Introduction
Oral squamous cell carcinoma (OSCC) caused 364,872
deaths (216,975 men and 147,897 women) in Japan in 2013 (1). Approximately 60% of head and neck cancer
patients are in the advanced stage (stage III and stage IV) of the
disease (2,3). As OSCCs are often diagnosed at the
advanced stage, survival rate of the patients is low. Also,
currently available clinical diagnosis, evaluation and advanced
treatment methods could not ensure increased survival rate of the
patients (2,3). Therefore, we should continue to
investigate for suitable biomarkers for early diagnosis or
prognostic prediction of OSCC. The development of useful biomarkers
must lead to the discovery of the novel therapeutic strategy or new
chemo-preventive agents.
The most crucial features of malignant tumors may be
non-predictable progression. Briefly, rapid growth and
proliferation rate, high invasive capacity and metastatic ability
are the characteristics of progressive tumor cells, while
regressive benign tumor cells show opposite characteristics.
Progressive (QRsP-11 clone) and regressive (QR-32 clone) murine
fibrosarcoma tumor models were successfully established by Okada
et al (4,5). The progressive clone QRsP-11 with
malignant characteristic was established from QR-32 regressive
clone, which is a weakly tumorigenic and non-metastatic cell clone.
In normal syngeneic mice, QR-32 cells were reported to regress
spontaneously if the cells were injected intravenously
(1×106 cells) or subcutaneously (up to 2×105
cells). However, subcutaneous injection of these cells with
co-implanted gelatin sponge results in progressive growth of the
cells. From these progressively-growing cells, QRsPs cell lines
were established which has the ability to progressively grow in
mice without the presence of gelatin sponge. QRsPs, e.g., -QRsP-11
is as malignant tumor cell clone which is more tumorigenic and
metastatic. Proteomic studies of QR-32 and QRsP-11 cells were
conducted by two-dimensional gel electrophoresis (2-DE) to evaluate
the differential expression patterns of proteins between these cell
types in order to understand various important factors in tumor
progression (6,7). Differential display analysis of the
nuclear protein expression of these cells revealed eight
differentially-regulated nuclear proteins between QR-32 and
QRsP-11, and Zing finger protein ZXDC is one of them (6). In addition, 11 differentially regulated
proteins were found by proteomic differential display analysis of
cytoplasmic proteins, including heat-shock protein (HSP)-90
(8). In the present study, we focused
on Calreticulin (CALR) which is one of those 11 proteins.
CALR is a 46 kDa protein which acts as a molecular
chaperone and a major Ca (2+)-binding (storage) protein in the
endoplasmic reticulum (ER) that ensures proper folding of
glycoproteins (9–11). CALR controls a number of diverse
biological processes in ER and other parts of the cell; such as
gene expression, transcriptional activities, cell proliferation,
phagocytosis, apoptosis and immune response (9,10). It can
also affect cell adhesion, wound healing and migration (9,10).
Although there are many studies on the role of CALR in a variety
cellular processes and functions, its role on human carcinogenesis
still remains unclear (11).
There have been studies on the alteration of CALR
expression in lung cancer, ovarian cancer, gibloblastoma,
gastrointentinal cancer, breast cancer, urinary tract cancer, which
indicates its role in promoting or suppressing various types of
cancers (9–11). Overespression of CALR has been
associated with a number of malignancies together with several
types of cancers (12–15). Elevated expressions of CALR in the
sera of patients with autoimmune diseases were also reported
(12). Again, differential expression
of CALR was reported in some cancer types, and in some cases CALR
low-expression was found to promote cancer growth (11,12). In
lung cancer, the expression level of CALR on tumor cell membrane
was apparently related with the pathological grade of the tumor
(12). Positive immunohistochemical
staining of CALR was associated with lymph node metastasis and high
microvessel density in gastric cancer; and resulted in poor patient
survival (15). However, the
importance of CALR expression in the regulation of OSCC tumor
growth or malignancy is largely unknown. The purpose of this study
was to determine whether CALR expression could be a useful
prognostic factor in patients with OSCC.
