Introduction
Malignant melanoma is the most serious type of skin
cancer and develops in melanocytes that produce melanin pigment
(1). The most common site of
malignant melanoma is the upper back, although it also occurs in
the arms and legs (2). Previous
studies have demonstrated that age, skin type and a family history
of melanoma significantly affect the development of malignant
melanoma. Surgery is the main treatment for early-stage malignant
melanoma (3). However, there is no
available treatment when melanoma passes early stage, or upon
recurrence, making it an incurable disease with a high rate of
metastasis (4,5). Moringa oleifera Lam is a tree of
the Moringaceae family that can reach a height of between 5 and 10
m (6). Moringa is cultivated
in Asia, Africa and Arabia, and is a good source of nutrition,
since the plant is rich in proteins and vitamins (7). It has various pharmacological effects,
including anti-hyperglycemic, anti-inflammatory and anticancer
functions (8). Moringa leaves
are the most nutritious part, as they are rich in β-carotene,
proteins, vitamin C, calcium and antioxidants (9). There are two types of cell death
process: Apoptosis and necrosis. Apoptosis is an important
physiological mechanism in which apoptotic cells cause immune
responses for removal of dead cells without destruction of
surrounding cells, leading to characteristic cell changes,
including cell shrinkage and membrane blebbing (10). This active process is mediated under
the control of gene regulation (11).
Reactive oxygen species (ROS) induce cancer and aging, formation of
lipid peroxides, destruction of proteins and nucleic acids, and
inhibition of various enzyme functions by attacking living cells.
ROS are also mediators of intracellular signaling (12). However, excessive ROS production
increases oxidative stress, resulting in cellular damage and
inhibition of cellular functions and the cell cycle to cause
apoptosis (13,14). Chemotherapeutic agents, including
anticancer agents, exert relatively marked toxic effects, although
certain cancer cells exhibit resistance. Once cancer cells acquire
resistance to a particular anticancer agent, they have resistance
to all anticancer agents operating via the same mechanism (15). Therefore, anticancer agents derived
from natural compounds have been developed with decreased side
effects and increased anticancer activity (16,17). It is
important for patients to strengthen their immune systems upon
occurrence of malignant melanoma. As synthetic anticancer agents
may weaken the immune system, more studies on natural products for
treatment of malignant melanoma are required. For the
identification of cytoprotective agents from natural resources, the
present study investigated the cytoprotective mechanisms underlying
Moringa oleifera fruit, against mitochondrial apoptosis with
respect to the induction of ROS formation, and c-Jun N-terminal
kinase (JNK) and extracellular-signal-regulated kinase (ERK)
activation in human melanoma A2058 cells.
Materials and methods
Plants and sample extraction
Moringa oleifera fruit (MOF) were collected
at Dar es Saalam, Tanzania, in September 2013. Botanical
identification was made by Professor Henry Joseph Hjndangalasi,
Department of Botany, Dar es Salaam University, Dar es Salaam,
Tanzania. Dried MOF (13.0 g) were soaked in 70% ethanol and
sonicated (40 kHz) for 3 h at room temperature. Extracts were
evaporated in a dry oven at 60°C and stored at −20°C until used for
the in vitro assay (yield, 0.2794 g).
Chemicals and reagents
MTT and propidium iodide were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Primary mouse
monoclonal antibodies against β-actin (catalog no. sc-47778;
dilution, 1:1,000) and rabbit monoclonal antibodies against B-cell
lymphoma-2 (Bcl-2; catalog no. sc-492; dilution, 1:1,000) and
Bcl-2-associated X protein (Bax; catalog no. sc-493; dilution,
1:1,000) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Rabbit monoclonal antibodies against cleaved
caspases-3 (dilution, 1:1,000; catalog no. 9661), −8 (dilution,
1:1,000; catalog no. 8592) and −9 (dilution, 1:1,000; catalog no.
7237) and poly (ADP-ribose) polymerase (PARP; dilution, 1:1,000;
catalog no. 5625) were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA). Horseradish peroxidase (HRP)-conjugated
goat anti-rabbit IgG (HRP; catalog no. sc-2004; dilution, 1:2,000)
and HRP-conjugated goat anti-mouse IgG (catalog no. sc-2005;
dilution, 1:2,000) secondary antibodies were purchased from Santa
Cruz Biotechnology, Inc. Primary antibodies against caspase-9
(catalog no. 9501; dilution, 1:1,000), caspase-3 (catalog no. 9664;
dilution, 1:1,000), JNK (catalog no. 9258; dilution, 1:1,000),
phosphorylated (p)-JNK (catalog no. 4668; dilution, 1:1,000), ERK
(catalog no. 4695; dilution, 1:1,000), p-ERK (catalog no. 4370;
dilution, 1:1,000), p38 (catalog no. 9212; dilution, 1:1,000) and
p-p38 (catalog no. 4511; dilution, 1:1,000) antibodies were
purchased from Cell Signaling Technology, Inc. All other chemicals
and reagents were of the highest analytical grade.
Cell culture
The human melanoma A2058 and human keratinocyte
HaCaT cell lines were purchased from the Korean Cell Line Bank
(Seoul, Korea) and Amore Pacific (Yongin, Gyeonggi-do, Republic of
Korea). respectively. Cells were maintained at 37°C in Dulbecco's
modified Eagle's medium (DMEM; Gibco, Thermo Fisher Scientific,
Inc., Waltham, MA, USA), supplemented with 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
and 100 µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.).
Cells were incubated at 37°C in an incubator with a humidified
atmosphere containing 5% CO2 and were subcultured every
2 to 3 days. Cell counts were performed using a hemocytometer from
Hausser Scientific (Horsham, PA, USA).
Cell viability assay
The cytotoxic effects of MOF extract on the A2058
and HaCaT cell lines were estimated colorimetrically using the MTT
method, which is based on the reduction of tetrazolium salt by
mitochondrial dehydrogenase in viable cells (18). The cells were seeded on a 96-well
plate (density, 2×106 cells/ml) and were then treated
with MOF extract at final concentrations of 0, 50, 75 and 100
µg/ml. After 72 h of incubation at 37°C, 50 µl MTT solution (2
mg/ml) was added to each well at a final concentration of 0.4
mg/ml. After 2 h of incubation at 37°C, the supernatants were
aspirated and replaced with 150 µl dimethyl sulfoxide to dissolve
the formazan product. The absorbance at 540 nm was then read using
a spectrophotometric plate reader (model 550; Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The results were calculated as
percentages relative to the unexposed control.
Nuclear staining with Hoechst
33258
The nuclear morphology of the cells was observed
using the DNA-specific blue fluorescent dye Hoechst 33258. The
viable cells were stained homogeneously, whereas the apoptotic
cells that exhibited chromatin condensation or nuclear
fragmentation were not stained (19).
The A2058 cells were treated with MOF extract at the various
concentrations (0, 50, 75, 100 µg/ml). Cells were then fixed for 30
min at 37°C in 100% methanol, washed with PBS and stained with 2
µg/ml Hoechst 33258 (Sigma-Aldrich; Merck KGaA). The cells were
observed under a BX51 fluorescence microscope (magnification, ×200)
and images were captured with a DP70 camera (Olympus Corporation,
Tokyo, Japan).
