Introduction
Pulmonary enteric adenocarcinoma (PEAC), a rare
pathological type of primary lung adenocarcinoma, shares similar
pathological morphology and immunohistochemical (IHC) markers with
those of metastatic colorectal carcinoma (MCC). In 1991, Tsao and
Fraser (1) initially proposed the
name ‘intestinal type of lung adenocarcinoma’; subsequently, it has
been referred to as ‘pulmonary intestinal-type adenocarcinoma’ or
‘pulmonary adenocarcinoma with enteric (or intestinal)
differentiation’. In 2005, PEAC was classified as pulmonary
mucinous adenocarcinoma by Yousem (2)
and Inamura et al (3). The
International Association for the Study of Lung Cancer
(IASLC)/American Thoracic Society (ATS)/European Respiratory
Society (ERS) International Multidisciplinary Classification of
Lung Adenocarcinoma, published in 2011, confirmed the definition of
PEAC (4). PEAC is a rare pathological
type. Between 1 May 2005 and 8 October 2015, <20 PEAC-related
references have been recorded by PubMed (www.ncbi.nlm.nih.gov/pubmed) and Wanfang (http://g.wanfangdata.com.cn/) (2,3,5–18).
Pancreatic neoplasms are mainly primary ductal
adenocarcinoma; the incidence of intrapancreatic metastases is
<10%: Frequencies reported by Smith et al (19) and Shi et al (20) are between 1.8 and 7.6%, and between 2
and 5%, respectively. ~6% of pancreatic metastases originated from
the lung cancer (21). The occurrence
of pancreatic metastases is a consequence of the terminal stage of
the disease and the invasion of numerous other organs (19).
Case presentation
A 62-year-old man with a ~20-year history of smoking
20 cigarettes/day was admitted to Ruijin Hospital (Shanghai, China)
on 28 September, 2015 owing to the discovery of masses in the left
chest wall and right abdominal wall for ~1 month and another in the
subcutaneous tissue of the right upper limb for ~1 week, but with
no respiratory symptoms. The patient had a history of hypertension
and diabetes for >10 years, but no history of malignancy. Blood
pressure and blood glucose were managed in a comparatively normal
range. Informed consent was obtained from the patient.
Masses were palpated in the subcutaneous tissue of
the left upper chest wall, right lower abdominal wall and right
upper extremity during the physical examination. The masses were