Materials and methods
Patients and patient samples
This study was carried out with tissue specimens
collected from 111 OSCC patients. All these patients were treated
at Yamaguchi University Hospital from April 2000 to March 2010 and
underwent curative surgery. Post-operative chemoradiation was used
in the case of tumors with positive surgical margin, and
post-operative radiation or chemoradiation was used for extranodal
invasion. Surgical resection alone was carried out for 93 cases,
surgical resection plus radiation for 6 cases, and surgical
resection plus chemoradiation for 12 cases. Primary oral cancer
tissue samples were obtained by biopsy before the patients received
any treatment. Phosphate-buffered 10% formalin was used to fix the
tissue samples and then the tissues were embedded in paraffin
blocks.
The tumors were staged according to the Tumor, Node
and Metastasis (TNM) classification of the Union Internationale
Contra le Cancer (UICC) (2002). Institutional Review Board (IRB)
approval from the Ethics Committee of the Yamaguchi University
Hospital was obtained for this study (Ref. H28-057). This being a
retrospective study, informed consent was waived by the IRB.
Immunohistochemical staining and the
analysis of staining
Formalin fixed and paraffin embedded (FFPE) samples
were used for the immunohistochemical analysis. FFPE tissue blocks
were sliced into 4-µm thick sections and attached on slides. These
FFPE slides were dried, then deparaffinized in xylene and immersed
in graded series (100–70%) of ethanol concentrations. After
phosphate buffered saline wash, the sections were heated in a
microwave oven in an antigen retrieval solution. The sections were
allowed to cool down and immersed in 0.3% hydrogen peroxide
solution in methanol for 30 min to quench any residual peroxidase
activity. After incubation with Dako REAL™ peroxidase-blocking
solution (S2023; Dako, Glostrup, Denmark) for 15 min, the sections
were incubated overnight at 4°C with rabbit polyclonal
anti-Calreticulin antibody (StressMarq Bioscience Inc., Victoria,
BC, Canada). Then the sections were incubated with a secondary
antibody solution for 60 min at room temperature, followed by
diaminobenzidine using REAL™ Envision™ Detection system kit (K5007,
Dako). The sections were lightly counterstained with hematoxylin,
dehydrated in graded series of ethanol, immersed in xylene and
coverslipped.
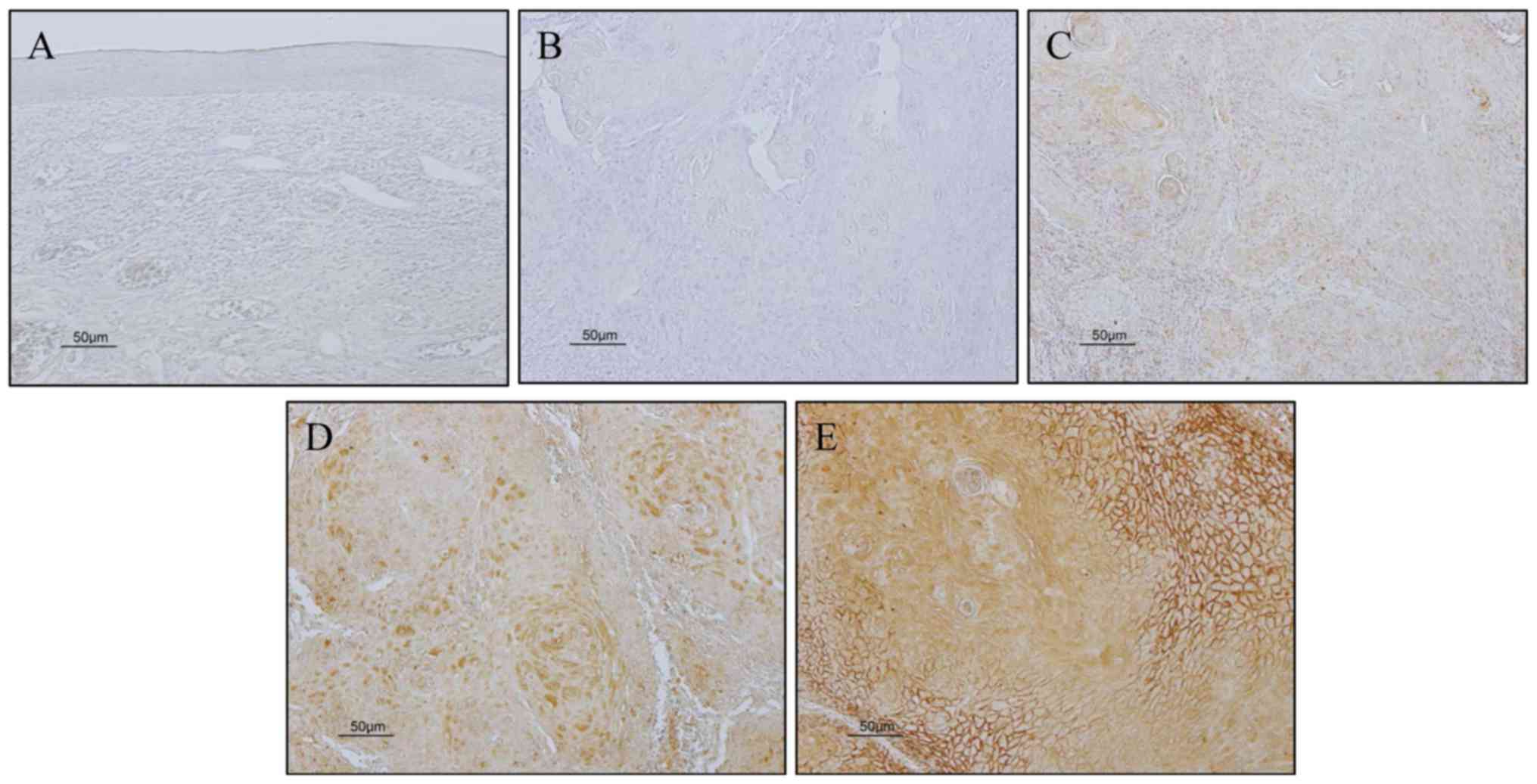
We evaluated CALR expression in our samples
according to the method described by Kuramitsu et al with
some modification (6). Briefly, we
compared the intensity of CALR staining in cancer cells from
cancerous tissue with that in the normal oral epithelium. The
percentage of positive cells was graded as 0, 0% immunopositive
cells; 1, <50% positive cells; 2, ≥50% positive cells. And the
staining intensity was graded as 0, negative; 1 weak; 2, moderate;