Determination of ROS levels
Intracellular ROS generation was assessed using the
stable nonpolar dye 2′,7′-dichlorodihydrofluorescein diacetate
(H2DCF-DA; Sigma-Aldrich; Merck KGaA), which readily
diffuses into the cells (20).
Following treatment with MOF extract (100 µg/ml) for 24 h at 37°C,
the A2058 cells were incubated at 37°C with 25 µM
H2DCF-DA for 30 min, and then washed twice with ice-cold
PBS. The ROS production was measured using a FACSCalibur flow
cytometer (BD Biosciences, San Jose, CA, USA). To cells grown in
6-well plates (1×106 cells/ml) for 24 h, an antioxidant
N-acetyl-L-cystein (NAC; 2 mM) was added for 1 h prior to exposing
them to 100 µg/ml MOF extract for 72 h at 37°C. A total of 25 µM of
H2DCF-DA was then added and the cells incubated for 30
min at 37°C, and then washed twice with ice-cold PBS. The ROS
production was evaluated using a FACSCalibur flow cytometer (BD
Biosciences).
Cell cycle analysis
Cell cycle analysis was performed to determine the
proportion of apoptotic sub-G1 hypodiploid cells (21). The A2058 cells were plated on 6-well
plates (1×106 cells/ml) and incubated for 24 h at 37°C.
The cells were treated with MOF extract (0, 50, 75, 100 µg/ml) and
incubated for 72 h at 37°C, following which they were trypsinized,
harvested and washed with PBS. The pellet was fixed using ice-cold
70% ethanol at 4°C for 30 min. The cells were then washed once with
PBS and resuspended in 50 µg/ml ice-cold propidium iodide (PI)
containing 50 µg/ml RNase A in PBS (pH 7.4) for 30 min in the dark.
Fluorescence emitted from the PI-DNA complex was quantified using a
FACSCalibur flow cytometer.
Apoptosis analysis
An Annexin V-PI double staining assay was performed
to differentiate early and late apoptosis stages. The assay was
determined using an ApoScan™ Annexin V-fluorescein isothiocyanate
(FITC) apoptosis detection kit (BioBud Co. Ltd., Seoul, Korea) in
the MOF extract-treated A2058 cells. Cells were trypsinized,
harvested and washed with PBS, and were subsequently were
resuspended in 500 µl 1X binding buffer (50 mM HEPES, 700 mM NaCl,
12.5 mM CaCl2, PH 7.4) and incubated with 1.25 µl
Annexin V-FITC (200 µg/ml) at room temperature for 15 min. The
supernatant was then removed following centrifugation at 400 × g
for 10 min at 4°C. The cells were then resuspended in 500 µl 1X
binding buffer, and cell suspensions were stained with 10 µl PI (30
µg/ml) at 4°C in the dark. Fluorescence was quantified using a
FACSCalibur flow cytometer. The number of cells in early and late
apoptosis was determined as the percentage of Annexin
V+/PI− or Annexin
V+/PI+ cells, respectively.
Western blot analysis
Western blot analyses were performed as previously
described (22). The cells were
cultured, harvested and lysed on ice for 30 min in lysis buffer
(120 mM NaCl, 40 mM, pH 8.0, and 0.1% nonyl
phenoxypolyethoxylethanol) and were then centrifuged at 13,000 × g
for 15 min at 4°C. Lysates from each sample were mixed with 5X
sample buffer (0.375 M Tris-HCl, 5% SDS, 5% 2-mercaptoethanol, 50%
glycerol and 0.05% bromophenol blue, pH 6.8) and were then heated
to 95°C for 5 min. Equal amounts of protein (25 µg) were separated
by SDS-PAGE (12% gel) and were transferred onto nitrocellulose
membranes. The membranes were then washed with TBS (10 mM Tris-HCl
and 150 mM NaCl) containing 0.05% Tween-20 (TBST), and were then
blocked in TBST containing 5% nonfat dried milk. The membranes were
then incubated with the aforementioned specific primary antibodies
overnight at 4°C. Subsequent to 3 washes in TBST, the membranes
were incubated with the appropriate HRP-conjugated secondary
antibodies for 1 h at room temperature. The membranes were then
washed 3 times in TBST with 15 min between each step, and protein
detection was performed using an enhanced chemiluminescence western
blotting detection ECL kit (Bio-Rad, Hercules, CA, USA). The
intensity of each band was analyzed using Image J software (version
k 1.45; National Institute of Health, Bethesda, MD, USA). The
expression level of individual proteins in the MOF-treated cells
was evaluated and expressed relative to that of the control, of
which the expression level was designated as 1.0.
Statistical analysis
SPSS software (version 22.0; IBM SPSS, Armonk, NY,
USA) was used to analyze the data. The results were subjected to
analysis of variance, followed by Tukey's range test in order to
analyze the differences between conditions. In each case, P<0.05
was considered to indicate a statistically significant difference.
All measurements were made in triplicate, and all values are
presented as the mean ± standard deviation.
Results
Cytotoxic effects of MOF extract in
A2058 and HaCaT cell lines
To examine the proliferation inhibitory effects of
MOF extract, human melanoma A2058 cells were exposed to MOF extract
at concentrations of 50, 75 and 100 µg/ml for 72 h, prior to the
cell viability being determined using an MTT assay. As presented in
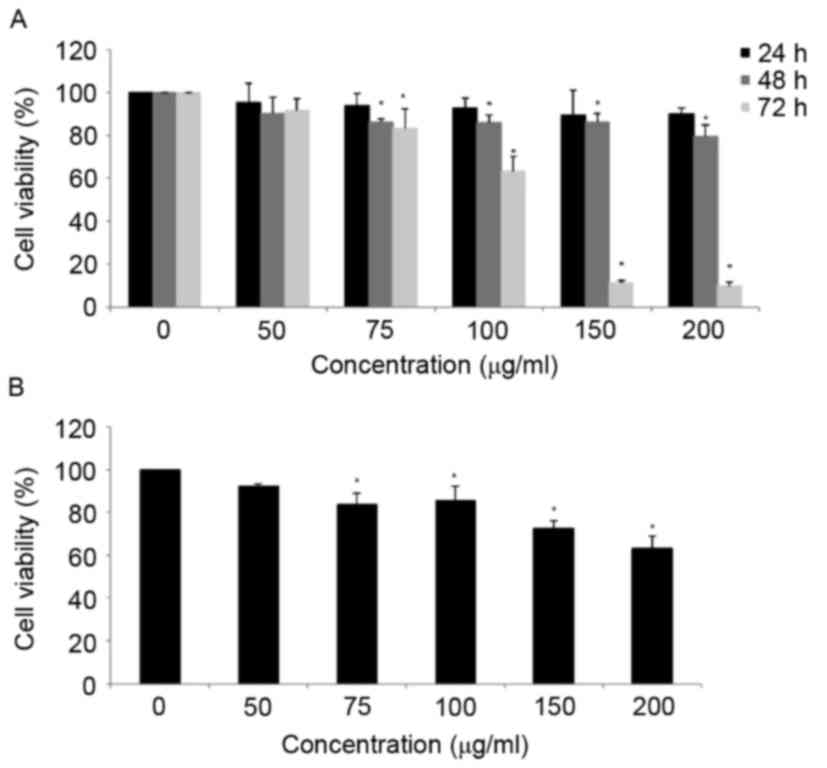
Fig. 1A, MOF extract decreased the
viability of A2058 cells in a dose-dependent manner. The viability
of MOF extract-treated A2058 cells was decreased to 91.6, 83.1 and
63.5% at concentrations of 50, 75 and 100 µg/ml for 72 h,
respectively. At concentrations of 150 and 200 µg/ml, the MOF
extract markedly decreased the cell proliferation to 11.3 and
10.1%, respectively. To compare the cytotoxic effects of MOF
extract in human keratinocyte HaCaT cells, the HaCaT cells were
treated with concentrations of MOF extract of 50, 75, 100, 150 and
200 µg/ml for 72 h. The results indicated that HaCaT cells were
more resistant to MOF extract-induced cytotoxicity compared with
human melanoma A2058 cells (Fig. 1B).
