Introduction
Colorectal cancer (CRC) is the third most common
cancer in males and the second in females worldwide (1). For patients in the early stages of CRC,
i.e., stages I and II, surgery is the most common treatment, and
the 5-year relative survival for patients with early-stage CRC is
90.3%. By contrast, for patients with advanced CRC, chemotherapy
and/or radiation therapy is required, and the 5-year survival rate
of patients with distant metastasis is 12.5% (2). The ideal anticancer drug would be one
that is affordable, has few side effects and is sufficiently potent
to achieve complete remission. Inhibitors of vascular endothelial
growth factor and monoclonal antibodies that inhibit epidermal
growth factor receptor have prolonged the survival of advanced CRC
patients (3,4). However, these drugs have severe side
effects, and thus, researchers have been searching for novel
biochemical compounds (5). Natural
products have become an important resource for novel drug discovery
(5), and >50% of anticancer drugs
are derived from natural products (6).
Essential oils are natural products extracted from
seeds, leaves and tree resin. These natural oils are used primarily
in perfumes, cosmetics and the food industry (7). Various of these oils have been
demonstrated to possess antibiotic, anti-inflammatory and
anticancer properties (7,8). The molecular mechanisms underlying their
anticancer effects have been revealed to occur via reactive oxygen
species (ROS) signaling pathways and cell cycle arrest (9,10).
Terpinen-4-ol (TP4O) is the main component of the essential oil
extracted from Melaleuca alternifolia (of the botanical
family Myrtaceae), a plant native to Australia that is also known
as the tea tree (11). Tea tree oil
(TTO) is famous for its scent, and has been demonstrated to have
antibacterial (11,12) and anti-inflammatory properties
(13). Previous studies have
investigated the anticancer effect of TP4O against human melanoma
cells (14), human non-small cell
lung cancer (15), human leukaemia
cells (16) and CRC cells (17). However, the anticancer effects of TPO4
remain unclear. In the present study, the anticancer effects of
TP40 against CRC cells, in particular the role of ROS, were
evaluated using the HCT116 and RKO cell lines.
Materials and methods
Chemicals and reagents
TP4O, Triton X-100, staurosporine and ebselen (EB)
were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
TP4O was dissolved in ethanol to prepare stock solutions. The
compound was administered at a final concentration of 0.0165%
ethanol. High-glucose (4.5 g/l) Dulbecco's modified Eagle's medium
(DMEM), phenol red-free DMEM and PBS were purchased from Wako Pure
Chemical Industries, Ltd. (Osaka, Japan). Eagle's minimum essential
medium (EMEM) was purchased from the American Type Culture
Collection (ATCC; Manassas, VA, USA).
5,5-dimethyl-l-pyrroline-N-oxide (DMPO) was purchased from Dojindo
Molecular Technologies, Inc. (Kumamoto, Japan). N-acetyl-L-cysteine
(NAC) and manganese (III) tetrakis (4-benzoic acid) porphyrin
chloride (MnTBAP) were purchased from EMD Millipore (Billerica, MA
USA) and Funakoshi Co., Ltd. (Tokyo, Japan), respectively.
Phenylmethylsulfonyl fluoride was purchased from Roche Diagnostics
GmbH (Mannheim, Germany).
Cell lines and cell culture
Human CRC cell lines (HCT116 and RKO) and a human
normal colon epithelial cell line (CCD 841 CoN) were purchased from
the ATCC. The cancer cell lines were cultured in DMEM, while CCD
841 CoN cells were cultured in EMEM. The media were supplemented
with 10% heat-inactivated foetal bovine serum (FBS) and 1%
antibiotics (100 U/ml penicillin). All cell lines were incubated at
37°C and maintained in an incubator with 5% CO2. All
cells were used while within a passage number that ranged between 8
and 21. Cells were seeded at 5,000 cells/well in 96-well plates for
the WST-8 and bromodeoxyuridine (BrdU) assays, at 10,000 cells/well
in 96-well plates for the lactate dehydrogenase (LDH) release
assay, at 2×105 cells/well in 6-well plates for the
caspase-3/7 and Annexin V assays, and at 1×106
cells/dish in 6-cm dishes for western blot analysis. Each cell line
was seeded in the above culture media and incubated for 24 h prior
to each experiment.
