbit polyclonal anti-N-cadherin (dilution, 1:300;
#18203; Abcam, Cambridge, MA, USA) and rabbit monoclonal
anti-β-catenin (dilution, 1:500; #32572; Abcam). Following
sufficient rinses by PBS three times (5 min each), sections were
immunostained with Polink-1 HRP DAB Detection system (PV-6000;
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing,
China). A light microscope and a digital color camera were used for
examination and image capture of the slides.
Immunohistochemistry results were diagnosed and
scored independently by two qualified pathologists without
knowledge of the information of all patients with NPC. Assessments
of N-cadherin and β-catenin proteins referred to methods described
by Hui et al (15). The
intensity of N-cadherin and β-catenin staining was scored as 0, 1,
2 and 3. Scores of positive cell percentage were assigned as 0
(<5%), 1 (5–25%), 2 (26–50%), and 3 (≥51%). The scores of each
view were multiplied to give a final score of 0–9, and the final
score of one sample was the mean of 10 microscopic fields. For
final statistical analysis, tumors were divided into two groups:
High expression of N-cadherin and β-catenin protein (scored 6–9)
and low expression of N-cadherin and β-catenin protein (scored
0–5).
Statistical analysis
Statistical procedures were analyzed using SPSS
version 15.0 (SPSS, Inc., Chicago, IL, USA). The χ2 test
was used to analyze the potential associations between the
expression of N-cadherin and β-catenin, and diverse NPC
clinicopathological variables. The Kaplan-Meier survival method was
applied to determine the prognostic value of N-cadherin and
β-catenin proteins. In addition, the Cox proportional hazards model
was used to perform multivariate analysis and to determine the
final independent prognostic factors. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of N-cadherin and β-catenin
mRNA is increased in NPC samples
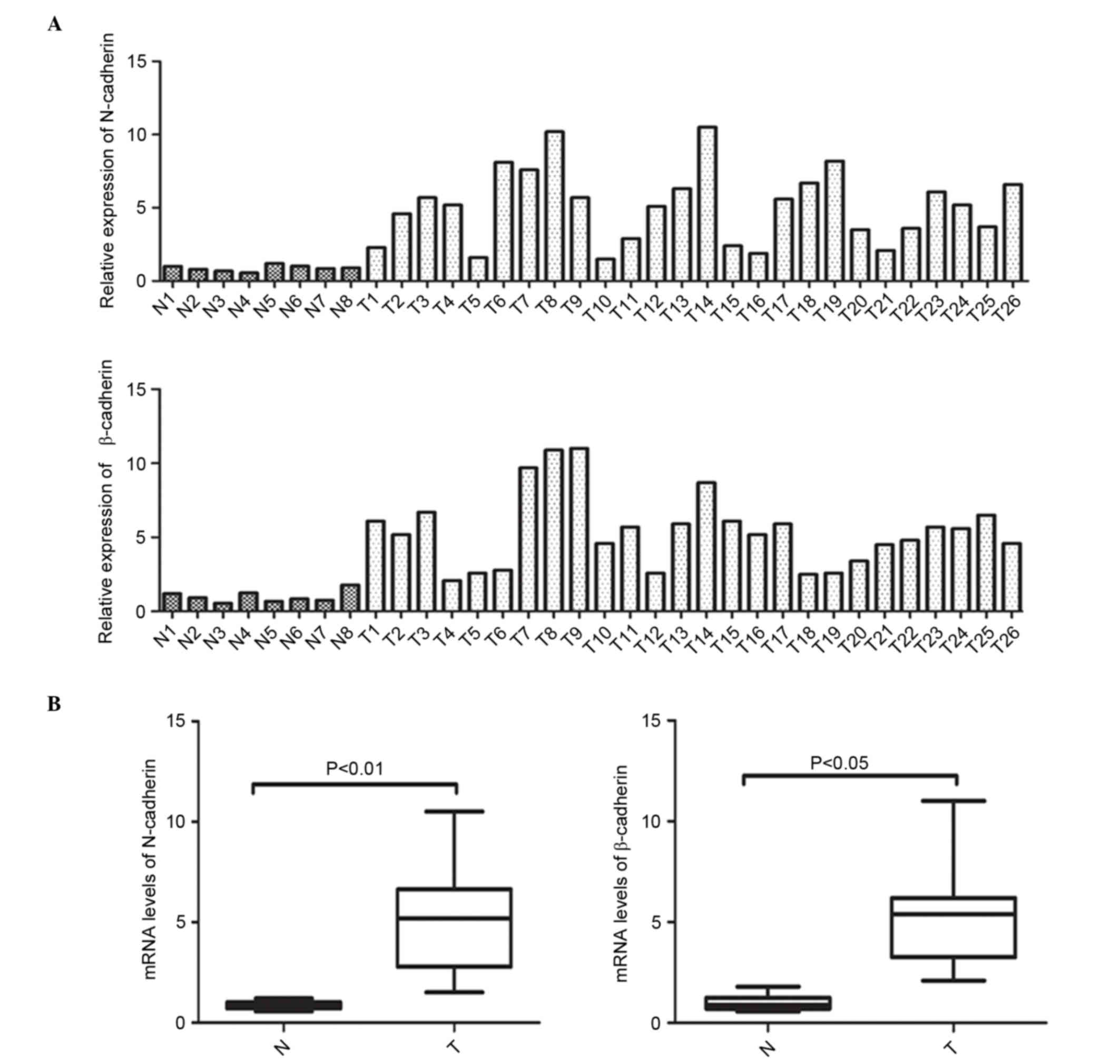
In order to clarify the abnormal expression of
N-cadherin and β-catenin at the transcriptional level, mRNA
expression of N-cadherin and β-catenin was initially measured in 26
NPC specimens and 8 normal NPE samples was initially measured via
RT-qPCR. Fig. 1A and B depict the
individual and average mRNA expression of N-cadherin and β-catenin
in the NPC and NPE tissues. These data clearly demonstrate that the
mRNA expression of N-cadherin (P<0.001) and β-catenin (P=0.0004)
was elevated in the NPC tissues when compared with the noncancerous
NPE specimens.
Protein expression of N-cadherin and
β-catenin is associated with NPC clinicopathological
parameters
Based on the aberrant mRNA expression N-cadherin and