Therefore, MOF extract concentrations of 50, 75 and 100 µg/ml were
selected for subsequent experiments.
Induction of apoptosis in A2058
cells
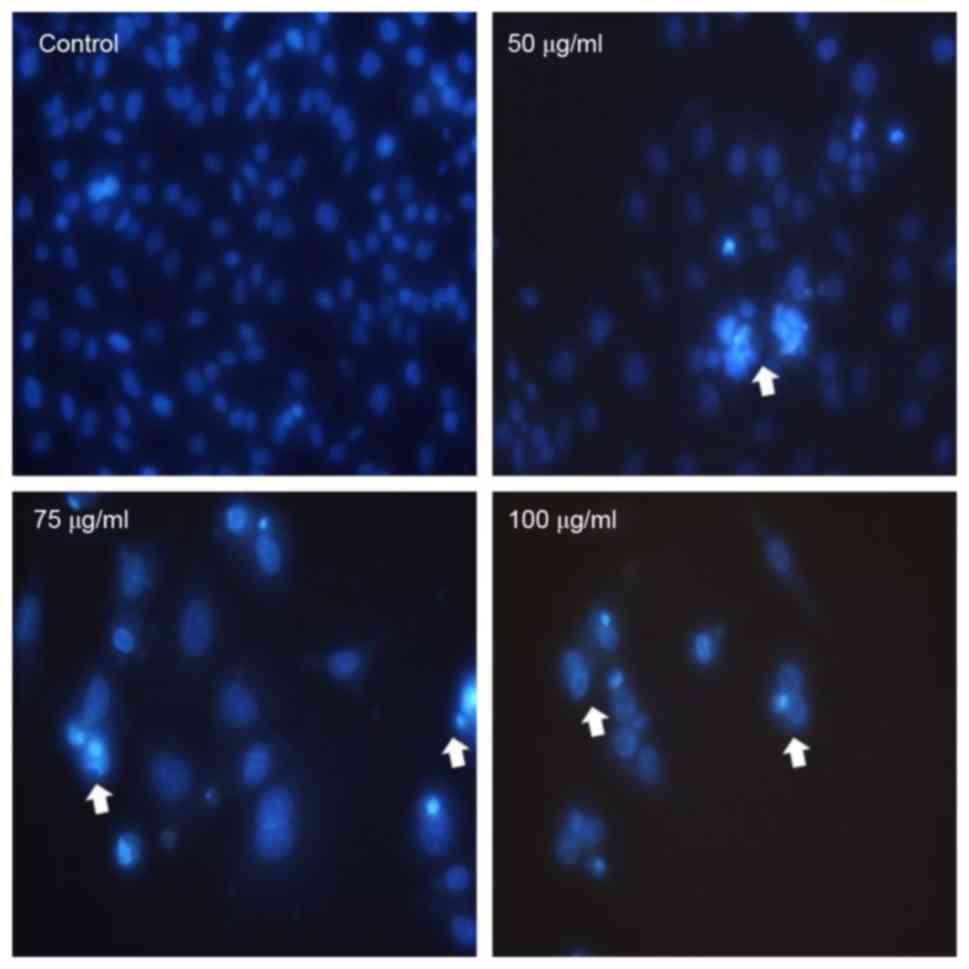
The apoptogenic property of MOF extract was
investigated through morphological changes in A2058 cells. Nuclear
Hoechst 33258 staining was performed in order to determine whether
the anti-proliferative effect of MOF extract was due to apoptosis.
As presented in Fig. 2, A2058 cells
treated with MOF extract exhibited a number of morphological
changes, including membrane blebbing, nuclear fragmentation,
chromatin condensation and an increased density of apoptotic bodies
compared with the untreated control cells. The number of apoptotic
cells increased in a dose-dependent manner, which is consistent
with the results of the MTT assay.