Evaluation of cell viability and
proliferation
To evaluate the effects of TP4O on cell viability
and proliferation, WST-8 and BrdU assays were performed. Briefly,
cells were pre-incubated for 24 h and treated with various
concentrations of TP4O for 24 h (0, 1, 10, 100, 1,000 or 10,000
µM). Cell viability was quantified using a WST-8 assay via the Cell
Counting kit-8 (Dojindo Molecular Technologies, Inc.) by measuring
the absorbance at 450 nm. Cell proliferation was quantified using a
BrdU assay kit purchased from Roche Diagnostics GmbH by measuring
the absorbance at 370 nm. The half maximal inhibitory concentration
(IC50) values for each cell line were determined over 24
h following TP4O treatment. Cell viability following treatment with
antioxidants was also measured. Following pre-incubation of the
cells for 24 h at 37°C, the culture medium was exchanged for medium
with antioxidants (20 mM NAC, 20 µM EB and 100 µM MnTBAP) 30 min
prior to TP4O administration. The results were obtained from three
independent experiments.
Evaluation of apoptosis
To evaluate apoptosis, the following flow cytometry
experiment was performed. After 24 h of pre-incubation at 37°C, the
cells were treated with various concentrations of TP4O (0, 100 or
1,000 µM). Treatment-induced caspase-3/7 activation was examined in
HCT116 and RKO cells using the Muse™ Cell Analyzer (Merck KGaA) and
Muse™ Caspase-3/7 Assay kit (Merck KGaA), according to the
manufacturer's protocol. Following 6 h of treatment, harvested
cells were mixed with the Muse™ Caspase-3/7 reagent, which contains
a DNA-binding dye that is linked to a DEVD peptide substrate and a
dead cell marker [7-aminoactinomycin D (7-AAD)]. Caspase-3/7
activity was detected with the fluorescence of a DNA-binding dye
from the Muse™ Caspase-3/7 Assay kit and cell viability was
detected with 7-AAD fluorescence using the Muse™ Cell Analyzer. The
results were obtained from three independent experiments.
For the Annexin V assay, cells were treated with
TP4O (0, 100 or 1,000 µM) for 12 h. Treatment-induced apoptosis was
examined using the Muse™ Annexin V & Dead Cell kit (Merck KGaA)
according to the manufacturer's protocol. Phosphatidylserine (PS)
was detected using Annexin V, and cell viability was detected using
a dead cell marker (7-AAD). The results were obtained from four
independent experiments.
LDH-release assay
An LDH-release assay was performed to assess
necrosis resulting from TP4O treatment. The LDH Cytotoxicity
Detection kit (Takara Bio, Inc., Otsu, Japan) quantifies the
activity of LDH released from damaged cells (18). Following 24 h of pre-incubation, the
culture medium was exchanged for FBS-free DMEM containing 1% Triton
X-100, various concentrations of TP4O (0, 10, 100 or 1,000 µM) and
200 nM staurosporine for 12 h. Following treatment, the plates were
centrifuged at 250 × g for 10 min at 4°C. The supernatant was
transferred to a clear 96-well plate, and 100 µl of reaction
mixture was then added to each well. The plates were incubated in
the dark for 30 min at 25°C, the absorbance was measured at 490 nm
and the percentage LDH release was calculated. To compare necrotic
cell death, samples with 1% Triton X-100 were used as positive
controls. To study apoptotic cell death, LDH release induced by 200
nM staurosporine was examined. The results were obtained from three
independent experiments.
Electron spin resonance (ESR)
measurement
ROS generation in cells was measured using ESR, as
previously described (19). Briefly,
106 cells were seeded on a glass cover slide
(49.0×5.0×0.2 mm) and incubated overnight as previously described.
