Introduction
Photodynamic therapy (PDT) has been developed as an
effective treatment for malignant tumors. PDT involves the
administration of a photosensitizer, which preferentially
accumulates in malignant tissue, and its subsequent activation by
light of the appropriate wavelength (1,2). The
interaction of light with the intracellular photosensitizer causes
the release of oxygen radicals, leading to cell death (3).
5-Aminolevulinic acid (5-ALA) is a precursor of
protoporphyrin IX (PpIX), a well-known photosensitizer, and is
converted in situ to PpIX via the heme biosynthetic pathway
(4). 5-ALA has been used successfully
for photodynamic diagnosis and PDT (5,6). Although
5-ALA-PDT has been intensively studied for several decades, the
mechanism underlying its cytotoxicity is not well known. It has
been demonstrated that 5-ALA-PDT leads to apoptosis when PpIX
accumulates in the mitochondria and to necrosis when it diffuses
into the cytoplasm (7). A previous
study reported that 5-ALA-PDT induced autophagic cell death through
the activation of adenosine monophosphate-activated protein kinase
(AMPK) (8).
The biguanide metformin
(N,N-dimethylimidodicarbo-nimidic diamide), a widely used drug for
the treatment of type 2 diabetes, reduces blood glucose levels by
suppressing gluconeogenesis in the liver and increasing glucose
uptake by the skeletal muscle (9,10).
Additionally, metformin has been demonstrated to significantly
inhibit tumor growth in several types of cancer and mouse tumor
models (11–13). It was reported that metformin can
control the proliferation of cancer cells, and this effect was
associated with a number of signaling pathways, including the
activation of AMPK and mitogen-activated protein kinase (MAPK)
signaling, and decreased mammalian target of rapamycin (mTOR) and
epidermal growth factor signaling (14).
Several studies have identified that metformin
enhances the cytotoxicity of chemotherapy by promoting the
AMPK-autophagy pathway (15–18). In addition, metformin has been
demonstrated to cause cell death in radiosensitized cancer cells
and radioresistant cancer stem cells by activating AMPK and
suppressing mTOR (19). These data
provide the rationale to study the role of metformin in other
cancer therapies.
To the best of our knowledge, no prior studies
investigating the effects of 5-ALA-PDT in combination with
metformin have been reported. In the present study, the effect of
metformin in combination with 5-ALA-PDT was evaluated in
vitro in KLN205 lung cancer cells. The results indicate that
metformin potentiates the efficacy of 5-ALA-PDT.
Materials and methods
Cell culture
KLN205 lung cancer cells were obtained from the
Institute of Development, Aging and Cancer of Tohoku University
(Sendai, Japan). KLN205 cells were maintained in RPMI-1640 medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% heat-inactivated fetal bovine serum (FBS;
Wako Pure Chemical Industries, Ltd., Osaka, Japan), penicillin
(0.05 mg/ml), streptomycin (0.05 mg/ml) and neomycin (0.1 mg/ml)
(all Invitrogen; Thermo Fisher Scientific, Inc.) in a humidified
incubator at 37°C with 5% CO2. KLN205 cells were
harvested from near-confluent cultures by a brief exposure to a
solution containing 0.25% trypsin and 1 mmol/l EDTA with phenol red
(Invitrogen; Thermo Fisher Scientific, Inc.). Trypsinization was
stopped using RPMI-1640 containing 10% FBS. The cells were
concentrated by centrifugation at 300 × g for 5 min at room
temperature and resuspended in RPMI-1640. The cells were used for
assays when they were in the logarithmic growth phase.
Chemicals
The 5-ALA was kindly donated by SBI Pharmaceuticals
Co., Ltd. (Tokyo, Japan); a stock solution of 100 mM 5-ALA in PBS
was kept at 4°C until required. Metformin hydrochloride was
purchased from Wako Pure Chemicals Industries, Ltd.; a stock
solution of 100 mM metformin hydrochloride in PBS was kept at 4°C
until use.