Effects on generation of ROS
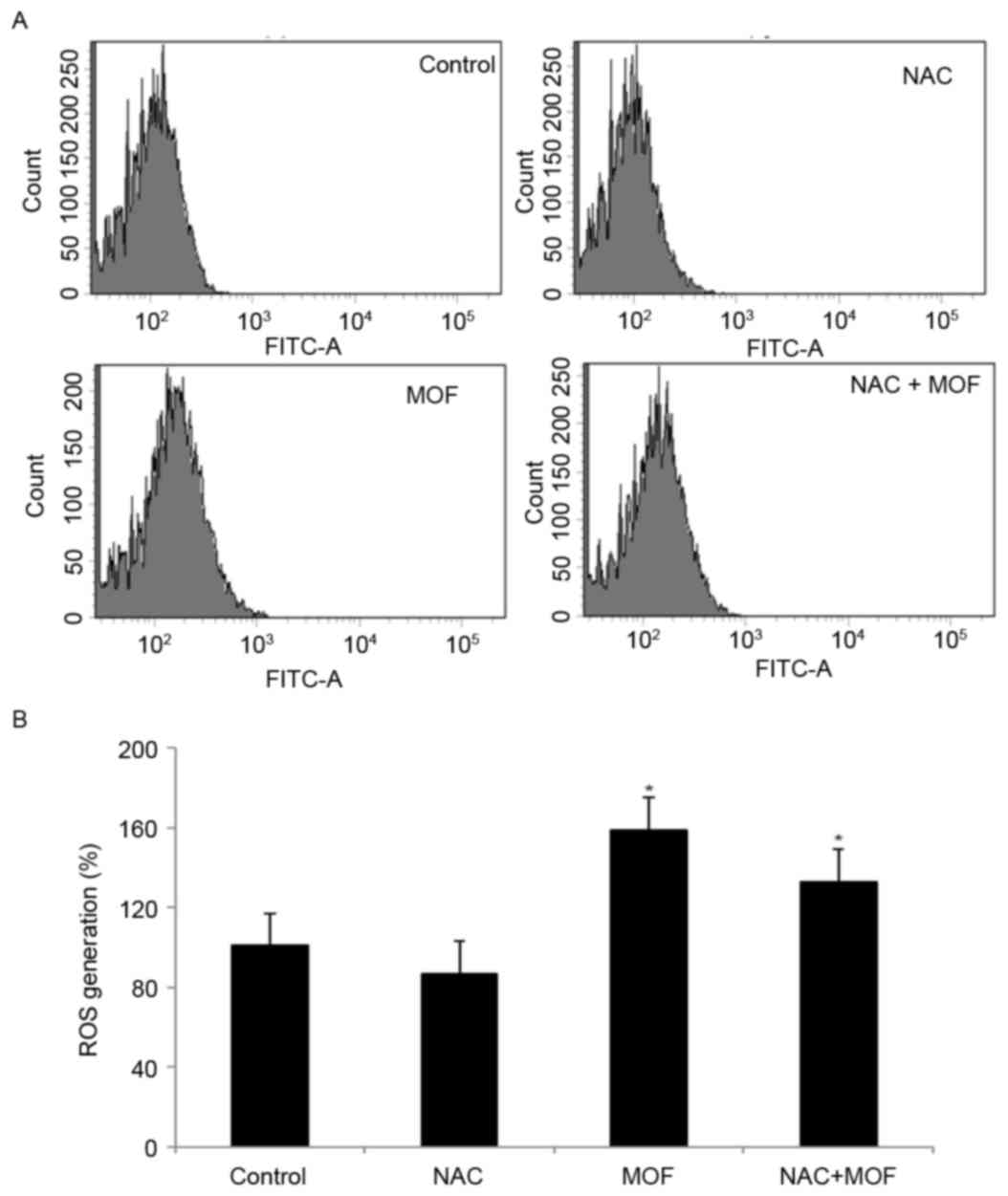
To investigate the possible underlying mechanisms of
MOF extract-induced apoptosis, ROS production was examined using
the specific fluorescent probe H2DCF-DA. Using flow
cytometry, MOF extract caused a rightward shift for
H2DCF-DA, indicating increased production of hydroxyl
radicals and hydrogen peroxide, compared with the control, followed
by cell death (Fig. 3A). However,
co-treatment with MOF extract and NAC slightly attenuated ROS
production, compared with MOF extract alone, indicating that NAC
may act as a scavenger of ROS (Fig.
3B). The results demonstrated that ROS is involved in MOF
extract-mediated apoptotic processes.
Effects on cell cycle progression in
A2058 cells
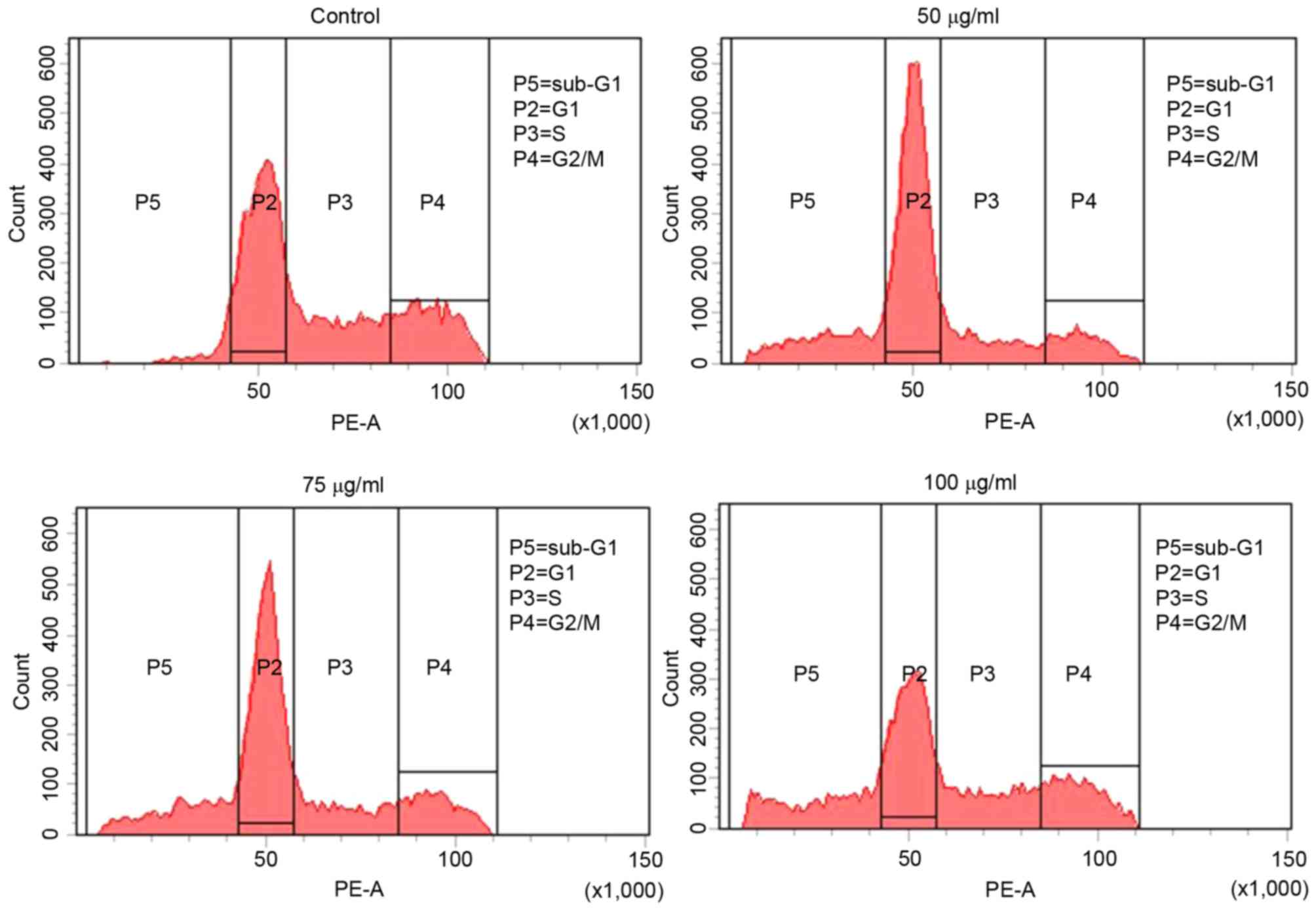
To investigate the effect of MOF extract on cell
cycle progression, flow cytometry was performed with 50, 75 and 100
µg/ml MOF extract. As presented in Fig.
4, 13.9% of control cells were in sub-G1 phase,
whereas 29.9, 31.1 and 57.5% of MOF extract-treated cells were in
sub-G1 phase, at concentrations of 50, 75 and 100 µg/ml,
respectively. This was accompanied by a significant decrease in the
numbers of A2058 cells in S phase of 28.5, 20.5 and 16.3%, and G2/M
phase of 23.1, 18.7 and 6.9 at 50, 75 and 100 µg/ml,
respectively.