Cells were treated with 0 or 1,000 µM TP4O for 15 min. ESR was
measured in a respiration buffer containing 10 mM DMPO as the
spin-trapping agent. All ESR spectra were obtained using a JES-RE1X
X-band spectrometer (JEOL, Ltd., Tokyo, Japan).
Detection of mitochondrial
superoxide
The source of superoxide was detected by
fluorescence microscopy. A total of 2.5×104 cells/well
were seeded in an 8-well cover glass chamber. After 24 h of
pre-incubation, cells were treated with 0 or 1,000 µM TP4O for 24
h. Culture medium was exchanged for phenol red-free DMEM, which
contained 5 µM MitoSOX Red™ Mitochondrial Superoxide Indicator
(Molecular Probes; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), and the cells were incubated for 10 min at 37°C. Following
one wash with PBS, the cells were incubated for a further 30 min at
37°C with phenol-free DMEM, which contained 100 nM MitoTracker
Green FM™ (Molecular Probes; Thermo Fisher Scientific, Inc.). Next,
the cells were washed with PBS and observed using a confocal
inverted fluorescence microscope (Olympus Corporation, Tokyo,
Japan). Localization of ROS derived from mitochondria was detected
by MitoSOX Red™ Mitochondrial Superoxide Indicator fluorescence,
and localization of mitochondria was detected by MitoTracker Green
FM™ fluorescence. Images of bifocal field, MitoSOX Red™
Mitochondrial Superoxide Indicator and MitoTracker Green FM™ were
merged.
Quantification of oxidative
stress
The quantitative measurement of cellular populations
undergoing oxidative stress was measured using the Muse™ Cell
Analyzer and Muse™ Oxidative Stress kit (Merck KGaA). Cells were
seeded at 2×105 cells/well in a 6-well plate and
incubated overnight. Following exposure to 0 or 1,000 µM TP4O for
24 h, according to the manufacturer's protocol, cells were
detached, resuspended at 1×106 cells/ml and incubated at
37°C for 30 min with Muse™ Oxidative Stress working solution, which
contained dihydroethidium (DHE). DHE is cell permeable, and has
been proposed to react with superoxide anions, thus undergoing
oxidation upon binding to DNA (20).
The number of oxidized cells were counted according to the
intensity of red fluorescence using the Muse™ Cell Analyzer. The
results were obtained from four independent experiments.
Western blot analysis
Cells were seeded at 1×106 cells/dish in
6-cm dishes. After 24 h, cells were treated with various
concentrations of TP4O (0, 100 or 1,000 µM) for 24 h. Cultured
cells were washed with PBS and lysed with lysis buffer that
contained 1% Protease Inhibitor Cocktail (100X; Cell Signaling
Technology, Inc., Danvers, MA, USA) and 1 mM phenylmethylsulfonyl
fluoride. Samples were kept on ice for 2 min, followed by
sonication for 5 min and centrifugation at 13,000 × g for 2 min at
4°C; the supernatants were then collected. The samples were
subjected to 15% SDS-PAGE and transferred to a polyvinylidene
fluoride membrane (Merck KGaA). After 30 min blocking with
Tris-buffered saline containing 0.1% Tween-20 (T-TBS) and 5% bovine
serum albumin (Iwai Chemicals Company, Ltd., Tokyo, Japan), primary
antibodies against superoxide dismutase 2 (SOD2; cat. no. 13141;
dilution, 1:1,000) glutathione peroxidase 1 (GPX1; cat. no. 3206;
dilution 1:500) and GAPDH (endogenous control; cat. no. 2118;
dilution, 1:1,000; all from Cell Signalling Technology, Inc.) were
incubated with membranes in Can Get Signal® Immunoreaction Enhancer
Solution 1 (Toyobo Co., Ltd., Osaka, Japan) overnight at 25°C.