3, strong (Fig. 1). The sum of the
assigned values of the percentage of positive cells and the
staining intensity was regarded as the immunoreactivity score.
Scores between 0–2 were regarded as low expression, scores between
3–5 were regarded as high expression. Immunoreactivity for CALR
expression was evaluated by three authors (K.H., T.T. and T.F.),
who had no knowledge of the patient's clinical status.
Statistical analysis
We used Chi-square test to determine the association
of CALR expression in the OSCC tissues with the clinical and
pathological variables. Overall survival (OS) and event-free
survival (EFS) were defined as the time from treatment initiation
to the date of death from any cause. To estimate the probability of
OS or EFS as a function of time, Kaplan-Meier method was used.
Log-rank test was used to compare the statistical differences in
the survival of subgroups of patients. To study the effects of CALR
expression on the OS, a multivariate survival analysis was carried
out using the Cox regression model. All P-values were based on
two-tailed statistical analysis, and P-values <0.05 were
considered statistically significant (P<0.05 and P<0.01). All
statistical analysis was done using the StatView software (version
5.0 J; SAS Institute Inc., Cary, NC, USA).
Results
Patients and tumor
characteristics
Table I shows the
characteristics of patients with OSCCs used in this study. This
study included a total of 111 OSCC patients. The median age of the
patients was 70 years (range, 18–96 years). The median follow-up
time was 5.4 years. We diagnosed 26 patients with stage I, 41 with
stage II, whereas stage III and IV were diagnosed in 12 and 32
patients, respectively.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | Total (n=111) No. of
patients | % |
|---|
| Age (years) |
|
Median | 70.0 |
|
|
Min-mac | 18–96 |
|
|
<65 | 41 | 36.9 |
|
>65 | 70 | 63.1 |
| Gender |
| Male | 60 | 54.1 |
|
Female | 51 | 45.9 |
| T classification |
| 1 | 26 | 23.4 |
| 2 | 55 | 49.5 |
| 3 | 8 | 7.2 |
| 4 | 22 | 19.8 |
| N classification |
| 0 | 82 | 73.9 |
| 1 | 12 | 10.8 |
| 2 | 14 | 12.6 |
| 3 | 3 | 2.7 |
| Stage |
| I | 26 | 23.4 |
| II | 41 | 36.9 |
| III | 12 | 10.8 |
| IV | 32 | 28.8 |
| Outcome |
|
Alive | 84 | 75.7 |
|
Death | 27 | 24.3 |
| CALR expression in
tumor cells cytoplasm |
| Low | 67 | 60.4 |
| High | 44 | 39.6 |
CALR expression in tumor cells and
clinicopathological features
Table II shows the
correlation between CALR expression in tumor cell and
clinicopathological factors of patients. Among 111 patients with
OSCC, we observed low expression of CALR in the tumor cells of 67
patients (60.4%) and high in 44 patients (39.6%). Positive
immunoreactivity of CALR in tumor cells was significantly
associated with T classification (P=0.0027), N classification
(P=0.0219), higher stage (P=0.0013) and fatal outcome (P=0.0014).