PDT
KLN205 cells at a density of 5×104
cells/ml were incubated with various 5-ALA concentrations (0, 0.6,
1.2, 2.5 and 5 mM) for 4 h at 37°C. Following the addition of fresh
medium, the cells were irradiated with a 630 nm laser light (0, 1,
5 and 10 J/cm2) emitted by a diode laser (Ceralas PDT
630 Diode Laser; CeramOptec GmbH, Bonn, Germany), using a Pioneer
Optics lensed fiber with a microlens delivery attachment (Pioneer
Optics Co., Inc., Windsor Lock, CT, USA).
Cell viability assay
KLN205 cells were seeded into 96-well plates at a
density of 1×104 cells/well, incubated overnight at
37°C, and subsequently incubated with metformin at various
concentrations (0, 0.1, 1 and 5 mM) for 24 h. Following the
addition of fresh medium, the cells were incubated at 37°C for 24
h. Following this, the cells were incubated with 5-ALA at various
concentrations (0, 0.6, 1.2, 2.5 and 5 mM) for 4 h at 37°C.
Following the addition of fresh medium, the cells were irradiated
with a 630 nm laser light (0, 1, 5 and 10 J/cm2).
Subsequently, the cells were incubated at 37°C for 24 h in the
dark. The viability of KLN205 cells was examined using the Cell
Counting Kit-8 (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) according to the manufacturer's instructions. The absorbance
(optical density) was measured at 450 nm with a
ChroMate® microplate reader (Awareness Technology, Inc.,
FL, USA). Each group included three replicates.
Fluorescent staining to examine
morphological changes in KLN205 cells
To assess nuclear morphological changes, KLN205
cells a density of 1×104 cells/well were cultured on 8
wells cell culture slides (SPL Life Sciences, Pocheon, Korea).
KLN205 cells were incubated with 1 mM metformin for 24 h at 37°C,
washed with fresh medium, and incubated for a further 24 h.
Following incubation, cells were incubated at 37°C with 5 mM 5-ALA
for 4 h, washed with fresh medium, and then irradiated with a
fluence of 5 J/cm2. Subsequently, the cells were
incubated at 37°C for 12 h in the dark. The cells were stained with
1 mM bisbenzimidazole (Hoechst 33342) for 15 min at room
temperature. Nuclear morphology was examined using an Olympus BX51
fluorescent microscope (Olympus Corporation, Tokyo, Japan).
Autophagy detection
To detect autophagy, KLN205 cells a density of
1×104 cells/well were cultured in 8 wells on cell
culture slides. KLN205 cells were incubated with 1 mM metformin for
24 h at 37°C. Following washing with fresh medium, the cells were
incubated at 37°C for 24 h. They were then further incubated with 5
mM 5-ALA for 4 h at 37°C. Subsequent to washing with fresh medium,
the cells were irradiated with a fluence of 5 J/cm2.
Subsequently, the cells were re-incubated at 37°C for 12 h in the
dark. Autophagy was detected with an Autophagy/Cytotoxicity Dual
Staining kit (Cayman Chemical Company, Ann Arbor, MI, USA)
according to the manufacturer's instructions. The kit employs
monodansylcadaverine (MDC), an autofluorescent substance
incorporated into multilamellar bodies via an ion trapping
mechanism and interactions with membrane lipids, as a probe for the
detection of autophagic vacuoles in cultured cells. Propidium
iodide (PI) was used as a marker of cell death. Images were
captured using an Olympus BX51 fluorescent microscope.
Statistical analysis
Data were analyzed using the Friedman test.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analyses were performed using Graphpad
Prism software (version 6.0; Graphpad Software, Inc., La Jolla, CA,
USA).