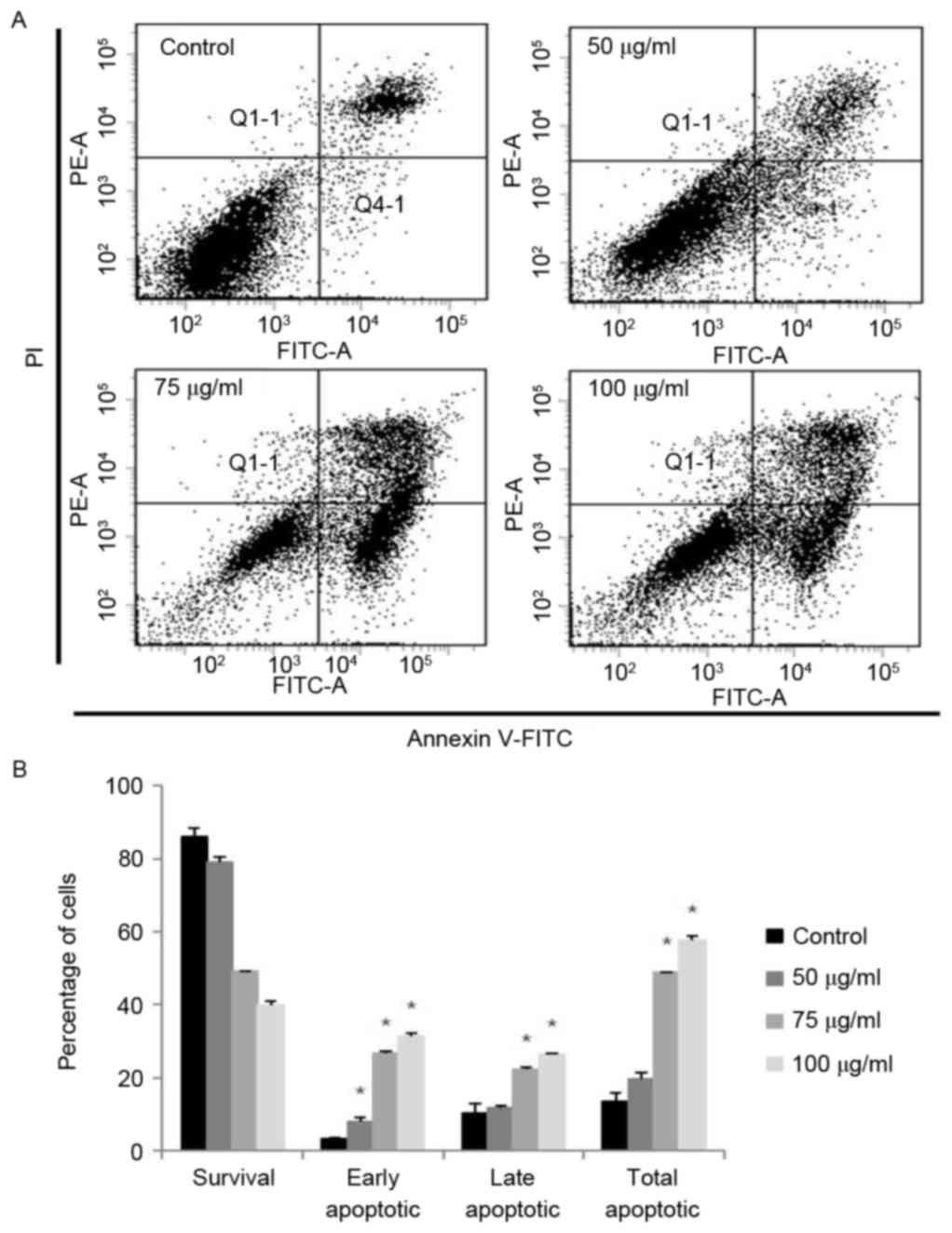
Effects on apoptosis in A2058
cells
In order to quantify the percentage of apoptotic
cells, flow cytometry analysis was performed using double staining
with Annexin V and PI. The Annexin V−/PI−
population was considered to account for unaffected cells, the
Annexin V+/PI− population represented early
apoptosis, the Annexin V+/PI+ population
represented late apoptosis and the Annexin
V−/PI+ population represented necrosis. The
results demonstrated that the treatment of the cells with MOF
extract significantly increased the percentage of apoptotic cells
compared with untreated control cells (Fig. 5). MOF extract-treated A2058 cells
exhibited increased proportions of early apoptotic cells to 6.5,
27.3 and 31.9% at 50, 75 and 100 µg/ml, respectively, compared with
3.2% for the control. The proportions of late apoptotic cells also
increased to 11.2, 21.6 and 26.6% at 50, 75 and 100 µg/ml,
respectively, compared with 9.0% for the control. The total number
of apoptotic cells increased from 12.2% in control cells to 17.7,
48.9 and 58.5% at 50, 75 and 100 µg/ml, respectively. The
proportion of early apoptotic cells was increased at 50 µg/ml
compared with that of late apoptotic cells. However, the population
of late apoptotic cells was increased compared with that of early
apoptotic cells. From these results, it is hypothesized that
anti-proliferative effects of MOF extract in A2058 melanoma cells
may be mediated by the induction of cell apoptosis in a
dose-dependent manner.
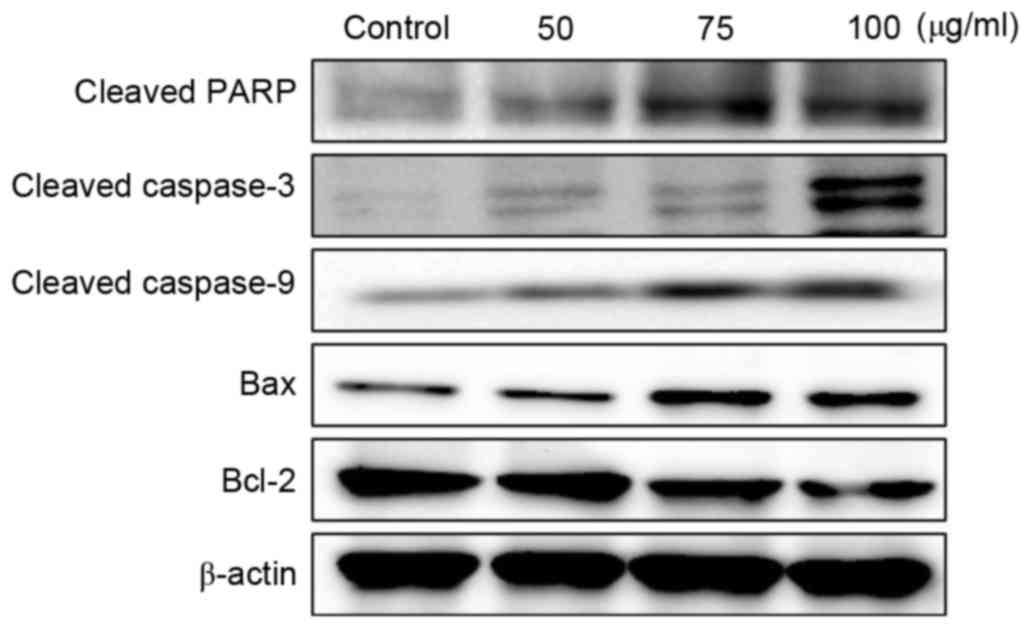
Effects on Bcl-2, Bax and caspase
expression in A2058 cells
In order to investigate the effects of MOF extract
on apoptosis in A2058 cells, the expression levels of apoptotic
regulatory proteins, including Bcl-2, Bax and caspases, were
examined. As presented in Fig. 6, MOF
extract increased Bax protein expression, but decreased the
expression of Bcl-2, in a dose-dependent manner. The disruption of
the mitochondrial plasma membrane by MOF extract was followed by
the activation of the cleaved caspase-3 and 9 and target protein,
PARP, respectively. These results, together with the Bax/Bcl-2
ratio, indicated that the MOF extract may induce apoptosis through
the regulation of apoptosis-associated protein expression in human
melanoma A2058 cells.