β-catenin, the expression levels of their encoded proteins were
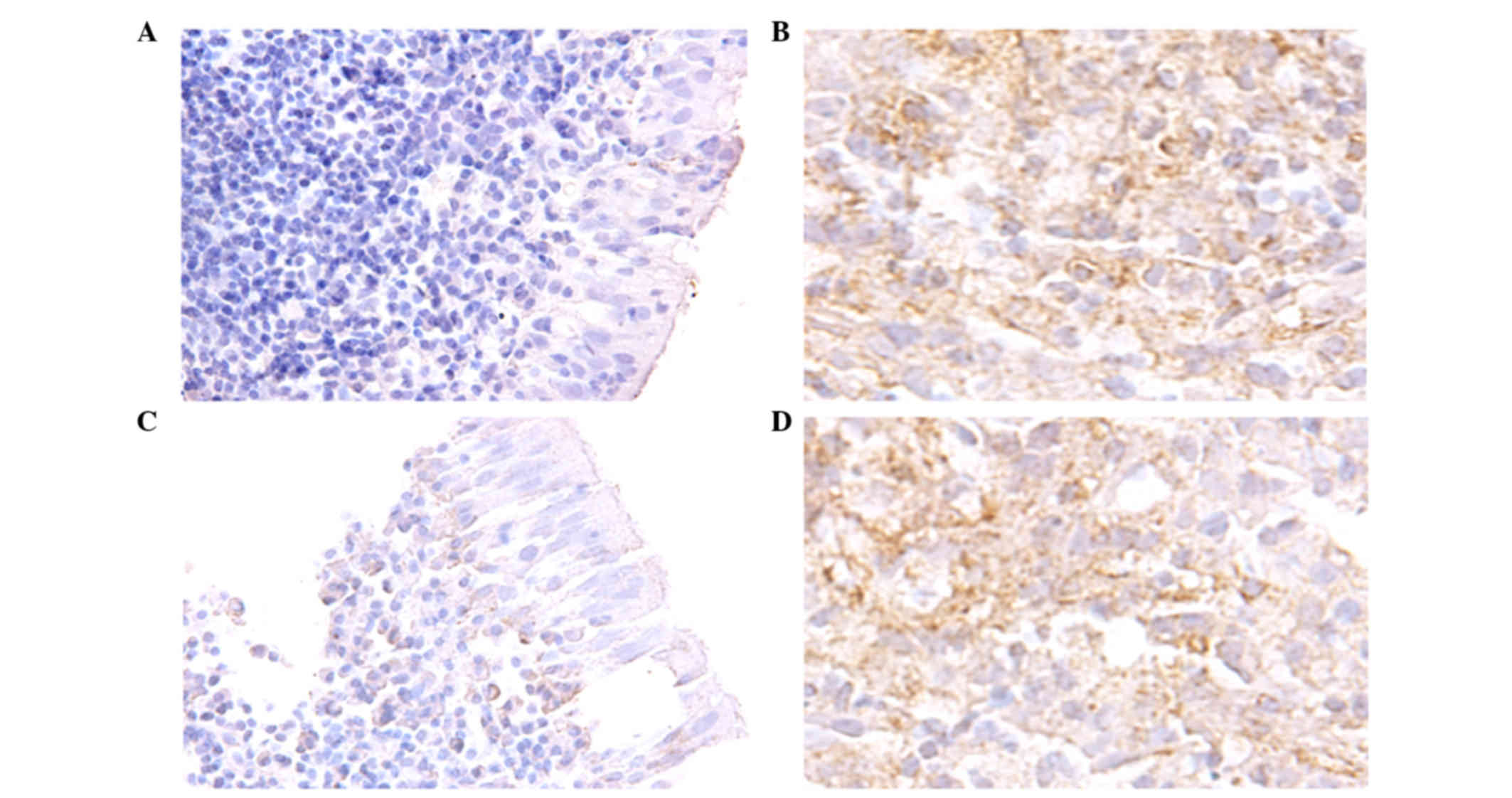
subsequently assayed via immunohistochemistry in 91
paraffin-embedded NPC tissues. Staining of N-cadherin and β-catenin
proteins demonstrated that they were primarily distributed in the
cytoplasm of NPC cells (Fig. 2).
Moreover, high N-cadherin (55/91 samples, 60.4%) and β-catenin
(59/91 samples, 64.8%) protein expression frequently occurred in
the NPC samples. Following this, correlations between N-cadherin
and β-catenin expression and NPC clinicopathological variables were
examined. Expression of N-cadherin and β-catenin protein was
positively correlated with lymph node metastasis status and
advanced clinical stages in patients with NPC (P=0.001) (Table I). However, no significant differences
were identified between expression levels of these proteins and
parameters, including age (P=0.339) and gender (P=0.651).
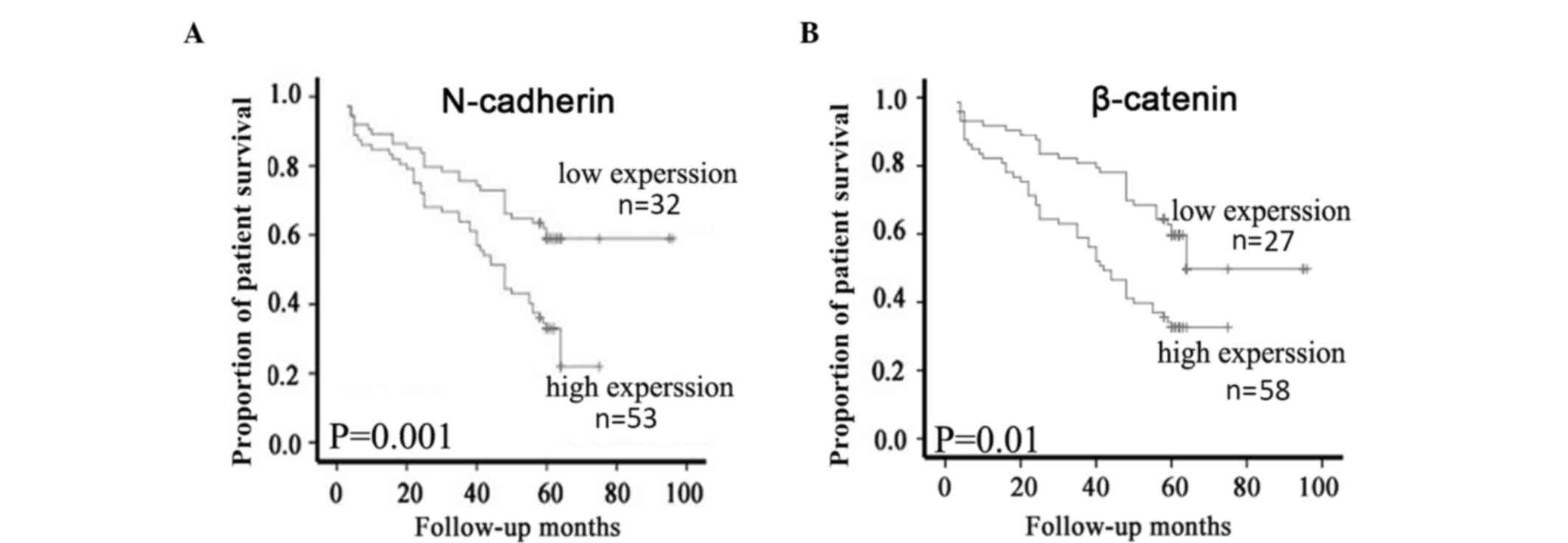
Survival analysis
Of the 91 patients with NPC, 85 (93.4%) had intact
follow-up information that was able to be further used for survival
analysis. According to the expression levels of N-cadherin and
β-catenin proteins, these cases were categorized into two groups
containing high and low N-cadherin or β-catenin expression.