Results
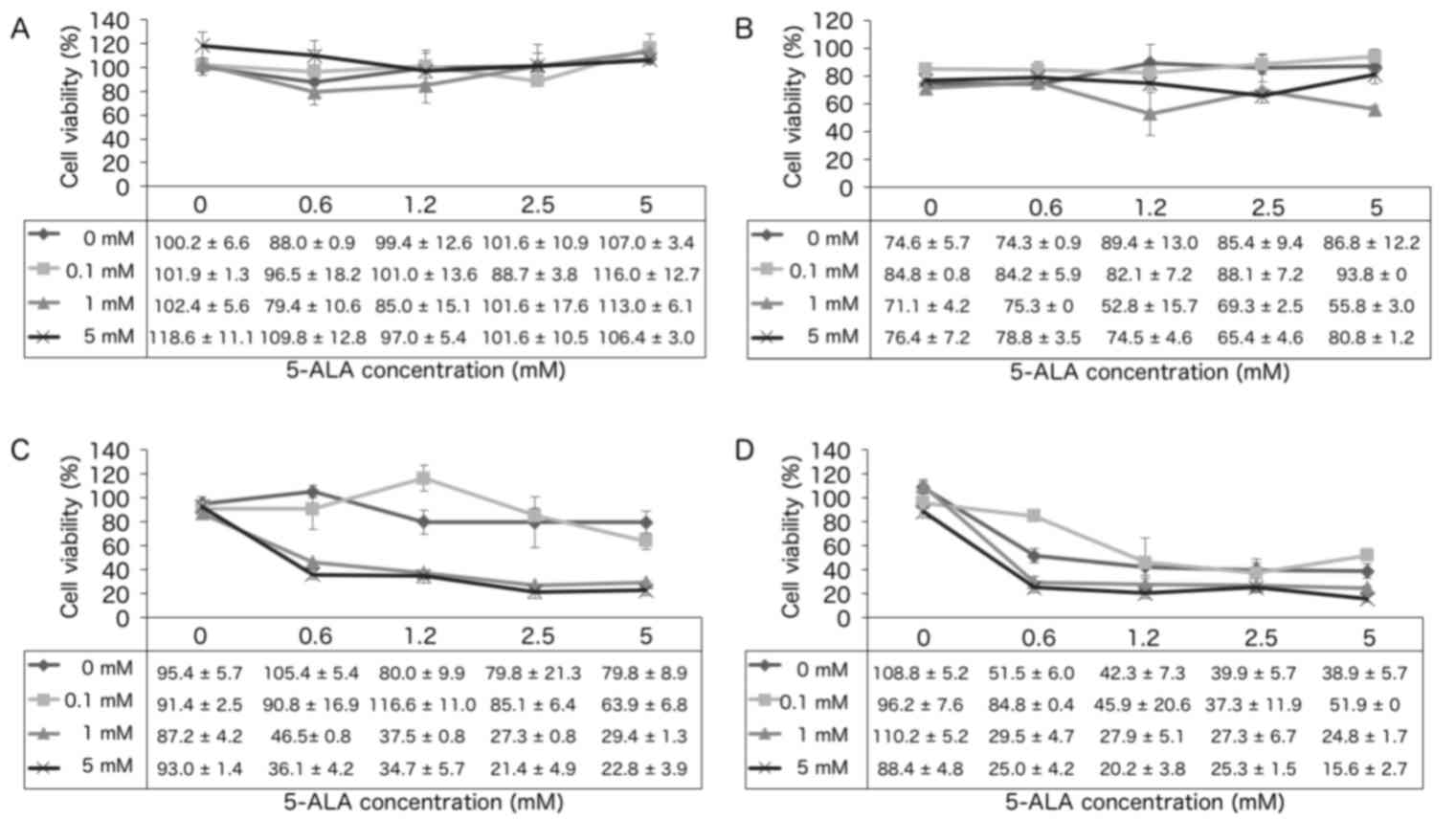
Cell survival assay
The survival of KLN205 cells following treatment
with 5-ALA-PDT in combination with 0, 0.1, 1 or 5 mM metformin
treatment was analyzed. In the absence of irradiation (0
J/cm2; Fig. 1A),
increasing concentrations of 5-ALA and metformin did not
significantly affect cell survival. However, at a fluence of 1, 5
and 10 J/cm2 (Fig. 1B-D),
the cytotoxic effect of 5-ALA-PDT was significantly increased in
the presence of metformin. For example, at a fluence of 5
J/cm2, 5-ALA-PDT treatment with 5 mM metformin resulted
in a significant increase in cytotoxicity compared with that
observed with 0 and 0.1 mM metformin (P=0.0198 and P=0.0424,
respectively; Fig. 1C). In the 10
J/cm2 group, 5-ALA-PDT with 5 mM metformin exhibited
increased cytotoxicity compared to that observed with 0.1 mM
metformin (P=0.0424; Fig. 1D).