After washing 3 times for 30 min with T-TBS, the membrane was
incubated with an anti-rabbit IgG, horseradish perxodiase-linked
antibody (cat. no. 7074; dilution, 1:1,000; Cell Signalling
Technology, Inc.) in Can Get Signal® Immunoreaction Enhancer
Solution 2 (Toyobo Co., Ltd.) for 2 h at 25°C. After washing 3
times for 15 min with T-TBS at 25°C, the bands were incubated with
ECL™ Western Blotting Detection reagents as according to the
manufacturer's protocol and detected with ImageQuant LAS 4000 mini
(both from GE Healthcare Life Sciences, Chalfont, UK). Results were
quantified using ImageQuant TL 7.0 software (GE Healthcare Life
Sciences).
Animals
A total of 14 male ICR-SCID mice aged 6–7 weeks old
and weighing 21–25 g were purchased from Charles River Laboratories
Japan, Inc. (Yokohama, Japan). The mice were provided with clean
water and food ad libitum, and housed in standardized,
pathogen-free conditions with a 14 h light/10 h dark cycle, a
temperature of 23.5±2.5°C and humidity of 52.5±12.5%. The mice were
used after an acclimation period of ≥7 days. All animal experiments
were performed with the approval of the Animal Ethics Committee of
the University of Tsukuba (Tsukuba, Japan; approval no. 13-385) and
according to the guidelines of this committee.
Xenograft model
To evaluate the effect of TP4O in vivo, a
subcutaneous tumor was induced. HCT116 cells (2×106
cells per mouse) were injected subcutaneously into the right flanks
of 14 mice. Tumor volume was calculated using the following
formula: 0.5 × length × width2. When tumor volume had
reached 80–100 mm3, the 12 qualifying mice were randomly
divided into two groups (n=6/group) and subcutaneously injected
with one of the following: 250 µl saline (control group) or 200
mg/kg TP4O dissolved in 250 µl saline (TP4O group). Each mouse was
injected once every 3 days (total, five times). The tumor volume
was recorded every 3 days. Body weight was recorded the first day
of the injection, on day 7 and at the end of experimentation (day
14). On day 14, blood samples were collected under anesthesia, the
mice were sacrificed and the tumors were excized. To evaluate TP4O
toxicity, serum alanine aminotransferase (ALT) and serum creatinine
levels were measured using an autoanalyzer (Dri-chem 7000 V;
Fujifilm, Tokyo, Japan).
Immunostaining
The tumor tissue was fixed with 10% formaldehyde.
Each 2-µm paraffin-embedded section was stained with an
anti-8-hydroxy-2′-deoxyguanosine (8-OHdG) antibody at 4°C overnight
(cat. no. MOG-20P; dilution, 1:100, Japan Institute for the Control
of Aging, Shizuoka, Japan) or with an anti-cleaved caspase-3
antibody according to the manufacturer's protocol (cat. no. 9661;
dilution, 1:300; Cell Signaling Technology, Inc.). 8-OHdG is a
marker for oxidative stress (21). To
accurately quantify 8-OHdG- and cleaved caspase-3-positive areas,
slides from three randomly selected high-power fields
(magnification, ×200) of each tumor were visualized using a BZ-X710
microscope (Keyence Corporation, Osaka, Japan). An automated
software analysis program, BZ-X analyser version 1.3.0.3 (Keyence
Corporation), was used to determine the percentage of stained areas
in the digital photomicrographs.
