Introduction
Choristoma of the nervous system is characterized by
the occurrence of residual dysplastic tissues outside of the
nervous system (1–4). Intraspinal choristomas are rare and, to
the best of our knowledge, only three cases have been reported
(2–4).
The most likely differential diagnoses for spinal choristoma
include lipoma, hamartoma and teratoma. Spinal hamartomas contain
mostly mature and well differentiated tissue from ectodermal and
mesodermal layers. Alternatively, if tissues from all 3 germ layers
are identified in the lesion, a teratoma with malignant potential
should be considered. The mechanism for the formation of spinal
choristoma is not well understood.
The present case involved a striated muscle-derived
choristoma, which was located in the lumbosacral spinal canal. This
presentation is rare and has not been previously reported, to the
best of our knowledge. The present report discusses the clinical
manifestations, imaging features, pathological changes and
differential diagnosis of the patient and reviews the associated
literature. Based on the findings, a striated muscle-derived
choristoma was diagnosed. Written informed consent was obtained
from the patient for the publication of this study.
Case report
A 26-year-old man was admitted to the Department of
Neurosurgery at The First Hospital of Jilin University, (Changchun,
China) on 3rd July 2011 with intermittent lumbago that
had persisted for 3 days. A physical examination indicated
percussive pain in the lower back but no symptoms in neurological
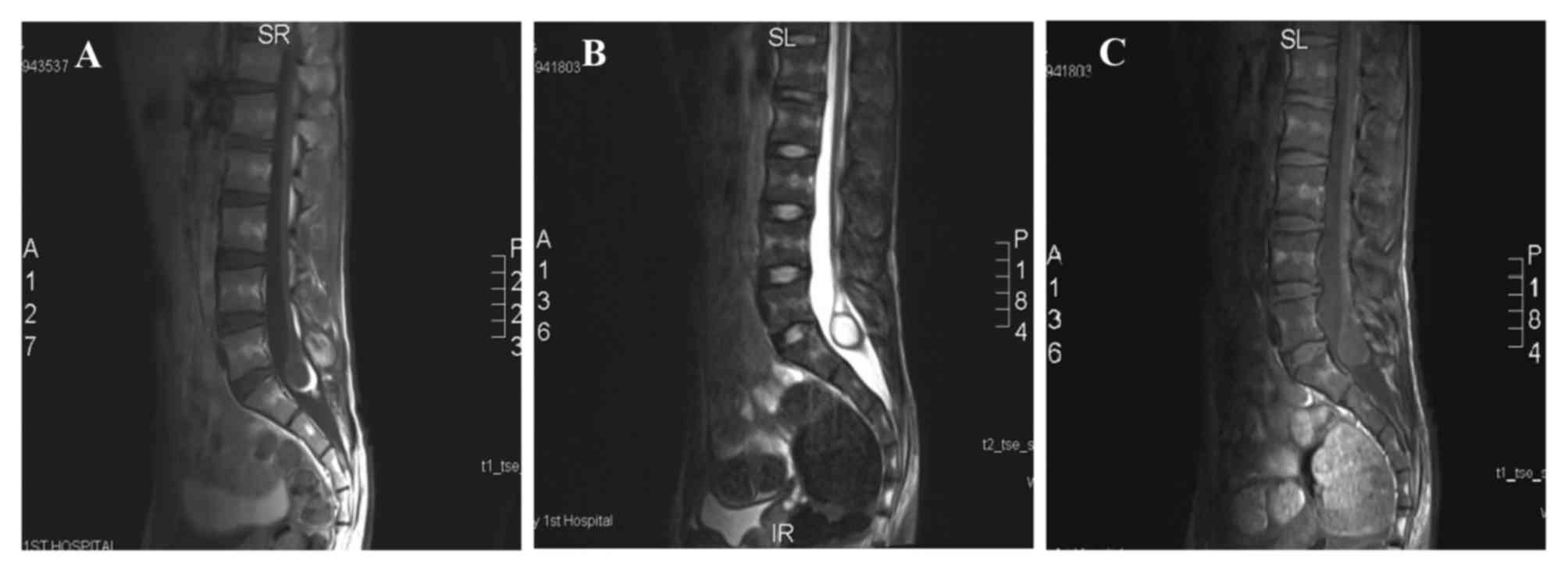