Morphological changes in KLN205
cells
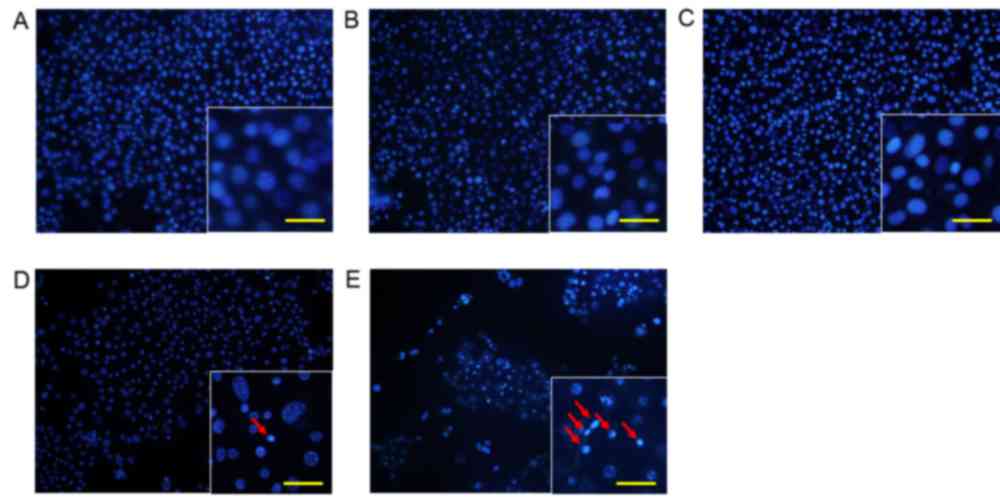
To analyze changes in cell morphology, KLN205 cells
were stained with Hoechst 33342 12 h following 5-ALA-PDT treatment
(5 mM 5-ALA, 5 J/cm2 fluence; Fig. 2). Cells treated with 5-ALA or 1 mM
metformin alone did not exhibit any changes in nuclear chromatin or
cell density (Fig. 2B and C). Cells
treated with 5-ALA-PDT alone exhibited minimal condensation of
nuclear chromatin and a slight decrease in cell density (Fig. 2D). However, cells treated with
5-ALA-PDT and metformin exhibited clear condensation of nuclear
chromatin and a marked decrease in cell density compared with cells
treated with 5-ALA-PDT alone (Fig.
2E).
Autophagy detection
To determine the effect of 5-ALA-PDT on autophagy
with and without metformin, KLN205 cells were co-stained with MDC
and PI 4 h following PDT (Fig. 3). A
basal level of autophagy was detected in untreated control cells,
and in cells treated with 1 mM metformin alone, as indicated by the
faint silver dot staining of autophagic vacuoles (Fig. 3A and B), although few dead cells were
observed. Cells treated with 5-ALA-PDT (5 mM 5-ALA, 5
J/cm2 fluence) with or without 1 mM metformin treatment
exhibited an increased intensity of silver dot staining and an
increased number of autophagic vacuoles compared with the control
group (Fig. 3C and D). PI-positive
intact nuclei (necrotic cells) were observed in cells treated with
5-ALA-PDT without metformin (Fig.
3C), whereas PI-positive condensed nuclei were observed in
cells treated with 5-ALA-PDT and metformin (Fig. 3D), indicating that these cells were in
a late stage of apoptosis.
Discussion
Previous reports have demonstrated that metformin
enhances the effect of chemotherapy and radiotherapy by promoting
the AMPK-autophagy signaling pathway (15–19).