between 1.0×1.0 and 1.5×1.0 cm, without redness, swelling or fever,
had an irregular shape, with a hard or pliable texture, and were
difficult to move and not tender. Lungs were clear to auscultation
bilaterally and without evidently dry or moist rales. Abdominal
signs and symptoms were negative. No abnormality was identified in
the extremities or following neurological examination.
Laboratory data identified that carbohydrate antigen
19–9 (CA19-9) was at a normal level; however, carcinoembryonic
antigen levels were increased compared with normal levels.
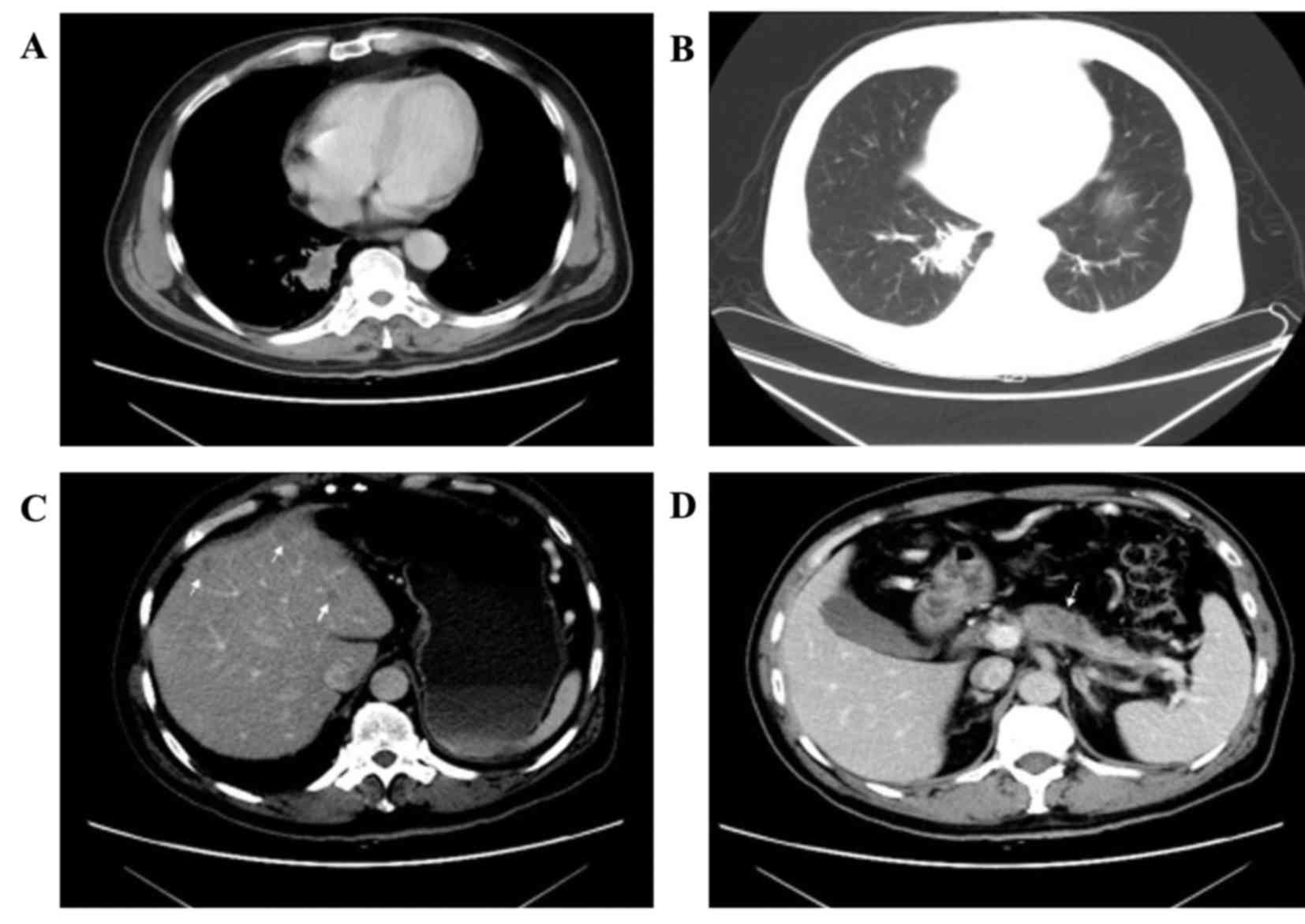
Computed tomography (CT) of the chest identified a
2.8×1.5 cm parenchymal lump with burrs and mild effusion in the
lower lobe of the right lung closing to the vertebral column, with
mild pleural thickening, but no evidently swollen mediastinal lymph
nodes observed (Fig. 1A and B). A
1.7×1.4 cm mass with irregular margin was revealed in the
subcutaneous tissue of the left chest. An abdominal enhanced CT
identified that there were multiple cystic-hypodense lesions with
no enhancement in the liver, but no dilatation was observed in the
intrahepatic bile duct (Fig. 1C). A
hypodense and homogeneous mass with relatively explicit margin was
revealed on the head of the mild atrophic pancreas and the mass was
enhanced compared with normal pancreatic tissue (Fig. 1D). No dilatation of the pancreatic
duct was identified. Furthermore, the bilateral adrenal glands were
swollen. No other focus was identified.
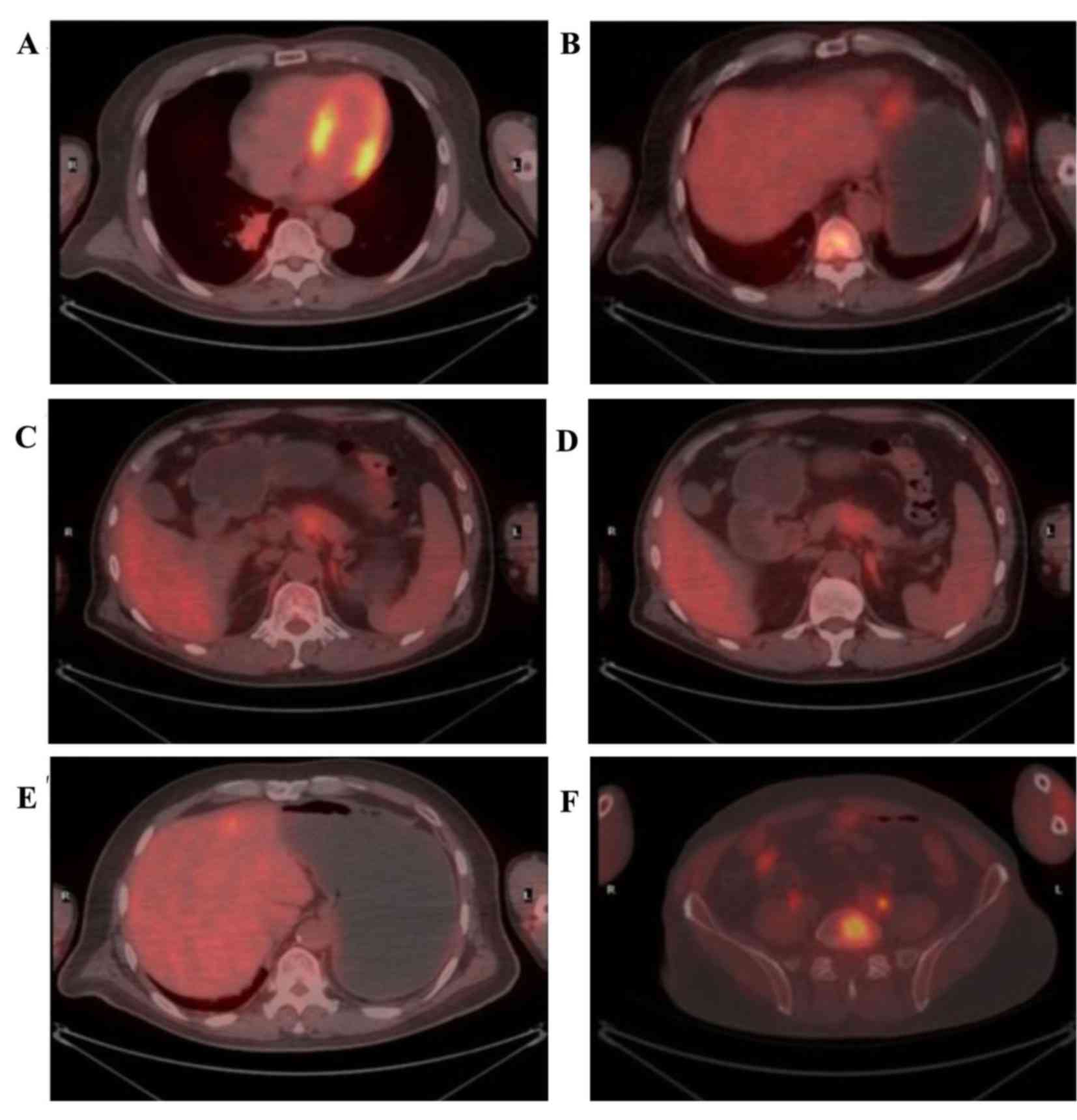
Positron emission tomography (PET)/CT was performed
to determine the metabolic status of the tumor. The standardized
uptake values (SUV) max of the masses in the right lung and left
chest were 6.2 and 4.7, respectively (Fig. 2A and B). In the pancreatic lesion,
which was a suspected metastatic tumor, the SUVearly was 3.7, and
the SUVdelayed was not markedly decreased compared with the
SUVearly (Fig. 2C and D). However,
the 18F-fluorodeoxyglucose (18F-FDG) uptake
by the pancreatic duct was not increased. The SUVmax of the lesions
in the left lobe of the liver and left adrenal exceeded normal
levels (Fig. 2E and F). In addition,
abnormally metabolic lesions were identified in many other organs;
however, no abnormal 18F-FDG uptake was identified in
the gastrointestinal organ.