examinations. A lumbar magnetic resonance imaging (MRI) scan and a
Gadolinium-enhanced scan revealed a low-lying, enlarged terminal
filament, with the terminal end located at the L5 level and a
broadened vertebral canal at the S1 level. An ovoid cystic-solid
lesion, ~2.8×2.0 cm in size, was visible at the end of the terminal
filament. High T1-weighted image (T1WI) and T2WI signals were
present in the solid portion of the lesion, with decreased signals
in a fat-saturated scan, and slightly lower T1WI signals and high
T2WI and fat-saturated signals in the cystic portion of the lesion
(Fig. 1). The enhancement scan showed
that the vertebral canal was broader at the S1 level. Mixed cystic
and solid lesions were observed at the filum terminale; the solid
portion exhibited hyper-intensity, whereas the cystic portion
exhibited slight hypo-intensity. The clinical diagnosis was of a
sacral spinal lesion at the S1 level (possibly a large teratoma)
and tethered cord syndrome. Surgery was conducted under
neurophysiological monitoring. The resected intraspinal tumor was
gray with moderate harness, lacked an abundant blood supply and
evidently adhered to the caudaequina. The tumor was removed from
the caudaequina and completely resected with KUSA ultrasonic
aspirator (Sonastar FS-1000-RF; Misonix, Inc., Farmingdale, NY,
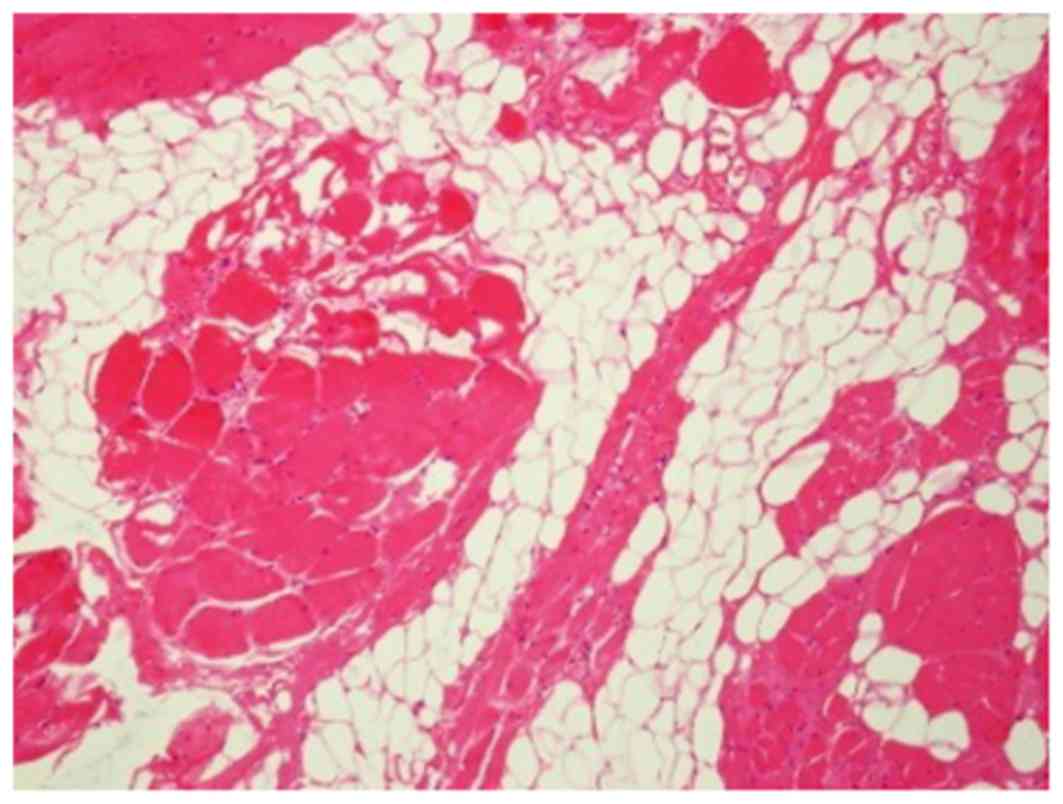
USA); the total size was ~3.0×2.5×2.0 cm. The pathological
examination (hematoxylin and eosin staining on formalin-fixed, 3
µm-thick sections) indicated the presence of mature striated
muscle, fat, scattered nerve and glandular-like tissue without cell
atypia (Fig. 2).