Therefore, it is expected that metformin would potentiate the
cytotoxicity of 5-ALA-PDT. The present study demonstrated that
KN205 cells that were pre-treated with ≥1 mM metformin exhibited
significantly greater cytotoxicity in response to 5-ALA-PDT. This
was most apparent at a PDT fluence of 5 J/cm2.
A previous study demonstrated that a combination of
10 mM metformin and 10 nM paclitaxel was more effective at
inhibiting cell growth compared with paclitaxel alone, due to an
increase in AMPK activation and the subsequent reduction of
signaling through the mTOR pathway (16). It has also been demonstrated that 1
and 5 mM metformin causes a significant increase in
radiosensitization at a radiation concentration >3 Gy (19). The combination of metformin and
irradiation was more efficient than radiation or metformin alone at
inactivating AMPK, and inactivating mTOR and its targets ribosomal
protein S6 kinase β-1 and eukaryotic translation initiation factor
4E-binding protein 1 (19). The
combination of 5-ALA-PDT and metformin used in the present study
may also activate AMPK and inactivate mTOR to initiate cell
death.
PDT induces cell death via apoptosis, autophagy and
necrosis. In KLN205 cells treated with 5-ALA-PDT and metformin,
marked condensation of nuclear chromatin and a decrease in cell
density were observed at 12 h following PDT, compared with cells
treated with 5-ALA-PDT alone. The morphological changes observed
indicate that a higher amount of apoptotic events occurred in cells
treated with 5-ALA-PDT and metformin compared with cells treated
with 5-ALA-PDT or metformin alone, suggesting that 5-ALA-PDT in
combination with metformin is more effective than either
monotherapy.
Increased numbers of autophagosomes were observed in
cells treated with 5-ALA-PDT with and without metformin compared
with untreated control cells (Fig.
3). However, treatment with 5-ALA-PDT and metformin did not
increase the number of autophagosomes compared with 5-ALA-PDT
treatment alone. A previous study demonstrated that increased
numbers of MDC-positive autophagosomes were found as early as 2 h
following 5-ALA-PDT administration, whereas little MDC
incorporation was observed in control cells (8). Additionally, 5-ALA-PDT-induced
autophagic cell death was mediated by AMPK. The results of the
present study suggest that the combination of 5-ALA-PDT and
metformin predominantly induces apoptosis, but also autophagy, via
AMPK activation.
In conclusion, the present study is the first to
demonstrate, to the best of our knowledge, that metformin
effectively potentiates the efficacy of 5-ALA-PDT. Further studies
are required to demonstrate the molecular mechanism underlying the
cell death induced by the combination of 5-ALA-PDT and
metformin.
Acknowledgements
The authors would like to thank Editage (www.editage.jp) for their English language
editing.
Glossary
Abbreviations
Abbreviations:
|
AMPK
|
adenosine monophosphate-activated
protein kinase
|
|
5-ALA
|
5-aminolevulinic acid
|
|
mTOR
|
mammalian target of rapamycin
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MDC
|
monodansylcadaverine
|
|
PDT
|
photodynamic therapy
|
|
PI
|
propidium iodide
|
|
PpIX
|
protoporphyrin IX
|
References
|
1
|
Sharman WM, Allen CM and van Lier JE:
Photodynamic therapeutics: Basic principles and clinical
applications. Drug Discov Today. 4:507–517. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Agostinis P, Berg K, Cengel KA, Foster TH,
Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel
D, et al: Photodynamic therapy of cancer: An update. CA Cancer J
Clin. 61:250–281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moore JV, West CM and Whitehurst C: The
biology of photodynamic therapy. Phys Med Biol. 42:913–935. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wachowska M, Muchowicz A, Firczuk M,
Gabrysiak M, Winiarska M, Wańczyk M, Bojarczuk K and Golab J:
Aminolevulinic acid (ALA) as a prodrug in photodynamic therapy of
cancer. Molecules. 16:4140–4164. 2011. View Article : Google Scholar
|
|
5
|
Ishizuka M, Abe F, Sano Y, Takahashi K,
Inoue K, Nakajima M, Kohda T, Komatsu N, Ogura S and Tanaka T:
Novel development of 5-aminolevurinic acid (ALA) in cancer
diagnoses and therapy. Int Immunopharmacol. 11:358–365. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nokes B, Apel M, Jones C, Brown G and Lang
JE: Aminolevulinic acid (ALA): Photodynamic detection and potential
therapeutic applications. J Surg Res. 181:262–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Amo T, Kawanishi N, Uchida M, Fujita H,
Oyanagi E, Utsumi T, Ogino T, Inoue K, Shuin T, Utsumi K and Sasaki
J: Mechanism of cell death by 5-aminolevulinic acid-based
photodynamic action and its enhancement by ferrochelatase
inhibitors in human histiocytic lymphoma cell line U937. Cell
Biochem Funct. 27:503–515. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji HT, Chien LT, Lin YH, Chien HF and Chen
CT: 5-ALA mediated photodynamic therapy induces autophagic cell
death via AMP-activated protein kinase. Mol Cancer Ther. 9:912010.