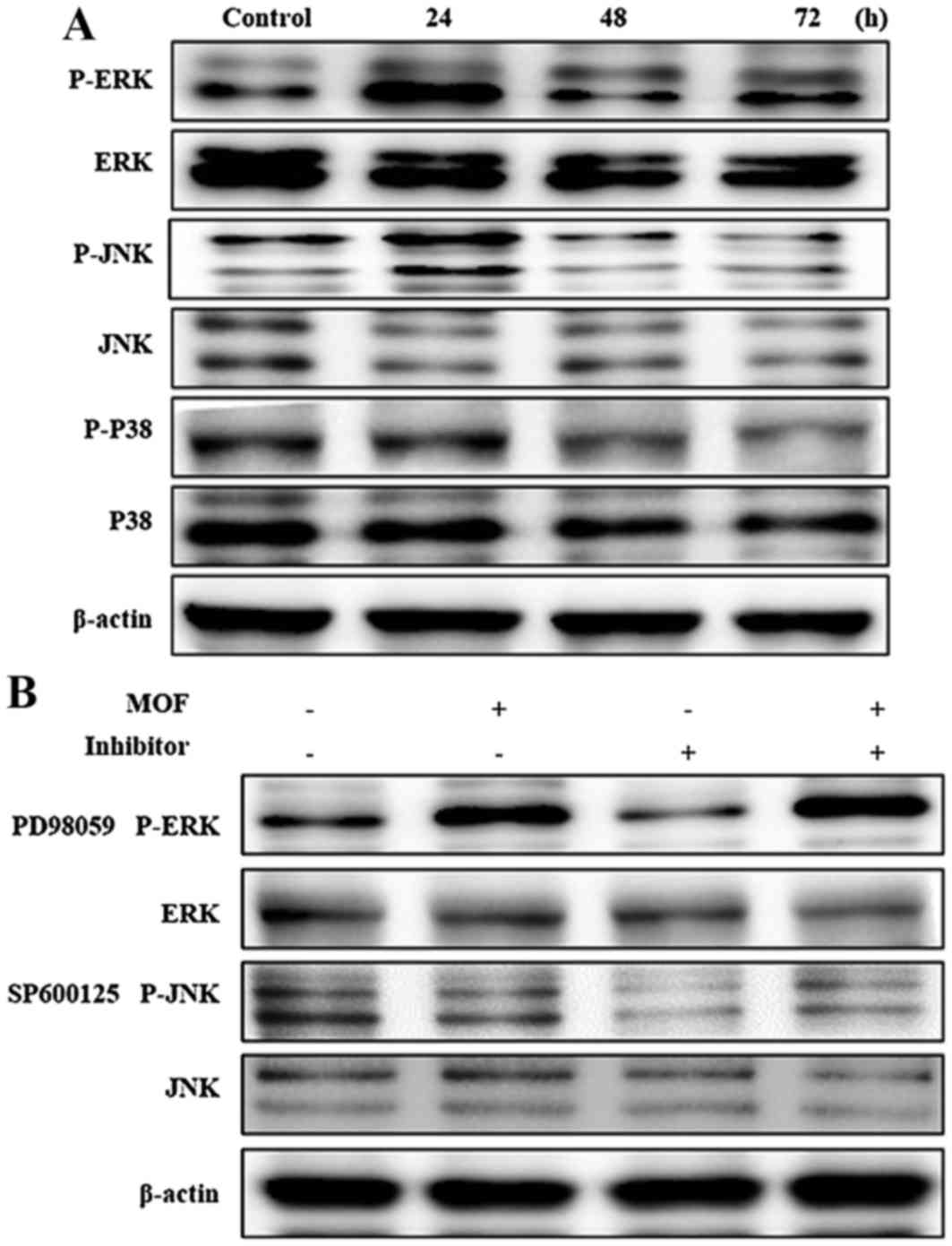
Effects on mitogen-activated protein
kinase (MAPK) expression in A2058 cells
The MAPKs, including JNK, ERK and p38 kinase, are
present in all eukaryotes and have been demonstrated to perform
central roles in regulating cell proliferation, differentiation and
apoptosis (23). In order to
investigate the effects of MOF extract in A2058 cells, the
phosphorylation of MAPKs was determined. As presented in Fig. 7A, expression levels of
non-phosphorylated ERK, JNK and p38 were unchanged with MOF
extract. By contrast, accumulation of p-ERK and p-JNK markedly
increased in a time-dependent manner. These results indicated that
MOF extract induces apoptosis through activation of ERK and JNK in
A2058 cells. In order to determine the effects of ERK and JNK on
MOF extract-induced A2058 cytotoxicity, the kinase-specific
inhibitors SP600125 and PD98058 were used. As presented in Fig. 7B, co-treatment with MOF extract and
inhibitors blocked MOF extract-mediated p-ERK and p-JNK
accumulation. The cumulative results indicated that ERK and JNK are
involved in the regulation of MOF extract-induced mitochondrial
apoptosis in A2058 cells.
Discussion
The incidence rate of malignant melanoma has
continued to increase by between 3 and 7% each year in Western
countries (24). In addition, as the
climate changes, exposure to harmful ultraviolet (UV) rays has
become a concern due to increasing incidence of various types of
skin cancer (25). As malignant
melanoma exhibits resistance to chemotherapy or immunotherapy,
complete treatment has become problematic (26,27).
Seeds, roots, leaves, flowers and bark of Moringa
oleifera have been used as materials for cosmetics and medicine
(28). As the anti-inflammatory,
anticancer and antioxidant effects of MOF extract have been
validated (29,30), the present study investigated the
effects of MOF extract on apoptosis and ROS accumulation in
malignant melanoma cells.
ROS are generated by active processes in normal
cells and are associated with various biological processes,
including cell differentiation and gene expression (31). Therefore, ROS homeostasis is important
for cell viability and survival. Although the association between
ROS and apoptosis has been reported to be contradictory, ROS
perform a role as an intermediate mediator that delivers signals
for apoptosis (32).