To exclude a diagnosis of colorectal adenocarcinoma,
rectocolonoscopy had been performed and no anomaly was
identified.
The neoplasm samples in the lower lobe of the right
lung and subcutaneous tissue of the left chest wall were obtained
using CT-guided fine-needle aspiration (FNA) and endoscopic
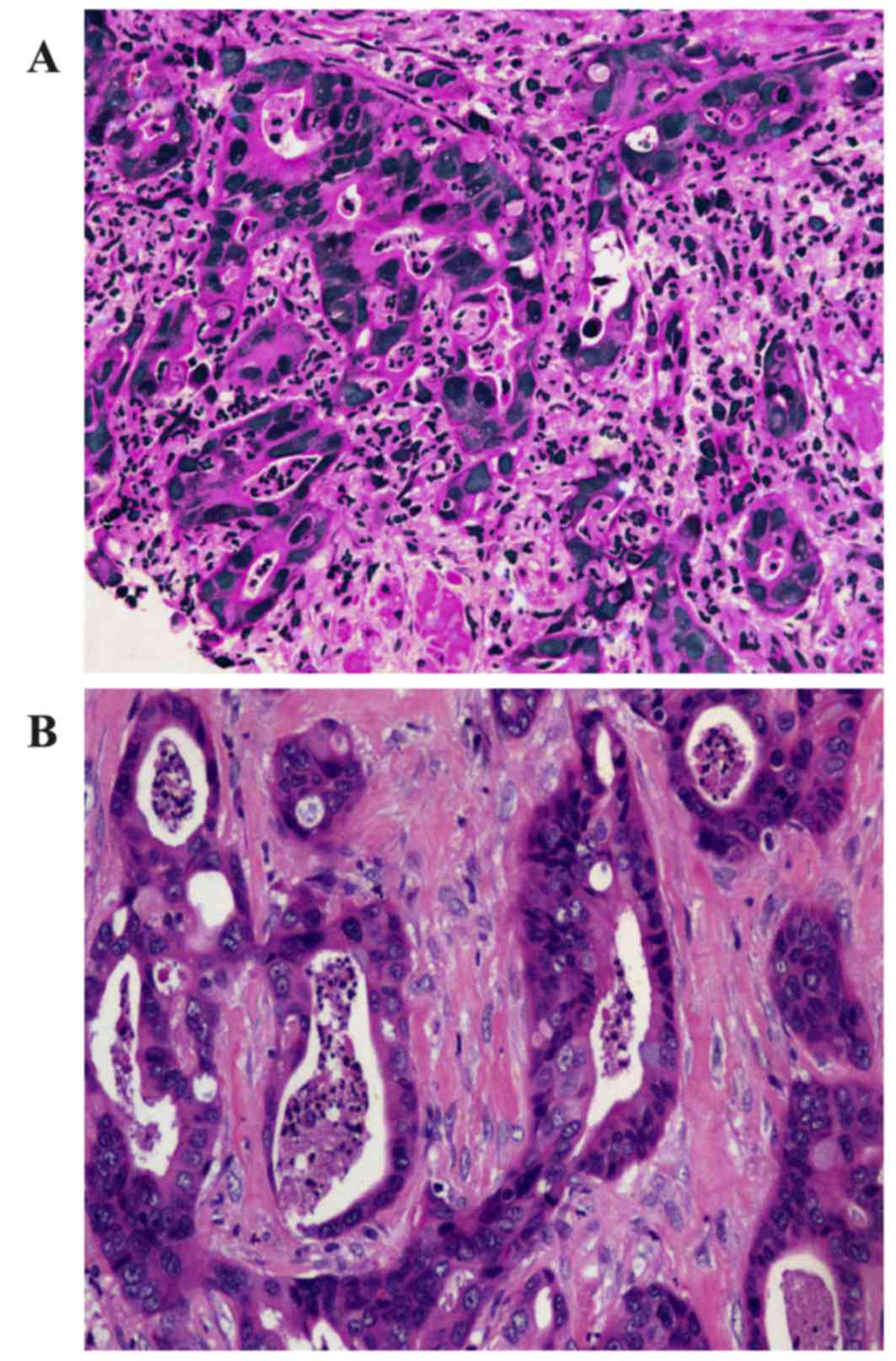
ultrasound (EUS)-guided FNA respectively. The cytological
examination of the two masses, which were stained using hematoxylin
and eosin, identified that the two masses possessed similar
pathological characteristics: Glandular or cribriform structures
with luminal necrosis, tall-columnar oncocytes with eosinophilic
cytoplasm, tall or ovoid nuclei arranging in pseudostratified
pattern and quantities of inflammatory cells infiltrating the
mesenchyma with fibrotic hyperplasia (Fig. 3). It was not possible to easily
distinguish the pathological features of the two lesions from those
of gastrointestinal adenocarcinoma. The antibodies used for IHC
detection are listed in Table I. The
tumor cells of the lung neoplasm was positive for cytokeratin 7
(CK7), negative for CK20, thyroid transcription factor 1 (TTF-1)
and CA19-9, and the expression of caudal type homeobox 2 (CDX2) was
weakly positive; by comparison, those of the chest neoplasm was
positive for CK7, negative for TTF-1, and CA19-9, and CK20 and CDX2
were weakly expressed (Table II;
Fig. 4).