The expression of carcinoembryonic antigen (CEA),
creatine kinase (CK), epithelial membrane antigen (EMA), glial
fibrillary acidic protein (GFAP), S-100, Vimentin and neurofilament
(NF) protein were detected by immunohistochemistry using the
EnVision method (1). Antibodies
included CEA (cat. no. 2A-0063), CK (cat. no. 2M-0067), EMA (cat.
no. ZM-0095), GFAP (cat. no. 2A-0529), S-100 (cat. no. 2M-0224),
Vimentin (cat. no. 2M-0260) (all from ZSGB-BIO, Beijing, China) and
NF (cat. no. MAB-0134) (Maixin Biological Co., Fuzhou, China). All
antibodies were diluted at a ratio of 1:100.
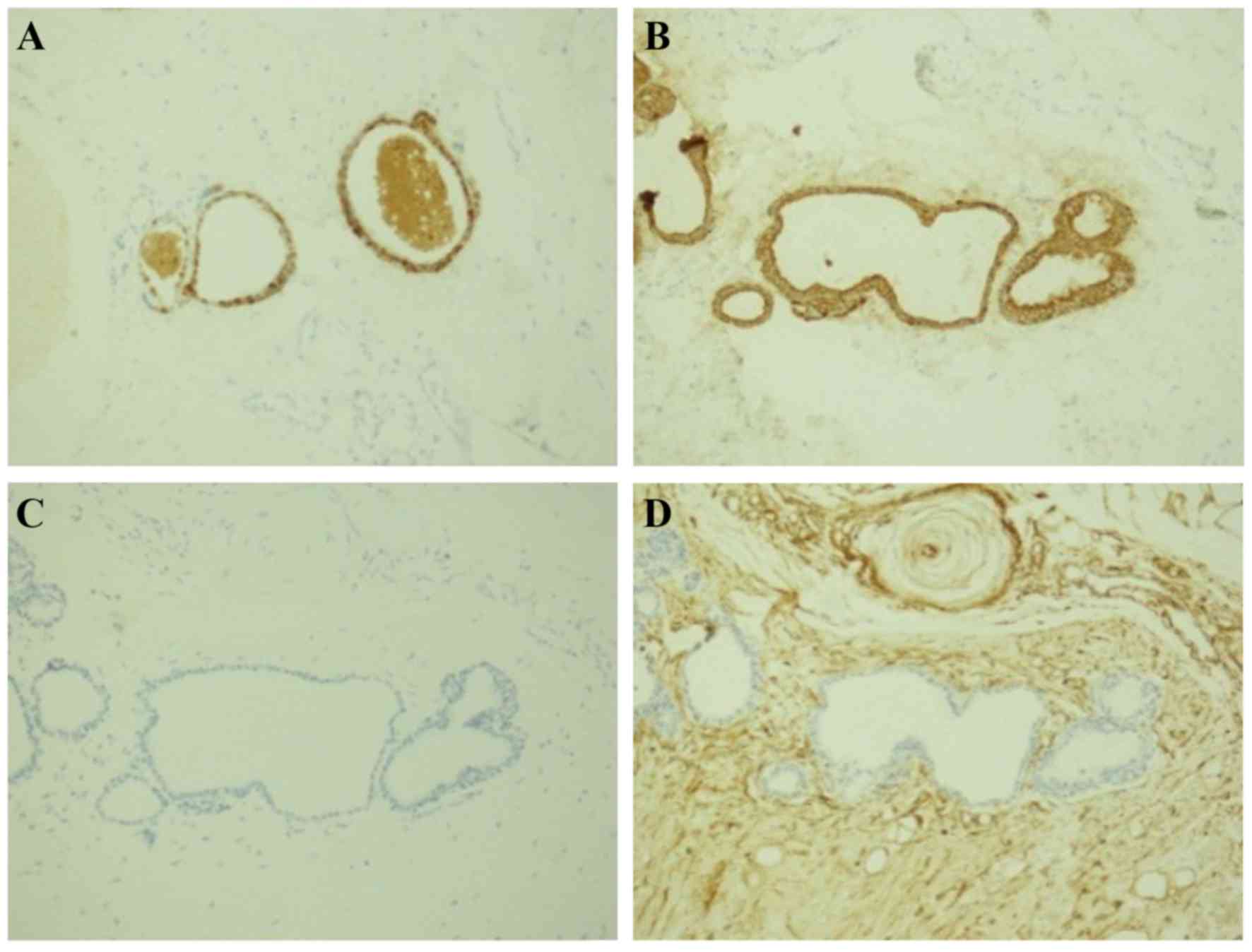
The immunohistochemical staining results were as
follows: CEA (glandular- +), CK (glandular +), EMA (glandular- +),
GFAP (−), S-100 (focal +), Vimentin (glandular- −), and NF (focal
+) (Fig. 3). The final diagnosis was
intraspinal choristoma at the S1 level. The patient recovered well
postoperatively and the lower back pain disappeared; however, the
patient succumbed to injuries sustained in a traffic accident 6
months after discharge.
Discussion
Choristoma of the nervous system is characterized by
the occurrence of residual dysplastic tissues outside the nervous
system in multiple systems and organs, including the head, eyes,
tongue, limbs, esophagus, stomach and hepatic arterial trunk
(2). Choristomas grow slowly and are
usually asymptomatic, with dysfunctions only occurring once the
size of the tumor increases. The majority of choristomas of the
central nervous system are derived from glial cells, including
astrocytes and oligodendrocytes, neurons, axons, the ependyma and
the choroid plexus, and are without evident mitosis or tumor-like
blood vessels. The first case involving multiple nests of
neuroglial tissue in the meninges of the spinal cord was reported
by Wolbach (3). Kurman et al
(4) identified the first case of
choristoma derived from the lumbosacral region. Chen et al
(5) reported the existence of a
growing mammary choristomain a girl at puberty, which resembled a
lumbosacral lipomyelomeningocele. Neuromuscular choristoma of the
oculomotor nerve has also been reported (1,6). The