Statistical analysis
The data are expressed as the mean ± standard
deviation. For comparison of >2 groups, analysis of variance
(ANOVA) was performed. If the ANOVA results were significant, a
Dunnett's test for comparison with the control was used. For
comparison of two groups, an unpaired Student's t-test was
performed. All indicated P-values were two-sided, and P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using GraphPad Prism software
version 5.01 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
TP4O inhibits CRC cell viability and
proliferation
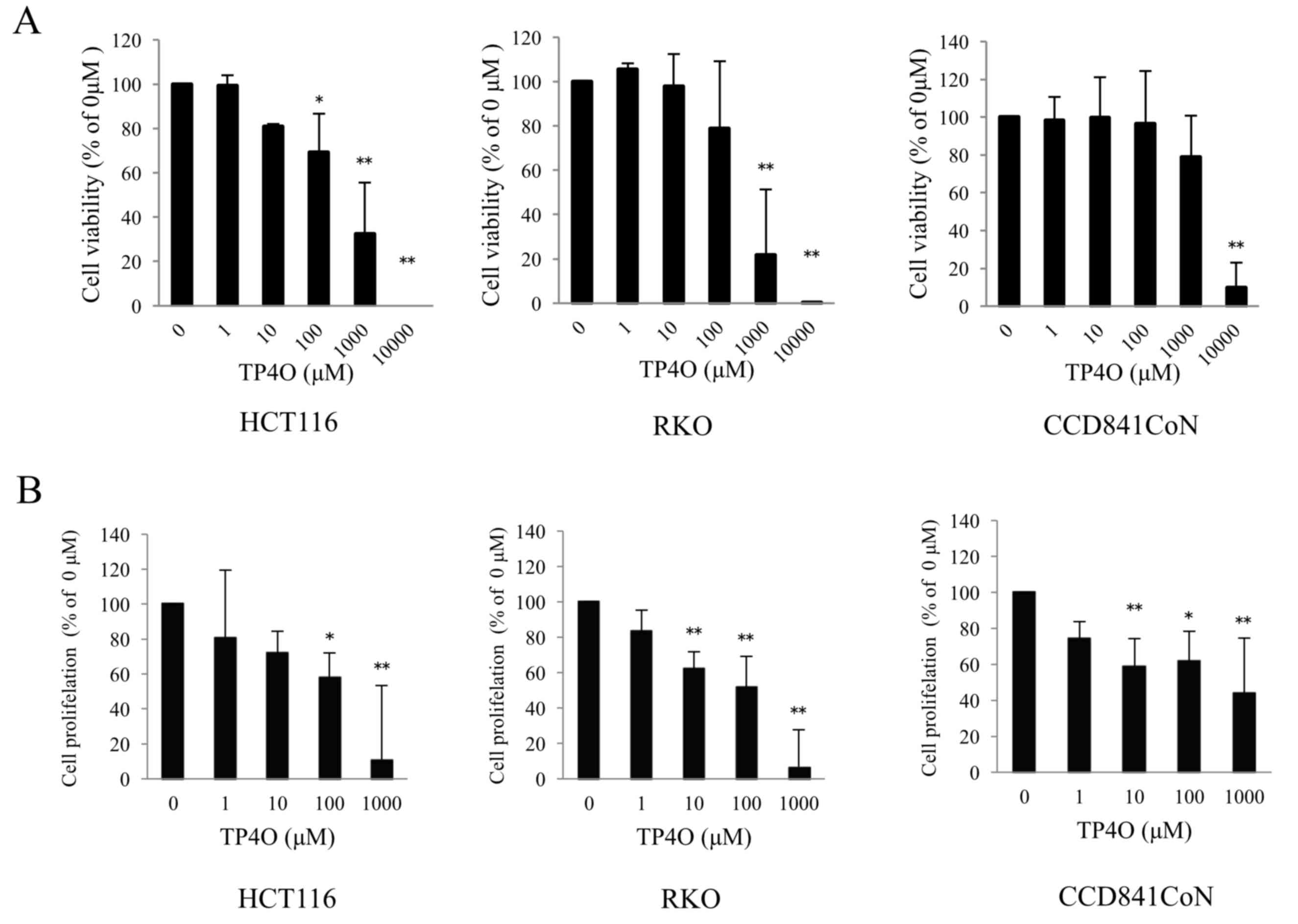
To evaluate cytotoxicity following TP4O treatment,
cell viability was measured using a WST-8 assay. TP4O significantly
decreased the viability of the human CRC cell lines HCT116 at 100,
1,000 and 10,000 µM, and RKO at 1,000 and 10,000 µM (Fig. 1A). The effect of TP4O was dose
dependent. The IC50 values of TP4O in HCT116 and RKO
cells were 661 and 381 µM, respectively. By contrast, the
IC50 value of TP4O in the normal human colon epithelial
cell line CCD 841 CoN was 5,347 µM. In addition, TP4O significantly
inhibited cell proliferation in a dose-dependent manner in CRC cell
lines and CCD 841 CoN cell line, particularly in the CRC cell lines
at 1,000 µM (Fig. 1B).