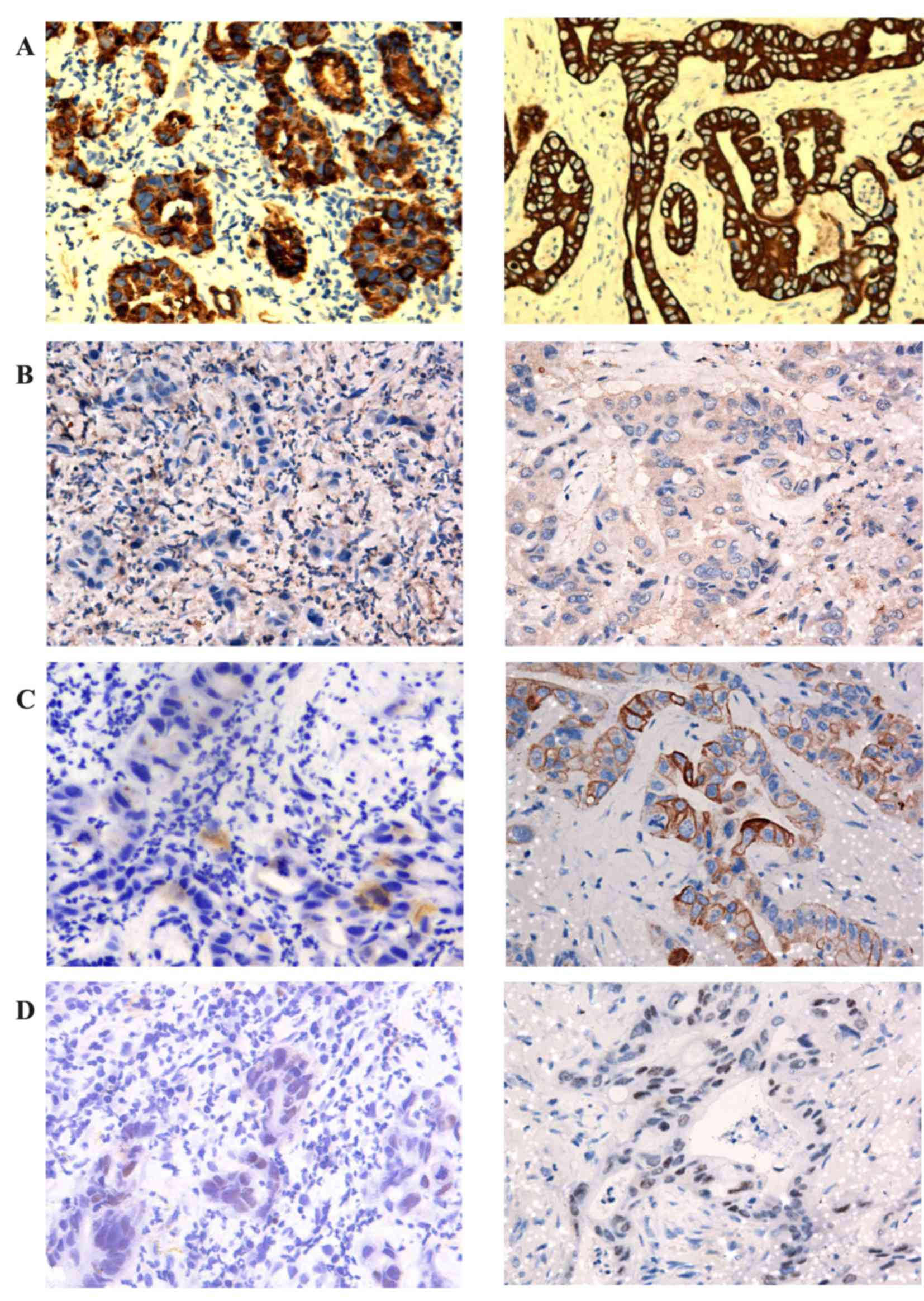 | Figure 4.Immunohistochemical results. Right
lung neoplasm (left) and left chest neoplasm (right) were stained
for (A) CK7, (B) TTF-1, (C) CK20 and (D) CDX2. IHC markers of right
lung neoplasm: CK7 (+), TTF-1 (−), CK20 (−), CDX2 (weakly
positive). IHC markers of left chest neoplasm: CK7 (+), TTF-1 (−),
CK20 (weakly positive) CDX2 (weakly positive). IHC,
immunohistochemical; CK, cytokeratin; TTF-1, thyroid transcription
factor 1; CDX2, caudal type homeobox 2 (IHC, ×200). |
 | Table I.Antibodies applied in the study of
PEAC. |
Table I.