However, gender and age of the patients were not correlated with
CALR positivity.
 | Table II.Correlation of CALR expression and
clinicopathological factors in OSCC. |
Table II.
Correlation of CALR expression and
clinicopathological factors in OSCC.
| CALR expression in
tumor cell |
|---|
| Characteristics | Low expression (n=67,
60.4%) | High expression
(n=44, 39.6%) | Total (n=111) | P-valuea |
|---|
| Age (years) |
|
|
| 0.7247 |
|
<65 | 25 | 16 | 41 |
|
|
>65 | 42 | 28 | 70 |
|
| Gender |
|
|
| 0.2817 |
| Male | 39 | 21 | 60 |
|
|
Female | 28 | 23 | 51 |
|
| T classification |
|
|
| 0.0027 |
| 1+2 | 56 | 25 | 81 |
|
| 3+4 | 11 | 19 | 30 |
|
| N classification |
|
|
| 0.0219 |
| 0 | 54 | 28 | 82 |
|
|
1+2+3 | 13 | 16 | 29 |
|
| Stage |
|
|
| 0.0013 |
| I+II | 48 | 19 | 67 |
|
|
III+IV | 19 | 25 | 44 |
|
| Outcome |
|
|
| 0.0014 |
|
Alive | 58 | 26 | 84 |
|
|
Death | 9 | 18 | 27 |
|
CALR expression in OSCCs and patient
survival
The overall median follow-up of the cohort was 5.4
years, 27 patients died. In addition, 11 patients had events
including recurrences and/or post-operative metastasis. Five-year
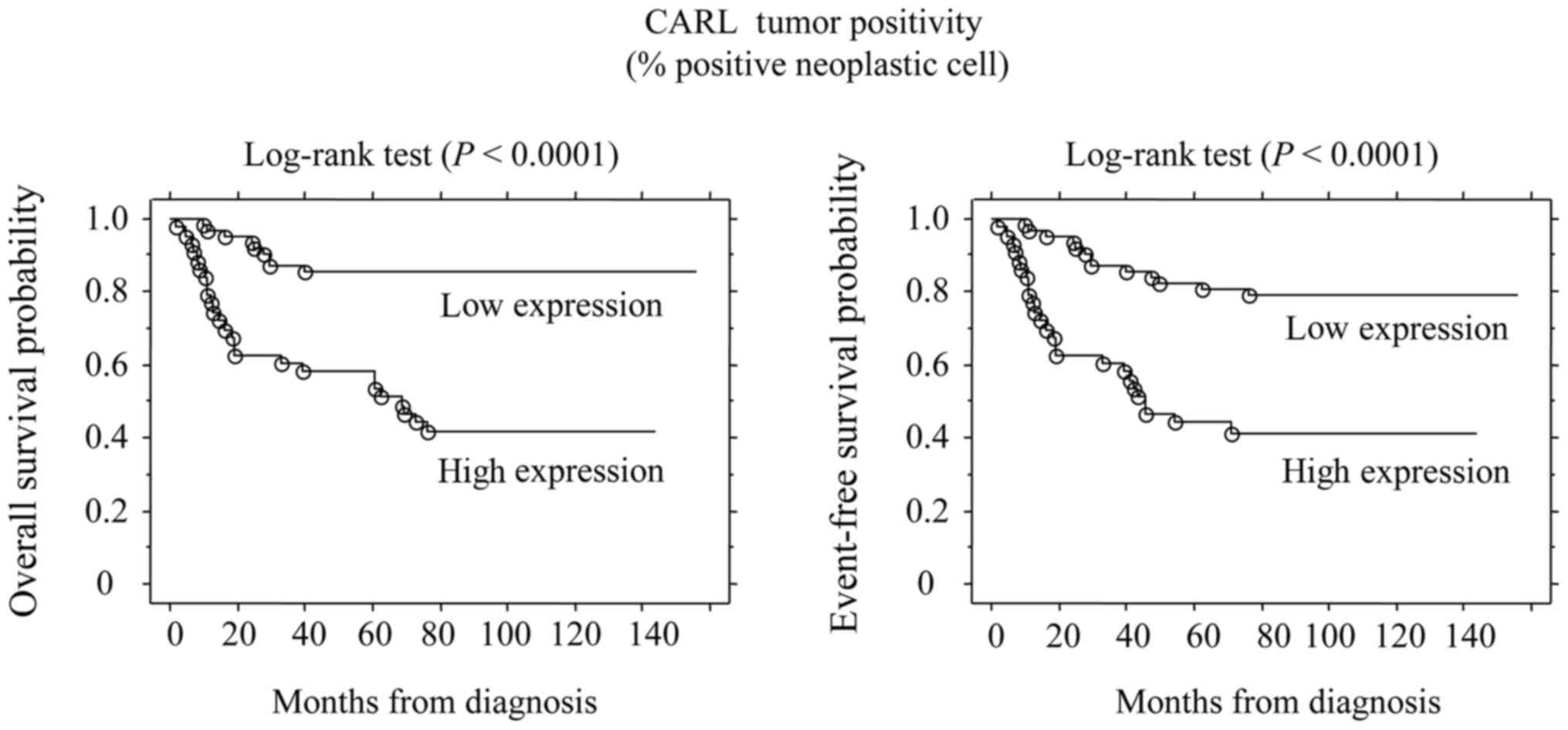
OS and EFS for the whole population patients were 75.7 and 65.8%,
respectively. The 5-year OS rates of CALR high- and low-expression
tumors were 59.1 and 86.6%, respectively. Also, the 5-year EFS
rates of CALR high- and low-expression tumors were 43.21 and 80.6%,
respectively. Log-rank test showed significant difference
(P<0.0001) in the OS rate between patients with high and low
CALR expression. In addition, CALR expression also was associated