present case involved a striated muscle-derived choristoma located
in the lumbosacral spinal canal, which contained striated muscle,
and adipose, nerve and glandular tissues. This type of lesion is
extremely rare and has not been previously reported.
The pathogenesis of choristoma is currently unclear
for the following reasons. First, the protrusion hypothesis
proposed by Cooper and Kernohan (7)
states that lesions protrude from pre-existing defects of the
mature brain or spinal cord. Second, the migration hypothesis by
Gyure et al (8) states that
abnormal embryonic neural epithelium migrates to the subarachnoid
space and differentiates from weeks 5 to 6 of the embryonic stage.
Third, the separation hypothesis by Harris et al (9) states that two outer valgi of the
primitive brain are isolated from brain precursors and exhibit
dysplasia during the early stages of neurogenesis or brain vesicle
formation. However, the pathogenesis of choristoma and the origin
of the ectopic tissue in the lumbosacral spinal region have not yet
been elucidated.
The differential diagnosis was ofteratomaor
hamartoma. Midline spinal cord teratomas are tumors derived from
the three germinal layers and generally contain cartilage, squamous
cells, skin appendages, cholesterol and other components, including
columnar mucosa and spinal nerves. MRI examinations of teratomas
reveal hyper-intense fat, isointense soft tissue and hypo-intense
calcification, generally without enhancement (of these findings,
calcification is particularly important) (10,11). In
the present case, the patient's tumor consisted of striated muscle
and gland tissue; therefore, a diagnosis of teratoma could be
excluded. A spinal cord hamartoma is composed of
well-differentiated tissues from the endoderm and ectoderm, in
which loose connective tissue and mucoid mesenchyme differentiate
into mature connective tissue, cartilage and fat (12–14). A
hamartoma is a benign lesion, similar to a choristoma. The two can
be differentiated as follows: A hamartoma is an excess of normal
tissue in a normal situation, while a choristoma is an excess of
tissue in an abnormal situation (5).