TP4O induces apoptotic cell death in
CRC cells
TP4O-induced apoptotic cell death was determined
using a caspase-3/7 activity assay and Annexin V assay. DNA
fragmentation and exposure of PS are typical phenomena observed in
apoptotic cell death (22).
Caspase-3/7 activity was enhanced in TP4O-treated cells in a
dose-dependent manner (Fig. 2A). The
distribution of apoptotic cells, which were located in the top- and
bottom-right quadrants, is presented in a bar graph; the percentage
of apoptotic cells was significantly increased in cells treated
with 1,000 µM TP4O compared with 0 µM TP4O (P<0.05; Fig. 2B). Although there was no statistical
difference, the percentage of Annexin V-positive cells tended to
increase in HCT116 and RKO cell cultures treated with 1,000 µM TP4O
(Fig. 2C and D). The lower level of
LDH release following TP4O treatment indicates that TP4O did not
induce necrotic LDH release in HCT116, RKO (Fig. 2E) or CCD 841 CoN (data not shown) cell
lines.
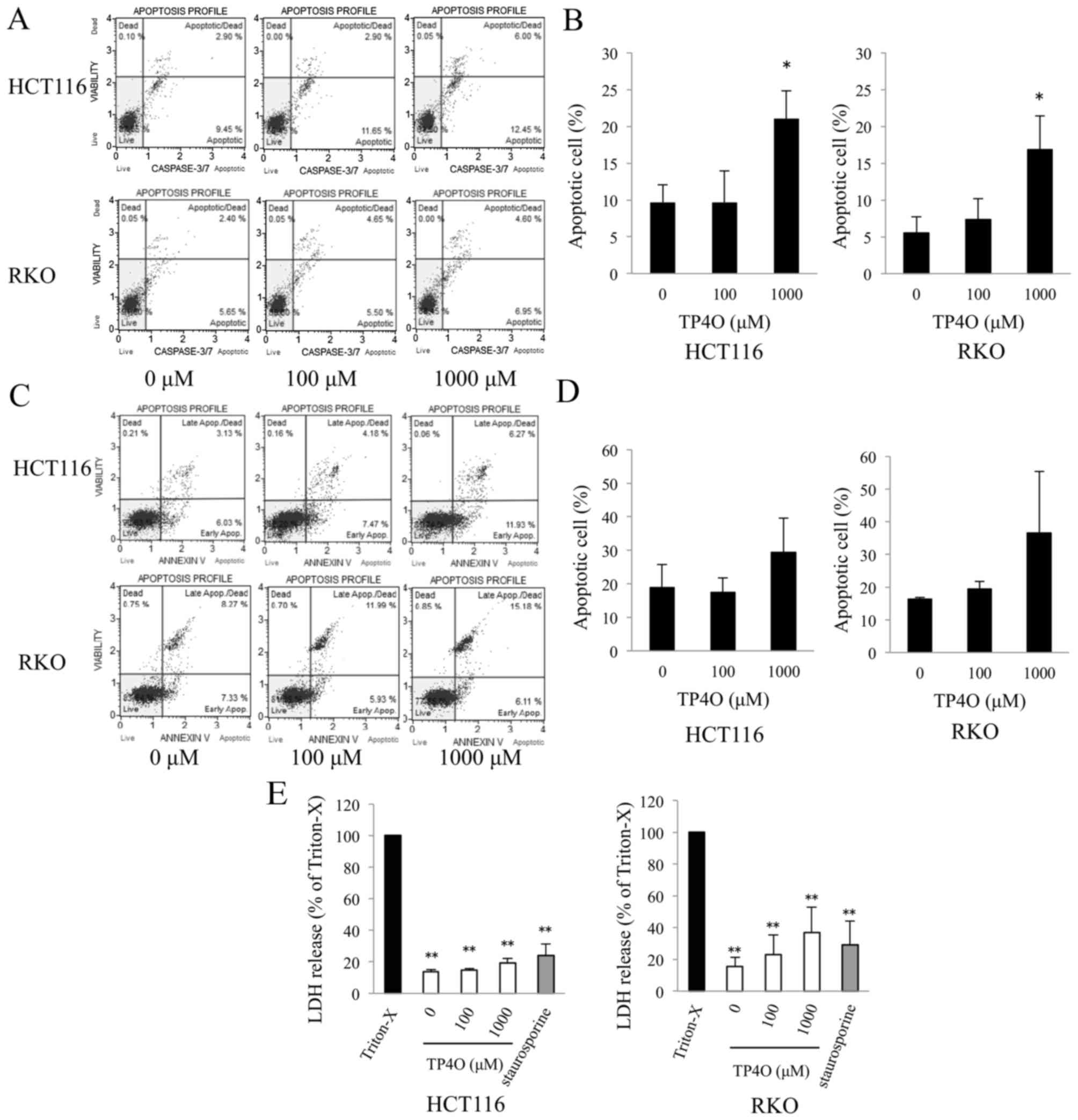 | Figure 2.TP4O induces the apoptosis of human
CRC cell lines. (A) HCT116 and RKO cells were treated with the
indicated concentrations of TP4O for 6 h. Caspase-3/7 activity and
cell viability were measured by flow cytometry. Caspase-3/7
activity was detected by fluorescence of a DNA-binding dye and cell
viability were detected with the detection of 7-aminoactinomycin D
fluorescence using the Muse™ Cell Analyzer. (B) The percentage of
apoptotic cells, which are located in the top- and bottom-right
quadrants, is presented. The values were compared with those
obtained with 0 µM TP4O. The data are presented as the mean ± SD.
*P<0.05. The results were obtained from three independent
experiments. (C) HCT116 and RKO cells were treated with the
indicated concentrations of TP4O for 12 h. The extent of apoptosis