Antibodies applied in the study of
PEAC.
| Antigen | Antibody | Catalog no. | Dilution |
|---|
| CK7 | OV-TL 12/30 | M7018 | 1:100 |
| TTF-1 | 8G7G3/1 | M3575 | 1:50 |
| CK20 | Ks20.8 | M7019 | 1:50 |
| CDX2 | EPR2764Y | M3617 | 1:100 |
| P63 | DAK-p63 | M7317 | 1:200 |
| CK5/6 | D5/16B4 | M7237 | 1:100 |
| CK19 | A53-B/A2.26 | M0888 | 1:100 |
| AE1/AE3
Cytokeratin | AE1/AE3 | M3515 | 1:200 |
| CK903 | 34βE12 | M0630 | 1:50 |
| CA19–9 | 1116-NS-19-9 | M3517 | 1:50 |
| Ki67 | MIB-1 | M7240 | 1:100 |
 | Table II.Immunohistochemical markers of right
lung lump and left chest mass. |
Table II.
Immunohistochemical markers of right
lung lump and left chest mass.
|
| Lump in right
lung | Mass in left
chest |
|---|
| CK7 | + | + |
| TTF-1 | − | − |
| CK20 | − | Weakly
positive |
| CDX2 | Weakly
positive | Weakly
positive |
| p63 | − | − |
| CK5/6 | − | − |
| CK19 | + | + |
| Cytokeratin |
| AE1/AE3 | + | + |
| CK903 | + | + |
| CA19–9 | − | − |
| Ki67 | 30% | 40% |
Discussion
Pulmonary enteric adenocarcinoma
According to the 2011 IASLC/ATS/ERS International
Multidisciplinary Lung Adenocarcinoma Classification, PEAC was
defined as a lung adenocarcinoma with enteric differentiation of
>50% (4). The enteric pattern
exhibits the features of colorectal adenocarcinoma, which has
glandular, papillary and/or cribriform structures with luminal
necrosis, tall-columnar cells with pseudostratified and atypical
nuclei and eosinophilic cytoplasm; in contrast with metastatic
colorectal adenocarcinoma (MCA), PEAC exhibits a histological
component that resembled primary lung adenocarcinoma (PLA),
including lepidic growth (4). PEAC is
consistently positive for CK7 (CK7-negative cases may occur) and
positive for TTF-1 in ~50% of cases, and exhibits >1 IHC marker
of enteric differentiation, including CDX2, CK20 and mucin 2 (MUC2)
(4). The IASLC/ATS/ERS Classification
pointed out that the tumor not exhibiting IHC markers of enteric
differentiation should be regarded as lung adenocarcinoma with
enteric morphology rather than PEAC (4).
According to the references published in PubMed and
Wanfang between 1 May 2005 and 8 October 2015, CK7, TTF-1, CK20 and
CDX2 are the principal IHC markers for distinguishing between PEAC,
MCA and PLA.
In MCC and PLA, the expression of IHC markers is
markedly consistent. Yousem (2)
demonstrated that all lesions in cases of MCA shared the same
immunoprofile, which was negative for TTF-1 and CK7, but markedly
positive for CK20 and CDX2 (75–100%). Inamura et al
(3) identified that TTF-1 and CK7
were all negative in the 14 samples of MCC; however, CDX2 and CK20
were markedly positive in 12 cases. Montezuma et al
(22) performed IHC detection in 25
cases of MCC and 198 cases of PLA, revealing that all MCC cases
were positive for CK20, but negative for TTF-1, and CK7 was
negative or weakly expressed; all PLA cases were strongly positive
for TTF-1, a number were positive for CK7, and only a limited
number were weakly positive for p63. TTF-1, which belongs to the
NK-2 homeobox family, is positive in PLA, but negative in MCC,
therefore it is recognized as the best single stain for PLA
(23).
References concerning IHC markers of PEAC, deposited
in the Wanfang database and PubMed between 1 May 2005 and 8 October
2015, were examined. As the studies by Geles et al (17) and Suzuki et al (18) lacked the specific information
concerning patients and the results of IHC, these studies were
excluded from the analysis. The results of the analysis
demonstrated that, in the 41 cases of PEAC recorded, the positive
rate of CK7 was 87.8%, the negative rates of CK20 and TTF-1 were
70.7 and 51.2%, respectively, and the positive rate of CDX2 ranged
between 51.2 and 65.9% (Table III).
CK7 and CK20 were identified to be comparatively accurate markers
that may be used to distinguish PEAC from MCC, whereas TTF-1
possesses marked accuracy in distinguishing between PEAC and PLA.
In addition to the aforementioned immune markers, PEAC and MCC are
heterogeneous in the expression of MUC1, MUC2 and MUC5 (2). Lin et al (9) reported that villin was able to function
as the IHC marker, which determined the occurrence of enteric
differentiation in cases of PEAC; however, PEAC was not able to be
distinguished from MCC on the basis of the expression pattern of
villin in the brush border.
 | Table III.Studies concerning IHC markers of
PEAC. |
Table III.
Studies concerning IHC markers of
PEAC.