The clinical neurological symptoms of intraspinal
choristoma vary according to the location and size of the lesions
(2–4).
The patient exhibited no symptoms in neurological examinations
other than intermittent back pain. For patients with a tethered
spinal cord, surgical intervention should be performed to prevent
progressive dysfunction of the spinal cord, as this condition
interferes with normal metabolism and can lead to progressive
ischemia and nerve damage (3,4). An intraspinal choristoma is a benign
lesion with a good prognosis, for which the early diagnosis and
treatment can prevent the progression of nerve dysfunction
(3,4).
The patient recovered well following surgery and the lower back
pain symptoms disappeared. Intraspinal choristomas are exceedingly
rare lesions. Preoperative diagnosis of intraspinal choristoma is
difficult and surgical specimens are usually required to confirm
the diagnosis.
References
|
1
|
Boyaci S, Moray M, Aksoy K and Sav A:
Intraocular neuromuscular choristoma: A case report and literature
review. Neurosurgery. 68:E551–E555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krolls SO, Jacoway JR and Alexander WN:
Osseous choristomas (osteomas) of intraoral soft tissue. Oral Surg
Oral Med Oral Pathol. 32:588–595. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wolbach SB: Congenital rhabdomyoma of the
heart. J Med Res. 16495–520. (7)1907.PubMed/NCBI
|
|
4
|
Kurman RJ, Funk RL and Kirshenbaum AH:
Spinal bifida with associated choristoma of Müllerian origin. J
Pathol. 99:324–327. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen X, Harter J, Iskandar BJ and Salamat
MS: Growing mammary choristoma masquerading as a lumbosacral
lipomyelomeningocele in a pubertal girl. J Neurosurg Pediatr.
8:321–324. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawamoto S, Matsuda H, Ueki K, Okada Y and
Kim P: Neuromuscular choristoma of the oculomotor nerve: Case
report. Neurosurgery. 60:E777–E778. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cooper IS and Kernohan JW: Heterotopic
glial nests in the subarachnoid space; histopathologic
characteristics, mode of origin and relation to meningeal gliomas.
J Neuropathol Exp Neurol. 10:16–29. 1951. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gyure KA, Morrison AL and Jones RV:
Intracranial extracerebral neuroglial heterotopia: A case report
and review of the literature. Ann Diagn Pathol. 3:182–186. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harris CP, Townsend JJ and Klatt EC:
Accessory brains (extracerebral heterotopias): Unusual prenatal
intracranial mass lesions. J Child Neurol. 9:386–389. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Castillo M, Smith MM and Armao D: Midline
spinal cord hamartomas: MR imaging features of two patients. AJNR
Am J Neuroradiol. 20:1169–1171. 1999.PubMed/NCBI
|
|
11
|
Brownlee RD, Clark AW, Sevick RJ and Myles
ST: Symptomatic hamartoma of the spinal cord associated with
neurofibromatosis type 1. Case report. J Neurosurg. 88:1099–1103.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hader WJ, Steinbok P, Poskitt K and
Hendson G: Intramedullary spinal teratoma and diastematomyelia.
Case report and review of the literature. Pediatr Neurosurg.
30:140–145. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ghostine S, Perry E, Vaynman S, Raghavan
R, Tong KA, Samudrala S, Johnson JP and Colohan A: The rare case of
an intramedullary cervical spinal cord teratoma in an elderly
adult: Case report and literature review. Spine (Phila Pa 1976).
34:E973–E978. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Borlot F, Soares MS, Espíndola AA, Reed
UC, Matushita H and Teixeira MJ: Intramedullary spinal teratoma: A
rare condition with a good outcome. Arq Neuropsiquiatr. 67(3A):
733–735. 2009. View Article : Google Scholar : PubMed/NCBI
|