MAPKs are protein kinases activated by a variety of
stimuli, and regulate cell proliferation, differentiation,
proliferation and death (33,34). MAPKs consist of JNK, p38 and ERK
proteins (35). In general, ERK is
activated by growth factors, cytokines and phorbol ester and is
involved in cell proliferation or differentiation (36). By contrast, JNK and p38 are activated
by proinflammatory cytokines, UV irradiation, heat, osmotic shock,
hydrogen peroxide and DNA damage induced by stress and is involved
in proliferation inhibition and apoptosis (37–40).
However, previous studies have reported that ERK, JNK and p38 are
involved in cell survival and death (41,42). The
present study revealed that JNK and ERK are involved in the
apoptotic effects of MOF extract.
Apoptosis, also termed programmed cell death, is
required to control the number of normal cells and is induced by
various types of damage (43). In
addition, apoptosis serves as an important control mechanism of
homeostasis, as it is involved in the removal of harmful cells, and
is induced by internal and external signaling (44). Upon apoptosis, cells are
morphologically characterized by membrane detachment, membrane
blebbing, nuclear condensation, exposure of phosphatidylserine to
the extracellular space and DNA fragmentation (45). Apoptosis may be initiated by one of
two pathways: The intrinsic or the extrinsic pathway (46). In the intrinsic pathway, loss of
mitochondrial transmembrane potential results in the release of
cytochrome c into the cytosol, formation of apoptotic
protease-activating factor 1 (Apaf-1) and activation of caspase-9.
Caspase-9 then activates downstream caspases-3, −6 and −7 (47,48). In
the extrinsic pathway, Fas-associated death domain protein and
caspase-8 are activated through Fas and tumor necrosis factor
receptors, which are death receptors (49). Bax and Bak proteins are widely known
for their cell killing activity (50). Alterations in the expression of Bcl-2
family proteins inhibit dimerization of Bcl-2 family members on the
outer mitochondrial membrane, which induces release of
mitochondrial proteins, including cytochrome c, Apaf-1,
second mitochondria-derived activator of caspase/direct inhibitor
of apoptosis-binding protein with low pI and apoptosis-inducing
factor, and promotes apoptosis (51,52).
Caspase-3 is generally inactive (pro-caspase-3) in cells and
activated by death signaling, which catalyzes proteolytic cleavage
of PARP as an apoptosis-specific marker (53,54). In
the present study, the Bax/Bcl-2 ratio was identified to have
increased and PARP was cleaved by caspase-3 via the caspase-9
signaling pathway.
The results of the present study revealed the
apoptotic effects of MOF extract via ROS production in melanoma
cells. Therefore, MOF extract inhibits the proliferation of human
melanoma A2058 cells by generating ROS, which regulate expression
of proteins involved in survival and apoptosis of cancer cells.
Acknowledgements
The present study was supported by the International
Research and Development Program of the National Research
Foundation, by the Ministry of Education, Science and Technology,
Republic of Korea (grant no. NRF-2014K1A3A1A09063352).
References
|
1
|
Singh S, Zafar A, Khan S and Naseem I:
Towards therapeutic advances in melanoma management: An overview.
Life Sci. Feb 23–2017.(Epub ahead of print). View Article : Google Scholar
|
|
2
|
Shayanfar N, Bahari L, Safaie-Naraghi Z,
Kamyab K, Gheytanchi E and Rezaei N: Negative HER2/neu
amplification using immunohistochemistry and chromogenic in situ
hybridization techniques in skin melanoma cases. Asian Pac J Cancer
Prev. 16:421–425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Read RL, Pasquali S, Haydu L, Thompson JF,
Stretch JR, Saw RP, Quinn MJ, Shannon K and Spillane AJ: Quality
assurance in melanoma surgery: The evolving experience at a large
tertiary referral centre. Eur J Surg Oncol. 41:830–836. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Markovic SN, Erickson LA, Rao RD, Weenig
RH, Pockaj BA, Bardia A, Vachon CM, Schild SE, McWilliams RR, Hand
JL, et al: Melanoma melanoma in the 21st century, part 2: Staging,
prognosis, and treatment. Mayo Clin Proc. 82:pp. 490–513. 2007;
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics for Hispanics/Latinos. CA Cancer J Clin. 62:283–298.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anwar F, Latif S, Ashraf M and Gilani AH:
Moringa oleifera A food plant with multiple medicinal uses.
Phytother Res. 21:17–25. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leone A, Fiorillo G, Criscuoli F,
Ravasenghi S, Santagostini L, Fico G, Spadafranca A, Battezzati A,
Schiraldi A, Pozzi F, et al: Nutritional characterization and
phenolic profiling of Moringa oleifera leaves grown in chad,
sahrawi refugee camps, and haiti. Int J Mol Sci. 16:18923–18937.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bharali R, Tabassum J and Azad MR:
Chemomodulatory effect of Moringa oleifera, Lam, on hepatic
carcinogen metabolising enzymes, antioxidant parameters and skin
papillomagenesis in mice. Asian Pac J Cancer Prev. 4:131–139.
2003.PubMed/NCBI
|
|
9
|
Siddhuraju P and Becker K: Antioxidant
properties of various solvent extracts of total phenolic
constituents from three different agroclimatic origins of drumstick
tree (Moringa oleifera Lam.) leaves. J Agric Food Chem.
51:2144–2155. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim DH, Park KW, Chae IG, Kundu J, Kim EH,
Kundu JK and Chun KS: Carnosic acid inhibits STAT3 signaling and
induces apoptosis through generation of ROS in human colon cancer
HCT116 cells. Mol Carcinog. 55:1096–1110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clarke PG and Clarke S: Historic
apoptosis. Nature. 378:2301995. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stadtman ER: Protein oxidation in aging
and age-related diseases. Ann N Y Acad Sci. 928:22–38. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sauer H, Wartenberg M and Hescheler J:
Reactive oxygen species as intracellular messengers during cell
growth and differentiation. Cell Physiol Biochem. 11:173–186. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuo PL, Chen CY and Hsu YL:
Isoobtusilactone A induces cell cycle arrest and apoptosis through
reactive oxygen species/apoptosis signal-regulating kinase 1
signaling pathway in human breast cancer cells. Cancer Res.