with EFS (P<0.0001) (Fig. 2).
Moreover, multivariate analysis also revealed that T3+T4
(P=0.0226), Stage (P=0.0048), N positive (P=0.0013) and high
expression of CALR (P<0.0001) was a predictor of reduced
survival, although no other varieties were identified (Table III).
 | Table III.Risk factors affecting overall
survival rate determined by Cox's proportional hazard model. |
Table III.
Risk factors affecting overall
survival rate determined by Cox's proportional hazard model.
| Variables | Hazard ratio | 95% CI |
P-valuea |
|---|
| Age (years) |
| <65
vs. >65 | 1.652 | 0.771–3.542 | 0.1966 |
| Gender |
| Male
vs. female | 1.351 | 0.689–2.650 | 0.3814 |
| T
classification |
| T1+T2
vs. T3+T4 | 2.216 | 1.118–4.391 | 0.0226 |
| N
classification |
| N0 vs.
N1+N2+N3 | 3.041 | 1.543–5.995 | 0.0013 |
| Stage |
| I+II
vs. III+IV | 2.673 | 1.349–5.296 | 0.0048 |
| CALR
expression |
| High
vs. low | 5.641 | 2.624–12.130 | <0.0001 |
Discussion
Calreticulin (CALR), an endoplasmic reticulum
(ER)-resident protein, plays an important role in multiple
biological processes such as Ca2+ homeostasis (9). CALR regulates cell proliferation, wound
healing, immune response, apoptosis and may other important
cellular processes (9,10). However, the role of CALR in OSCC
development has not been fully clarified. Especially, it remains
unclear whether CALR may act as a biomarker for OSCC patients or
not. Thus, we investigated the expression of CALR in 111 OSCC
tissue samples using immunohistochemistry to evaluate whether CALR
can be a useful biomarker for OSCC in the present study.