| Author | No. of cases | TTF-1 | CK7 | CK20 | CDX2 | (Refs.) |
|---|
| Yousem | 6 | 6(+); 0(−) | 6(+); 0(−) | 0(+); 6(−) | 0(+); 6(−) | (2) |
| Inamura et
al | 7 | 3(+); 4(−) | 7(+); 0(−) | 3(+); 4(−) | 5(+); 2(−) | (3) |
| Maeda et
al | 1 | 1(+); 0(−) | 1(+); 0(−) | 0(+); 1(−) | NA | (5) |
| Li et
al | 1 | 0(+); 1(−) | 0(+); 1(−) | 1(+); 0(−) | 1(+); 0(−) | (6) |
| Hatanaka et
al | 1 | 0(+); 1(−) | 0(+); 1(−) | 1(+); 0(−) | 1(+); 0(−) | (7) |
| Qureshi et
al | 1 | 0(+); 1(−) | 1(+); 0(−) | 1(+); 0(−) | 1(+); 0(−) | (8) |
| Lin et
al | 1 | 0(+); 1(−) | 1(+); 0(−) | 1(+); 0(−) | NA | (9) |
| Stojsic et
al | 2 | 0(+); 2(−) | 0(+); 2(−) | 2(+); 0(−) | 2(+); 0(−) | (10) |
| Wang et
al | 9 | 4(+); 5(−) | 9(+); 0(−) | 2(+); 7(−) | 6(+); 3(+) | (11) |
| László et
al | 1 | 0(+); 1(−) | 0(+); 1(−) | 1(+); 0(−) | 1(+); 0(−) | (12) |
| Handa et
al | 1 | 1(+); 0(−) | 1(+); 0(−) | 0(+); 1(−) | 0(+); 1(−) | (13) |
| Metro et
al | 1 | 0(+); 1(−) | 1(+); 0(−) | 0(+); 1(−) | 1(+); 0(−) | (14) |
| Wei et
al | 4 | 2(+); 2(−) | 4(+); 0(−) | 0(+); 4(−) | NA | (15) |
| Wang et
al | 5 | 3(+); 2(−) | 5(+); 0(−) | 5(−); 0(+) | 3(+); 2(−) | (16) |
| Totals | 41 | 20(+),
48.80%; | 36(+), 87.80%; | 12(+),
29.30%; | 21(+),
51.2–65.9%; |
|
|
|
| 21(−),
51.20% | 5(−),
12.20% | 29(−),
70.70% | 14(−), 34.1–48.8%;
6NA |
|
To further confirm PEAC, in addition to the
morphological detection of IHC markers, rectocolonoscopy must be
performed to exclude MCA or MCC (10).
In the present case study, the right lung neoplasm
and the left chest neoplasm exhibited similar morphological
characteristics to an enteric pattern. Additionally, the two
lesions possessed similar expressions of the IHC marker: CK7(+),
TTF-1(−) and CDX-2 (weakly positive). Furthermore, rectocolonoscopy
was performed and no anomaly was identified. These results led to
the conclusion that the two neoplasms are of the same pathological
type and the left chest neoplasm is a metastasis from the primary
lung neoplasm. Furthermore, the rectocolonoscopy examination
assisted in excluding the possibility of the presence of MCA or
MCC, and the negative expression of CA19-9 demonstrates that the
two masses are not the metastases of a pancreatic neoplasm.
The two lesions are markedly positive for CK7 and
negative for TTF-1. The aforementioned analysis identified that CK7
and CK20 are comparatively accurate markers used to distinct PEAC
from MCC (MCC is typically negative for CK7 and positive for CK20,
whereas PEAC is typically positive for CK and negative for CK20).
TTF-1 possesses marked accuracy in distinguishing PEAC from PLA
(PEAC is typically negative for TTF-1 and PLA is typically positive
for TTF-1). As aforementioned, the IASLC/ATS/ERS Classification
pointed out that the tumor not exhibiting IHC markers of enteric
differentiation should be regarded as lung adenocarcinoma with
enteric morphology rather than PEAC (4). As the two neoplasms exhibit the same
pathological type and the metastases in the left chest are positive
for CDX2, the uncertain CDX2 in the primary lung neoplasm is most
likely to be positive. Therefore, the primary lung neoplasm is PEAC
rather than primary lung adenocarcinoma with enteric
morphology.
In addition, the 2011 IASLC/ATS/ERS Classification
proposed that the enteric differentiation in lung adenocarcinoma
should be >50% (4). However, the
studies obtained from the PubMed and Wanfang databases did not
demonstrate enteric differentiation of >50%. Furthermore,
samples, obtained using CT-guided FNA, are limited, therefore a
level of 50% is difficult to confirm, so the practical relevance of
50% requires reconsideration.
Metastasis to the pancreas
Pancreatic masses are typically primary neoplasms,
and the incidence of pancreatic metastases reported in previous
studies is <10%; however, pancreatic metastases are being
observed with increased frequency at high-volume pancreatic
surgeries (19,20). The underlying molecular mechanism for
the occurrence of metastases in the pancreas is poorly understood.
According to previous research, one reason may be the
transformation of biological behavior of the tumor cells, which is
a result of the alterations of certain molecules and genes during
chemotherapeutic regimens (24).
Another reason may lie in the fact that certain tumor cells may
have a marked affinity for the parenchyma of the pancreas
regardless of whether or not the neoplasm has been treated
(24).
The definitive separation of primary and secondary
pancreatic neoplasms depends primarily on the examination of
pathological tissue, which is typically collected using EUS-guided
FNA in living patients (25).
Nevertheless, pathological samples of pancreatic metastases remain
difficult to obtain. Furthermore, CT exhibits certain typical
radiographic characteristics in separating the pancreatic
metastases from primary pancreatic carcinomas (20).
The most common manifestation was hypodense or
isodense mass on unenhanced CT. In terms of enhancement pattern,
the homogeneous lesions with well-defined margins exhibited
hypoattenuation, compared with the normal enhanced pancreas. The
rare presentation was pancreatic infiltration with mild enhancement
instead of focal mass, which is similar to focal pancreatitis
(20). However, unlike pancreatic
ductal adenocarcinoma (PDAC), the majority of metastases tended to
be well circumscribed, although they lacked true capsules. Tan
et al (26) identified other
unique features of secondary alterations caused by PDAC that are
rarely observed in pancreatic metastasis, including dilatation of
the upstream pancreatic duct or pancreatic parenchymal atrophy;
furthermore, multiple lesions occurring in the pancreas are strong
evidence of pancreatic metastases. Shi et al (20) identified that pancreatic metastases
from non-small cell lung cancer and gastrointestinal carcinoma
exhibited similar CT imaging characteristics, and they hypothesized
that this may have an association with pathological type, since the
majority were adenocarcinoma.