67:7406–7420. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burris HA III: Overcoming acquired
resistance to anticancer therapy: Focus on the PI3K/AKT/mTOR
pathway. Cancer Chemother Pharmacol. 71:829–842. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alcazar O, Achberger S, Aldrich W, Hu Z,
Negrotto S, Saunthararajah Y and Triozzi P: Epigenetic regulation
by decitabine of melanoma differentiation in vitro and in vivo. Int
J Cancer. 131:18–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Triozzi PL, Aldrich W, Achberger S,
Ponnazhagan S, Alcazar O and Saunthararajah Y: Differential effects
of low-dose decitabine on immune effector and suppressor responses
in melanoma-bearing mice. Cancer Immunol Immunother. 61:1441–1450.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carmichael J, DeGraff WG, Gazder AF,
Minnan JD and Mitchell JB: Evaluation of a tetrazolium-based
semiautomated colorimetric assay: Assessment of chemosensitivity
testing. Cancer Res. 47:936–942. 1987.PubMed/NCBI
|
|
19
|
Lizard G, Fournel S, Genestier L, Dgedin
N, Chaput C, Flacher M, Mutin M, Panaye G and Revillard JP:
Kinetics of plasma membrane and mitochondrial alterations in cells
undergoing apoptosis. Cytometry. 21:275–283. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cathcart R, Schwiers E and Ames BN:
Detection of picomole levels of hydroperoxides using a fluorescent
dichlorofluorescein assay. Anal Biochem. 134:111–116. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nicoletti I, Migliorati G, Pagliacci MC,
Grignani F and Riccardi C: A rapid and simple method for measuring
thymocyte apoptosis by propidium iodide staining and flow
cytometry. J Immunol Methods. 139:271–279. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ryu MJ and Chung HS: [10]-Gingerol induces
mitochondrial apoptosis through activation of MAPK pathway in
HCT116 human colon cancer cells. In Vitro Cell Dev Biol Anim.
51:92–101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stadheim TA and Kucera GI: c-Jun
N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is
required for mitoxantrone- and anisomycin-induced apoptosis in
HL-60 cells. Leuk Res. 26:55–65. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Erdei E and Torres SM: A new understanding
in the epidemiology of melanoma. Expert Rev Anticancer Ther.
10:1811–1823. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Diffey B: Climate change, ozone depletion
and the impact on ultraviolet exposure of human skin. Phys Med
Biol. 49:R1–R11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jerant AF, Johnson JT, Sheridan CD and
Caffrey TJ: Early detection and treatment of skin cancer. Am Fam
Physician. 62:357–368. 2000.PubMed/NCBI
|
|
27
|
Lopez RF, Lange N, Guy R and Bentley MV:
Photodynamic therapy of skin cancer: Controlled drug delivery of
5-ALA and its esters. Adv Drug Deliv Rev. 56:77–94. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rebecca HSU, Sharon M, Arbainsyah A and de
Witte L: Moringa oleifera: Medicinal and Socio-Economic
UsesInternational Course on Economic Botany. National Herbarium
Leiden; The Netherlands: pp. 2–6. 2006
|
|
29
|
Guevara AP, Vargas C, Sakurai H, Fujiwara
Y, Hashimoto K, Maoka T, Kozuka M, Ito Y, Tokuda H and Nishino H:
An antitumor promoter from Moringa oleifera Lam. Mutat Res.
440:181–188. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mahajan SG and Mehta AA: Effect of Moringa
oleifera Lam. seed extract on ovalbumin-induced airway inflammation
in guinea pigs. Inhal Toxicol. 20:897–909. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rhee SG: Cell signaling. H2O2, a necessary
evil for cell signaling. Science. 312:1882–1883. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ozben T: Oxidative stress and apoptosis:
Impact on cancer therapy. J Pharm Sci. 96:2181–2196. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McCubrey JA, Lahair MM and Franklin RA:
Reactive oxygen species-induced activation of the MAP kinase
signaling pathways. Antioxid. Redox Signal. 8:1775–1789. 2006.
View Article : Google Scholar
|
|
34
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hanawa N, Shinohara M, Saberi B, Gaarde
WA, Han D and Kaplowitz N: Role of JNK translocation to
mitochondria leading to inhibition of mitochondria bioenergetics in
acetaminophen-induced liver injury. J Biol Chem. 283:13565–13577.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dérijard B, Hibi M, Wu IH, Barrett T, Su
B, Deng T, Karin M and Davis RJ: JNK1: A protein kinase stimulated
by UV light and Ha-Ras that binds and phosphorylates the c-Jun
activation domain. Cell. 76:10251994. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kyriakis JM, Banerjee P, Nikolakaki E, Dai
T, Rubie EA, Ahmad MF, Avruch J and Woodgett JR: The
stress-activated protein kinase subfamily of c-Jun kinases. Nature.
369:156–160. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Raingeaud J, Gupta S, Rogers J, Dickens M,
Han J, Ulevitch R and Davis RJ: Pro-inflammatory cytokines and
environmental stress cause p38 mitogen-activated protein kinase
activation by dual phosphorylation on tyrosine and threonine. J
Biol Chem. 270:7420–7426. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Davis RJ: Signal transduction by the JNK
group of MAP kinases. Cell. 103:239–252. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ichijo H: From receptors to
stress-activated MAP kinases. Oncogene. 18:6087–6093. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xia Z, Dickens M, Raingeaud J, Davis RJ
and Greenberg ME: Opposing effects of ERK and JNK-p38 MAP kinases
on apoptosis. Science. 270:1326–1331. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Strasser A, O'Connor L and Dixit VM:
Apoptosis signaling. Ann Rev Biochem. 69:217–245. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pathak S, Sharma CH, Jayaram HN and Singh
N: Apoptotic signaling induced by benzamideriboside: An in vitro
study. Mol Cell Biochem. 328:67–73. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gschwind M and Huber G: Apoptotic cell
death induced by beta-amyloid 1–42 peptide is cell type dependent.
J Neurochem. 65:292–300. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kiechle FL and Zhang X: Apoptosis:
Biochemical aspects and clinical implications. Clin Chim Acta.
326:27–45. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Brenner D and Mak TW: Mitochondrial cell
death effectors. Curr Opin Cell Biol. 21:871–877. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cain K, Bratton SB and Cohen GM: The
Apaf-1 apoptosome: A large caspase-activating complex. Biochimie.
84:203–214. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bold RJ, Termuhlen PM and McConkey DJ:
Apoptosis, cancer and cancer therapy. Surg Oncol. 6:133–142. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shamas-Din A, Bindner S, Zhu W, Zaltsman
Y, Campbell C, Gross A, Leber B, Andrews DW and Fradin C: tBid
undergoes multiple conformational changes at the membrane required
for Bax activation. J Biol Chem. 288:22111–22127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Alves NL, van Lier RA and Eldering E:
Withdrawal symptoms on display: Bcl-2 members under investigation.
Trends Immunol. 28:26–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nagappan A, Park KI, Park HS, Kim JA, Hong
GE, Kang SR, Lee H, Kim EH, Lee WS, Won CK and Kim GS: Vitamin C
induces apoptosis in AGS cells by down-regulation of 14–3-3σ via a
mitochondrial dependent pathway. Food Chem. 135:1920–1928. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Guon TE and Chug HS: Hyperoside and rutin
of nelumbo nucifera induce mitochondrial apoptosis through a
caspase-dependent mechanism in HT-29 human colon cancer cells.
Oncol Lett. 11:2463–2470. 2016.PubMed/NCBI
|
|
54
|
Kim KN, Ham YM, Moon JY, Kim MJ, Jung YH,
Jeon YJ, Lee NH, Kang N, Yang HM, Kim D and Hyun CG: Acanthoic acid
induces cell apoptosis through activation of the p38 MAPK pathway
in HL-60 human promyelocytic leukaemia. Food Chem. 135:2112–2117.
2012. View Article : Google Scholar : PubMed/NCBI
|