We found that among 111 patients with OSCC, CALR
expression in tumor cells was low in 67 patients (60.4%) and high
in 44 patients (39.6%). In addition, CALR positivity in tumor cell
was significantly associated with T classification, N
classification, higher stage and fatal outcome (Table II). Briefly, CALR may be a useful
prognostic factor for patients with OSCC according to above our
findings. It has been reported that soluble CALR level in sera
could be a useful biomarker for detecting lung cancer, and its
level in urine might be important for the prediction and diagnosis
of bladder cancer (12,13). In addition, overexpression of CALR was
correlated with postoperative tumor metastasis in patients with
breast cancer (14). Moreover, it was
reported that overexpression of CALR might be associated with
increased proliferation, migration and angiogenesis of gastric
cancer cells (15). Furthermore,
elevated expression of CALR was observed in tumor tissues of
various cancers including hepatocellular carcinoma (16), colon adenocarcinoma (17) and urinary cancer (13). CALR may be closely associated to tumor
malignancy in various cancers.
However, it was also reported that CALR might
inhibit growth and/or metastasis of prostate cancer cells in
vitro and in vivo (11).
Also, cell surface CALR has been considered as an ‘eat-me’ signal,
and cell surface CALR might promote phagocytic uptake of cancer
cells by immune systems (18,19). Therefore, the influence of CALR on
tumor malignancy may depend on localization of tumor cells as well
as cell types and clinical stages. Although there are almost no
report on CALR expression affecting OSCC carcinogenesis and
patients' outcome, we believe CALR can be a useful biomarker for
OSCC.
Our findings showed that high expression of CALR in
tumor cell was associated with poor survival of patients (Fig. 2), as well as poor prognosis. Thus,
high expression of CALR may promote tumor progression in OSCC.
These results suggest that OSCC patients with low expression of
CARL may benefit from standard therapy based on surgical resection.
Therefore, investigation of CALR expression in biopsy material may
lead to select effective treatments for patients with OSCC. In
addition, we took the tissue samples from the center of the tumor
mass. Sometimes we took a number of samples from different parts of
the tumor or different tumors (if they seem heterogeneous) from the
same patient if needed, and analyzed them. Therefore, we think our
biopsy tissue samples reflected almost all part of the tumors for a
particular patient.
This study showed the importance of CALR as a
prognostic marker in OSCC. Our data on the correlation of CALR
expression levels and T classification, N classification, higher
stage and fatal outcome were similar to most of the previous
reports. In order to clarify the detailed mechanism of action of
CALR in OSCC, further investigations are necessary.
This study was funded partly by a Grant-in-Aid
(Grant number 16K20581) from the Japanese Ministry of Education,
Science and Culture.
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
Institutional review board (IRB) of Yamaguchi University Hospital
(Ref. H28-057).
For retrospective studies, formal consent from
patients is not required. Therefore, informed consent was waived by
the IRB.
Acknowledgements
We thank Dr Dan Cui (Department of Pathology,
Yamaguchi University Graduate School of Medicine) for her valuable
suggestions and technical support in immunohistochemical
analysis.
References
|
1
|
Cancer Information Service, . Cancer
Statistics in Japan-2014. http://ganjoho.jp/reg_stat/statistics/brochure/backnumber/2014_jp.htmlMarch
14–2016
|
|
2
|
National Comprehensive Cancer Network
(NCCN), . NCCN Clinical Practice Guidelines in Oncology: Head and
Neck Cancers, Ver. 1. 2012.http://www.nccn.org/clinical.aspMarch
14–2016
|
|
3
|
Inagi K, Takahashi H, Okamoto M, Nakayama
M, Makoshi T and Nagai H: Treatment effects in patients with
squamous cell carcinoma of the oral cavity. Acta Otolaryngol Suppl.
547:25–29. 2002. View Article : Google Scholar
|
|
4
|
Okada F, Hosokawa M, Hamada JI, Hasegawa
J, Kato M, Mizutani M, Ren J, Takeichi N and Kobayashi H: Malignant
progression of a mouse fibrosarcoma by host cells reactive to a
foreign body (gelatin sponge). Br J Cancer. 66:635–639. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ishikawa M, Hosokawa M, Oh-hara N, Niho Y
and Kobayashi H: Marked granulocytosis in C57BL/6 mice bearing a
transplanted BMT-11 fibrosarcoma. J Natl Cancer Inst. 78:567–571.