PET/CT, combining metabolic detection with
anatomical imaging, may reflect the metabolic level and
proliferative index of the tumor. It has been reported that PET/CT
is superior to CT in diagnosis of pancreatic cancer (27). Improved diagnostic value for
pancreatic cancer may be obtained when 18F-FDG uptake of
the anomaly is increased compared with that of normal parenchyma or
that of normal liver. The threshold value of SUVmax in
distinguishing pancreatic cancer from benign lesions has not yet
been determined. Nishiyama et al (28) demonstrated that dual-phase
18F-FDG imaging may improve diagnostic efficacy in
distinguishing pancreatic cancer from mass-forming pancreatitis,
whereas Kato et al (29)
indicated that differentiation between metastasis-free pancreatic
cancer and mass-forming pancreatitis was difficult by
18F-FDG-PET/CT due to considerable overlap between the
SUVmax of these two diseases.
With the exception of radiographic characteristics,
Tan et al (26) proposed that
the existence of other malignant lesions was important for the
diagnosis of pancreatic metastasis. The presence of CA19-9 may also
contribute to the correct diagnosis of pancreatic metastasis.
CA19-9 possesses 81% sensitivity and 89% specificity for the
diagnosis of primary pancreatic adenocarcinoma rather than
secondary pancreatic lesions, particularly when the level of CA19-9
is increased progressively (20).
The present case has demonstrated typical
radiographic characteristics of pancreatic metastases from PEAC,
which differ from those of primary pancreatic lesions. The serum
tumor marker CA19-9 in the present case was also at a normal level.
Indeed, certain patients with primary pancreatic tumors have normal
CA19-9 levels; however, if CA19-9 is maintained at a normal level,
the possibility of the occurrence of primary pancreatic tumors
decreases. Furthermore, it has been confirmed that the neoplasm in
the left chest originated from a right lung neoplasm, and the IHC
marker CA19-9 is negative in the two neoplasm, which further
decreases the possibility of the identification of a primary
pancreatic neoplasm. A number of other metastases have also been
presented following examination using PET/CT; generally speaking,
it is more likely that the metastases originate from one primary
neoplasm rather than two. Although EUS-guided FNA was not applied
in the present case, owing to the poor physical condition of the
patient, according to the above analysis, the lesion in the
pancreas is considered to be a metastasis originating from
PEAC.
PEAC is an aggressive cancer, which is characterized
by rapid growth and early metastatic behavior. To realize
personalized therapy of primary lung carcinoma, further
investigations are required for further understanding of PEAC.
However, owing to a lack of cases of PEAC, it remains challenging
to study PEAC. Pancreatic metastasis is also observed infrequently,
but it typically affects the treatment decision, so increased
efforts should be made to improve our knowledge of the underlying
molecular mechanism of pancreatic metastasis and improve the
radiographic diagnosis efficacy of PEAC since EUS-guided FNA is an
invasive technique that should be limited in use.
Acknowledgements
The present study was supported by the Public Health
Discipline Establishment in Shanghai (grant no. 12GWZX1002) and the
Key Discipline Construction Project by Shanghai Health Development
Planning(grant no. 2015ZB0503).
References
|
1
|
Tsao MS and Fraser RS: Primary pulmonary
adenocarcinoma with enteric differentiation. Cancer. 68:1754–1757.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yousem SA: Pulmonary intestinal-type
adenocarcinoma does not show enteric differentiation by
immunohistochemical study. Mod Pathol. 18:816–821. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Inamura K, Satoh Y, Okumura S, Nakagawa K,
Tsuchiya E, Fukayama M and Ishikawa Y: Pulmonary adenocarcinomas
with enteric differentiation: Histologic and immunohistochemical
characteristics compared with metastatic colorectal cancers and
usual pulmonary adenocarcinomas. Am J Surg Pathol. 29:660–665.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger K, Yatabe Y, Powell CA, Beer D, Riely G,
Garg K, et al: International Association for the Study of Lung
Cancer/American Thoracic Society/European Respiratory Society:
International multidisciplinary classification of lung
adenocarcinoma: Executive summary. Proc Am Thorac Soc. 8:pp.
381–385. 2011; View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maeda R, Isowa N, Onuma H and Miura H:
Pulmonary intestinal-type adenocarcinoma. Interact Cardiovasc
Thorac Surg. 7:349–351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li HC, Schmidt L, Greenson JK, Chang AC
and Myers JL: Primary pulmonary adenocarcinoma with intestinal
differentiation mimicking metastatic colorectal carcinoma: Case
report and review of literature. Am J Clin Pathol. 131:129–133.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hatanaka K, Tsuta K, Watanabe K, Sugino K
and Uekusa T: Primary pulmonary adenocarcinoma with enteric
differentiation resembling metastatic colorectal carcinoma: A
report of the second case negative for cytokeratin 7. Pathol Res
Pract. 207:188–191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qureshi A and Furrukh M: Enteric
adenocarcinoma lung: A rare presentation in an Omani woman. BMJ
Case Rep. 2013:bcr2012007667. 2013. View Article : Google Scholar
|
|
9
|
Lin D, Zhao Y, Li H and Xing X: Pulmonary
enteric adenocarcinoma with villin brush border immunoreactivity: A
case report and literature review. J Thorac Dis. 5:E17–E20.