Kaplan-Meier survival analyses demonstrated that patients with NPC
with high N-cadherin protein expression had a significantly lower
overall survival (OS) rate than those with low N-cadherin
expression (P=0.001; Fig. 3A).
Similarly, high β-catenin protein expression was significantly
associated with a lower OS rate compared with low expression of
β-catenin protein (P=0.01; Fig. 3B).
Finally, Cox multivariate analysis determined that N-cadherin
expression (P=0.017) and clinical stage (P=0.024) were independent
factors with prognostic value in patients with NPC (Table II).
 | Table II.Cox regression analysis of the
progression-free survival and overall survival rates. |
Table II.
Cox regression analysis of the
progression-free survival and overall survival rates.
|
| Progression-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Gender
(female/male) | 1.665 | 0.710–3.416 | 0.251 | 1.436 | 0.815–3.317 | 0.342 |
| Age
(≤48/>48) | 1.16 | 0.816–1.221 | 0.914 | 0.875 | 0.657–1.149 | 0.764 |
| β-catenin
(high/low) | 1.417 | 0.857–2.124 | 0.612 | 1.214 | 0.716–2.151 | 0.273 |
| N-cadherin
(high/low) | 2.453 | 1.325–3.455 | 0.013a | 2.887 | 1.561–5.176 | 0.017a |
| Clinical stage (I
and II/III and IV) | 2.612 | 1.724–3.294 | 0.021 | 2.429 | 1.816–3.169 | 0.024a |
Discussion
The present study measured the expression of
N-cadherin and β-catenin in NPC, which indicated that the mRNA and
protein expression of the two were significantly elevated in the
NPC tissues. Moreover, the data demonstrated that increased protein
expression of N-cadherin and β-catenin was positively correlated
with NPC lymph node metastasis and predicted a poorer prognosis in
patients with NPC.
The cadherin family is comprised of various proteins
and includes E-cadherin (epithelial), N-cadherin (neural),
P-cadherin (placental) and nonclassical cadherins, such as
OB-Cadherin, VE-Cadherin, K-cadherin, LI-cadherin, BR-cadherin,
M-cadherin, R-cadherin and T-cadherin (16). E-cadherin has been studied as an
epithelial cell molecule in a various forms of human cancer,
including NPC (17). A number of
studies have demonstrated that downregulated E-cadherin is
associated with cancer metastasis and poor prognosis (18–20).
Studies performed in vitro and in vivo have
established the hypothesis that E-cadherin is a vital molecule in
the EMT process (21).
By contrast, the well-known mesenchymal molecule
N-cadherin has scarcely been investigated in NPC. In the present
study, mRNA and protein expression of N-cadherin were markedly
increased in the NPC tissues when compared with the noncancerous
NPE tissues. Moreover, N-cadherin upregulation was highly
correlated with lymph node metastasis and poor survival in patients
with NPC (22). The aforementioned
results are consistent with previous reports examining other forms
of malignancy, including breast cancer, lung cancer and oral
squamous cell carcinoma (23–25). However, it is important to note that
decreased expression of N-cadherin has also been reported in
several human malignancies, such as osteosarcoma (26), ovarian carcinoma (27), glioblastoma (28) and renal carcinoma (29). The opposing expression profile in
these forms of cancer suggests that N-cadherin has a distinct
expression pattern based on the diverse background of cancer, and
its exact role in different types of cancer requires further
study.