1987.PubMed/NCBI
|
|
6
|
Kuramitsu Y, Hayashi E, Okada F, Tanaka T,
Zhang X, Ueyama Y and Nakamura K: Proteomic analysis for nuclear
proteins related to tumour malignant progression: A comparative
proteomic study between malignant progressive cells and regressive
cells. Anticancer Res. 30:2093–2099. 2010.PubMed/NCBI
|
|
7
|
Kuramitsu Y, Hayashi E, Okada F, Zhang X,
Ueyama Y and Nakamura K: Two-dimensional gel electrophoresis using
immobilized pH gradient strips and flamingo™ fluorescent gel stain
identified non-nuclear proteins possibly relates to tumor malignant
progression. Anticancer Res. 31:1259–1263. 2011.PubMed/NCBI
|
|
8
|
Hayashi E, Kuramitsu Y, Okada F, Fujimoto
M, Zhang X, Kobayashi M, Iizuka N, Ueyama Y and Nakamura K:
Proteomic profiling for cancer progression: Differential display
analysis for the expression of intracellular proteins between
regressive and progressive cancer cell lines. Proteomics.
5:1024–1032. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sheng W, Chen C, Dong M, Zhou J, Liu Q,
Dong Q and Li F: Overexpression of calreticulin contributes to the
development and progression of pancreatic cancer. J Cell Physiol.
229:887–897. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Michalak M, Groenendyk J, Szabo E, Gold LI
and Opas M: Calreticulin, a multi-process calcium-buffering
chaperone of the endoplasmic reticulum. Biochem J. 417:651–666.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alur M, Nguyen MM, Eggener SE, Jiang F,
Dadras SS, Stern J, Kimm S, Roehl K, Kozlowski J, Pins M, et al:
Suppressive roles of calreticulin in prostate cancer growth and
metastasis. Am J Pathol. 175:882–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu R, Gong J, Chen J, Li Q, Song C, Zhang
J, Li Y, Liu Z, Dong Y, Chen L and Jin B: Calreticulin as a
potential diagnostic biomarker for lung cancer. Cancer Immunol
Immunother. 61:855–864. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kageyama S, Isono T, Iwaki H, Wakabayashi
Y, Okada Y, Kontani K, Yoshimura K, Terai A, Arai Y and Yoshiki T:
Identification by proteomic analysis of calreticulin as a marker
for bladder cancer and evaluation of the diagnostic accuracy of its
detection in urine. Clin Chem. 50:857–866. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Erić A, Juranić Z, Milovanović Z, Marković
I, Inić M, Stanojević-Bakić N and Vojinović-Golubović V: Effects of
humoral immunity and calreticulin overexpression on postoperative
course in breast cancer. Pathol Oncol Res. 15:89–90. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen CN, Chang CC, Su TE, Hsu WM, Jeng YM,
Ho MC, Hsieh FJ, Lee PH, Kuo ML, Lee H and Chang KJ: Identification
of calreticulin as a prognosis marker and angiogenic regulator in
human gastric cancer. Ann Surg Oncol. 16:524–533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoon GS, Lee H, Jung Y, Yu E, Moon HB,
Song K and Lee I: Nuclear matrix of calreticulin in hepatocellular
carcinoma. Cancer Res. 60:1117–1120. 2000.PubMed/NCBI
|
|
17
|
Brünagel G, Shah U, Schoen RE and
Getzenberg RH: Identification of calreticulin as a nuclear matrix
protein associated with human colon cancer. J Cell Biochem.
89:238–243. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Obeid M, Tesniere A, Ghiringhelli F, Fimia
GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T,
Casares N, et al: Calreticulin exposure dictates the immunogenicity
of cancer cell death. Nat Med. 13:54–61. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chao MP, Jaiswal S, Weissman-Tsukamoto R,
Alizadeh AA, Gentles AJ, Volkmer J, Weiskopf K, Willingham SB,
Raveh T, Park CY, et al: Calreticulin is the dominant
pro-phagocytic signal on multiple human cancers and is
counterbalanced by CD47. Sci Transl Med. 2:63ra942010. View Article : Google Scholar : PubMed/NCBI
|