2013.PubMed/NCBI
|
|
10
|
Stojsic J, Kontic M, Subotic D, Popovic M,
Tomasevic D and Lukic J: Intestinal type of lung adenocarcinoma in
younger adults. Case Rep Pulmonol. 2014:2821962014.PubMed/NCBI
|
|
11
|
Wang CX, Liu B, Wang YF, Zhang RS, Yu B,
Lu ZF, Shi QL and Zhou XJ: Pulmonary enteric adenocarcinoma: A
study of the clinicopathologic and molecular status of nine cases.
Int J Clin Exp Pathol. 7:1266–1274. 2014.PubMed/NCBI
|
|
12
|
László T, Lacza A, Tóth D, Molnár TF and
Kálmán E: Pulmonary enteric adenocarcinoma indistinguishable
morphologically and immunohistologically from metastatic colorectal
carcinoma. Histopathology. 65:283–287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Handa Y, Kai Y, Ikeda T, Mukaida H, Egawa
H and Kaneko M: Pulmonary enteric adenocarcinoma. Gen Thorac
Cardiovasc Surg. 64:749–751. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Metro G, Valtorta E, Siggillino A,
Lauricella C, Cenci M, Ludovini V, Minenza E, Prosperi E, Ricciuti
B, Rebonato A, et al: Enteric-type adenocarcinoma of the lung
harbouring a novel KRAS Q22K mutation with concomitant KRAS
polysomy: A case report. Ecancermedicalscience. 9:5592015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei QZ, Liu JH, Zhang ZX, Yang Q and Zhao
T: Expression and significance of TTF-1, CK7, CK5/6, P63 and CD20
in mucin producing lung cancer. Clin J Diffic and Compl Cas.
9:822–824. 2010.(In Chinese).
|
|
16
|
Wang CX, Xu Y, Liu B, Zhang J, Yu B, Shi
SS and Zhou XJ: Clinicopathologic features and differential
diagnosis of pulmonary intestinal-type adenocarcinoma. J Clin Exp
Pathol. 29:1101–1104. 2013.(In Chinese).
|
|
17
|
Geles A, Gruber-Moesenbacher U,
Quehenberger F, Manzl C, Al Effah M, Grygar E, Juettner-Smolle F
and Popper HH: Pulmonary mucinous adenocarcinomas: Architectural
patterns in correlation with genetic changes, prognosis and
survival. Virchows Arch. 467:675–686. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suzuki M, Yazawa T, Ota S, Morimoto J,
Yoshino I, Yamanaka S, Inayama Y, Kawabata Y, Shimizu Y, Komatsu M,
et al: High-grade fetal adenocarcinoma of the lung is a tumour with
a fetal phenotype that shows diverse differentiation, including
high-grade neuroendocrine carcinoma: A clinicopathological,
immunohistochemical and mutational study of 20 cases.
Histopathology. 67:806–816. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smith AL, Odronic SI, Springer BS and
Reynolds JP: Solid tumor metastases to the pancreas diagnosed by
FNA: A single-institution experience and review of the literature.
Cancer Cytopathol. 123:347–355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi HY, Zhao XS and Miao F: Metastases to
the pancreas: Computed tomography imaging spectrum and clinical
features: A retrospective study of 18 patients with 36 metastases.
Medicine (Baltimore). 94:e9132015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sweeney AD, Fisher WE, Wu MF, Hilsenbeck
SG and Brunicardi FC: Value of pancreatic resection for cancer
metastatic to the pancreas. J Surg Res. 160:268–276. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Montezuma D, Azevedo R, Lopes P, Vieira R,
Cunha AL and Henrique R: A panel of four immunohistochemical
markers (CK7, CK20, TTF-1 and p63) allows accurate diagnosis of
primary and metastatic lung carcinoma on biopsy specimens. Virchows
Arch. 463:749–754. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Travis WD and Rekhtman N: Pathological
diagnosis and classification of lung cancer in small biopsies and
cytology: Strategic management of tissue for molecular testing.
Semin Respir Crit Care Med. 32:22–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ballarin R, Spaggiari M, Cautero N, de
Ruvo N, Montalti R, Longo C, Pecchi A, Giacobazzi P, de Marco G,
D'Amico G, et al: Pancreatic metastases from renal cell carcinoma:
The state of the art. World J Gastroenterol. 17:4747–4756. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karakan T, Cengiz M, İbiş M, Akyürek N and
Ünal S: Pancreatic metastasis in a case of small cell lung
carcinoma diagnosed by EUS. Turk J Gastroenterol. 26:53–55. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tan CH, Tamm EP, Marcal L, Balachandran A,
Charnsangavej C, Vikram R and Bhosale P: Imaging features of
hematogenous metastases to the pancreas: Pictorial essay. Cancer
Imaging. 11:9–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang XY, Yang F, Jin C and Fu DL: Utility
of PET/CT in diagnosis, staging, assessment of resectability and
metabolic response of pancreatic cancer. World J Gastroenterol.
20:15580–15589. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nishiyama Y, Yamamoto Y, Monden T,
Sasakawa Y, Tsutsui K, Wakabayashi H and Ohkawa M: Evaluation of
delayed additional FDG PET imaging in patients with pancreatic
tumour. Nucl Med Commun. 26:895–901. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kato K, Nihashi T, Ikeda M, Abe S, Iwano
S, Itoh S, Shimamoto K and Naganawa S: Limited efficacy of
(18)F-FDG PET/CT for differentiation between metastasis-free
pancreatic cancer and mass-forming pancreatitis. Clin Nucl Med.
38:417–421. 2013. View Article : Google Scholar : PubMed/NCBI
|