β-catenin is a critical component in the
cadherin-catenin complex, which is essential in connecting actin
filaments of cells to the cell-cell interface at adherens junctions
(30). The aberrant expression and
dysfunction of the N-cadherin/β-catenin complex leads to increased
malignant capacity in cancer cells, including increased cell
motility and invasion (31). Multiple
signaling pathways, such as phosphoinositide 3-kinase/protein
kinase B and nuclear factor-κβ, have been implicated in the
mechanisms behind these unfavorable alterations in the complex
(32,33). However, these mechanisms require
further study in different forms of human cancer.
Finally, the prognostic significance of N-cadherin
and β-catenin proteins in patients with NPC was investigated. To
the best of our knowledge, the present study is the first to
demonstrate that high expression of N-cadherin or β-catenin is
strongly correlated with adverse prognosis in patients with NPC.
These results indicate that N-cadherin and β-catenin may be used as
a valuable biomarker during surveillance of patients with NPC.
In conclusion, the present study demonstrated that
N-cadherin and β-catenin mRNA and protein expression are positively
correlated with lymph node metastasis and prognosis in patients
with NPC. As the current study was a retrospective clinical
association analysis based on archival tissue specimens, further
in vitro and in vivo studies are required to enable a
comprehensive understanding of the potential function and related
mechanism of the N-cadherin/β-catenin complex in NPC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81170912 and
81300819).
References
|
1
|
Smith A, Teknos TN and Pan Q: Epithelial
to mesenchymal transition in head and neck squamous cell carcinoma.
Oral Oncol. 49:287–292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hennessy BT, Gonzalez-Angulo AM,
Stemke-Hale K, Gilcrease MZ, Krishnamurthy S, Lee JS, Fridlyand J,
Sahin A, Agarwal R, Joy C, et al: Characterization of a naturally
occurring breast cancer subset enriched in
epithelial-to-mesenchymal transition and stem cell characteristics.
Cancer Res. 69:4116–4124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chung S, Yao J, Suyama K, Bajaj S, Qian X,
Loudig OD, Eugenin EA, Phillips GR and Hazan RB: N-cadherin
regulates mammary tumor cell migration through Akt3 suppression.
Oncogene. 32:422–430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li G, Satyamoorthy K and Herlyn M:
N-cadherin-mediated intercellular interactions promote survival and
migration of melanoma cells. Cancer Res. 61:3819–3825.
2001.PubMed/NCBI
|
|
6
|
Miao Y, Li AL, Wang L, Fan CF, Zhang XP,
Xu HT, Yang LH, Liu Y and Wang EH: Overexpression of NEDD9 is
associated with altered expression of E-cadherin, β-catenin and
N-cadherin and predictive of poor prognosis in non-small cell lung
cancer. Pathol Oncol Res. 19:281–286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mao J, Fan S, Ma W, Fan P, Wang B, Zhang
J, Wang H, Tang B, Zhang Q, Yu X, et al: Roles of Wnt/β-catenin
signaling in the gastric cancer stem cells proliferation and
salinomycin treatment. Cell Death Dis. 5:e10392014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tothill RW, Tinker AV, George J, Brown R,
Fox SB, Lade S, Johnson DS, Trivett MK, Etemadmoghadam D, Locandro
B, et al: Novel molecular subtypes of serous and endometrioid
ovarian cancer linked to clinical outcome. Clin Cancer Res.
14:5198–5208. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tulalamba W and Janvilisri T:
Nasopharyngeal carcinoma signaling pathway: An update on molecular
biomarkers. Int J Cell Biol. 2012:5946812012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tao Q and Chan AT: Nasopharyngeal
carcinoma: Molecular pathogenesis and therapeutic developments.
Expert Rev Mol Med. 9:1–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian Y, Tian Y, Zhang W, Wei F, Yang J,
Luo X, Zhou T, Hou B, Qian S, Deng X, et al: Junctional adhesion
molecule-A, an epithelial-mesenchymal transition inducer,
correlates with metastasis and poor prognosis in human
nasopharyngeal cancer. Carcinogenesis. 36:41–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Greene FL, Page DL, Fleming ID, Fritz AG,
Balch CM, Haller DG and Morrow M: AJCC cancer staging manual.
Springer Science & Business Media; 2002, View Article : Google Scholar
|
|
14
|
Li G, Liu Y, Liu C, Su Z, Ren S, Wang Y,
Deng T, Huang D, Tian Y and Qiu Y: Genome-wide analyses of long
noncoding RNA expression profiles correlated with radioresistance
in nasopharyngeal carcinoma via next-generation deep sequencing.