View Article : Google Scholar
|
|
9
|
Ben Sahra I, Le Marchand-Brustel Y, Tanti
JF and Bost F: Metformin in cancer therapy: A new perspective for
an old antidiabetic drug? Mol Cancer Ther. 9:1092–1099. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Del Barco S, Vazquez-Martin A, Cufí S,
Oliveras-Ferraros C, Bosch-Barrera J, Joven J, Martin-Castillo B
and Menendez JA: Metformin: Multi-faceted protection against
cancer. Oncotarget. 2:896–917. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dowling RJ, Zakikhani M, Fantus IG, Pollak
M and Sonenberg N: Metformin inhibits mammalian target of
rapamycin-dependent translation initiation in breast cancer cells.
Cancer Res. 67:10804–10812. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ben Sahra I, Laurent K, Loubat A,
Giorgetti-Peraldi S, Colosetti P, Auberger P, Tanti JF, Le
Marchand-Brustel Y and Bost F: The antidiabetic drug metformin
exerts an antitumoral effect in vitro and in vivo through a
decrease of cyclin D1 level. Oncogene. 27:3576–3586. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cantrell LA, Zhou C, Mendivil A, Malloy
KM, Gehrig PA and Bae-Jump VL: Metformin is a potent inhibitor of
endometrial cancer cell proliferation-implications for a novel
treatment strategy. Gynecol Oncol. 116:92–98. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dowling RJ, Goodwin PJ and Stambolic V:
Understanding the benefit of metformin use in cancer treatment. BMC
Med. 9:332011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rattan R, Graham RP, Maguire JL, Giri S
and Shridhar V: Metformin suppresses ovarian cancer growth and
metastasis with enhancement of cisplatin cytotoxicity in vivo.
Neoplasia. 13:483–491. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rocha GZ, Dias MM, Ropelle ER,
Osório-Costa F, Rossato FA, Vercesi AE, Saad MJ and Carvalheira JB:
Metformin amplifies chemotherapy-induced AMPK activation and
antitumoral growth. Clin Cancer Res. 17:3993–4005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin CC, Yeh HH, Huang WL, Yan JJ, Lai WW,
Su WP, Chen HH and Su WC: Metformin enhances cisplatin cytotoxicity
by suppressing signal transducer and activator of transcription-3
activity independently of the liver kinase B1-AMP-activated protein
kinase pathway. Am J Respir Cell Mol Biol. 49:241–250. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin YC, Wu MH, Wei TT, Lin YC, Huang WC,
Huang LY, Lin YT and Chen CC: Metformin sensitizes anticancer
effect of dasatinib in head and neck squamous cell carcinoma cells
through AMPK-dependent ER stress. Oncotarget. 5:298–308.
2014.PubMed/NCBI
|
|
19
|
Song CW, Lee H, Dings RP, Williams B,
Powers J, Santos TD, Choi BH and Park HJ: Metformin kills and
radiosensitizes cancer cells and preferentially kills cancer stem
cells. Sci Rep. 2:3622012. View Article : Google Scholar : PubMed/NCBI
|