BMC Cancer. 16:7192016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hui L, Zhang S, Dong X, Tian D, Cui Z and
Qiu X: Prognostic significance of twist and N-cadherin expression
in NSCLC. PLoS One. 8:e621712013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nollet F, Kools P and van Roy F:
Phylogenetic analysis of the cadherin superfamily allows
identification of six major subfamilies besides several solitary
members. J Mol Biol. 299:551–572. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng Z, Pan J, Chu B, Wong YC, Cheung AL
and Tsao SW: Downregulation and abnormal expression of E-cadherin
and beta-catenin in nasopharyngeal carcinoma: Close association
with advanced disease stage and lymph node metastasis. Hum Pathol.
30:458–466. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guilford P: E-cadherin downregulation in
cancer: Fuel on the fire? Mol Med Today. 5:172–177. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He X, Chen Z, Jia M and Zhao X:
Downregulated E-cadherin expression indicates worse prognosis in
Asian patients with colorectal cancer: Evidence from meta-analysis.
PloS One. 8:e708582013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fang Y, Liang X, Jiang W, Li J, Xu J and
Cai X: Cyclin b1 suppresses colorectal cancer invasion and
metastasis by regulating e-cadherin. PloS One. 10:e01268752015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Canel M, Serrels A, Frame MC and Brunton
VG: E-cadherin-integrin crosstalk in cancer invasion and
metastasis. J Cell Sci. 126:393–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo WR, Wu AB, Fang WY, Li SY and Yao KT:
Nuclear expression of N-cadherin correlates with poor prognosis of
nasopharyngeal carcinoma. Histopathology. 61:237–246. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagi C, Guttman M, Jaffer S, Qiao R, Keren
R, Triana A, Li M, Godbold J, Bleiweiss IJ and Hazan RB: N-cadherin
expression in breast cancer: Correlation with an aggressive
histologic variant-invasive micropapillary carcinoma. Breast Cancer
Res Treat. 94:225–235. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, Liu G, Kang Y, Dong Z, Qian Q and
Ma X: N-cadherin expression is associated with acquisition of EMT
phenotype and with enhanced invasion in erlotinib-resistant lung
cancer cell lines. PloS One. 8:e576922013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Di Domenico M, Pierantoni GM, Feola A,
Esposito F, Laino L, DE Rosa A, Rullo R, Mazzotta M, Martano M,
Sanguedolce F, et al: Prognostic significance of N-cadherin
expression in oral squamous cell carcinoma. Anticancer Res.
31:4211–4218. 2011.PubMed/NCBI
|
|
26
|
Marie PJ: Role of N-cadherin in bone
formation. J Cell Physiol. 190:297–305. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alaee M, Danesh G and Pasdar M:
Plakoglobin Reduces the in vitro Growth, Migration and Invasion of
Ovarian Cancer Cells Expressing N-Cadherin and Mutant p53. PLoS
One. 11:e01543232006. View Article : Google Scholar
|
|
28
|
Musumeci G, Magro G, Cardile V, Coco M,
Marzagalli R, Castrogiovanni P, Imbesi R, Graziano AC, Barone F, Di
Rosa M, et al: Characterization of matrix metalloproteinase-2 and
−9, ADAM-10 and N-cadherin expression in human glioblastoma
multiforme. Cell Tissue Res. 362:45–60. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tani T, Laitinen L, Kangas L, Lehto VP and
Virtanen I: Expression of E- and N-cadherin in renal cell
carcinomas, in renal cell carcinoma cell lines in vitro and in
their xenografts. Int J Cancer. 64:407–414. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nagafuchi A and Takeichi M: Cell binding
function of E-cadherin is regulated by the cytoplasmic domain. EMBO
J. 7:36791988.PubMed/NCBI
|
|
31
|
Rappl A, Piontek G and Schlegel J:
EGFR-dependent migration of glial cells is mediated by
reorganisation of N-cadherin. J Cell Sci. 121:4089–4097. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Méndez-Samperio P, Pérez A and Rivera L:
Mycobacterium bovis Bacillus Calmette-Guérin (BCG)-induced
activation of PI3K/Akt and NF-kB signaling pathways regulates
expression of CXCL10 in epithelial cells. Cell Immunol. 256:12–18.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|