was measured with Annexin V staining. (D) The percentage of
apoptotic cells, which are located in the top- and bottom-right
quadrants, is presented. The values were compared to with those
obtained with 0 µM TP4O. The data are presented as the mean ± SD.
The results were obtained from four independent experiments. (E)
HCT116 and RKO cells were treated with the indicated concentrations
of TP4O for 12 h, and the LDH level in the supernatant was
measured. The values were compared with those obtained with 1%
Triton X-100 as the control (100%). To determine whether LDH
release was associated with necrosis or apoptosis, cells were
treated with Triton X-100 or staurosporine, respectively. The data
are represented as the mean ± SD. **P<0.01. The results were
obtained from three independent experiments. TP4O, terpinen-4-ol;
CRC, colorectal cancer; IC50, half maximal inhibitory
concentration; SD, standard deviation; LDH, lactate dehydrogenase;
Apop., apoptosis. |
TP4O generates ROS in CRC cells, and
TP4O-induced cell death is rescued by antioxidant reagents
In the presence of DMPO, the hydroxyl radical can be
detected as as four spikes in ESR (23). ESR measurements revealed that
treatment with 1,000 µM TP4O for 15 min induced the generation of
the hydroxyl radical in CRC cells (Fig.
3A), whereas the hydroxyl radical was barely detectable in
CCD841 CoN cells (data not shown). The increase in oxidative stress
was quantified by DHE reaction. TP4O administration led to an
increase in the number of cells with high ROS (Fig. 3B). There was a significant increase in
oxidative stress in HCT116 cells (P<0.05; Fig. 3C). This result implies that TP4O
enhances the accumulation of intracellular ROS in HCT116 cells.
There was a non-significant increase observed in RKO cells
following the administration of TP4O.
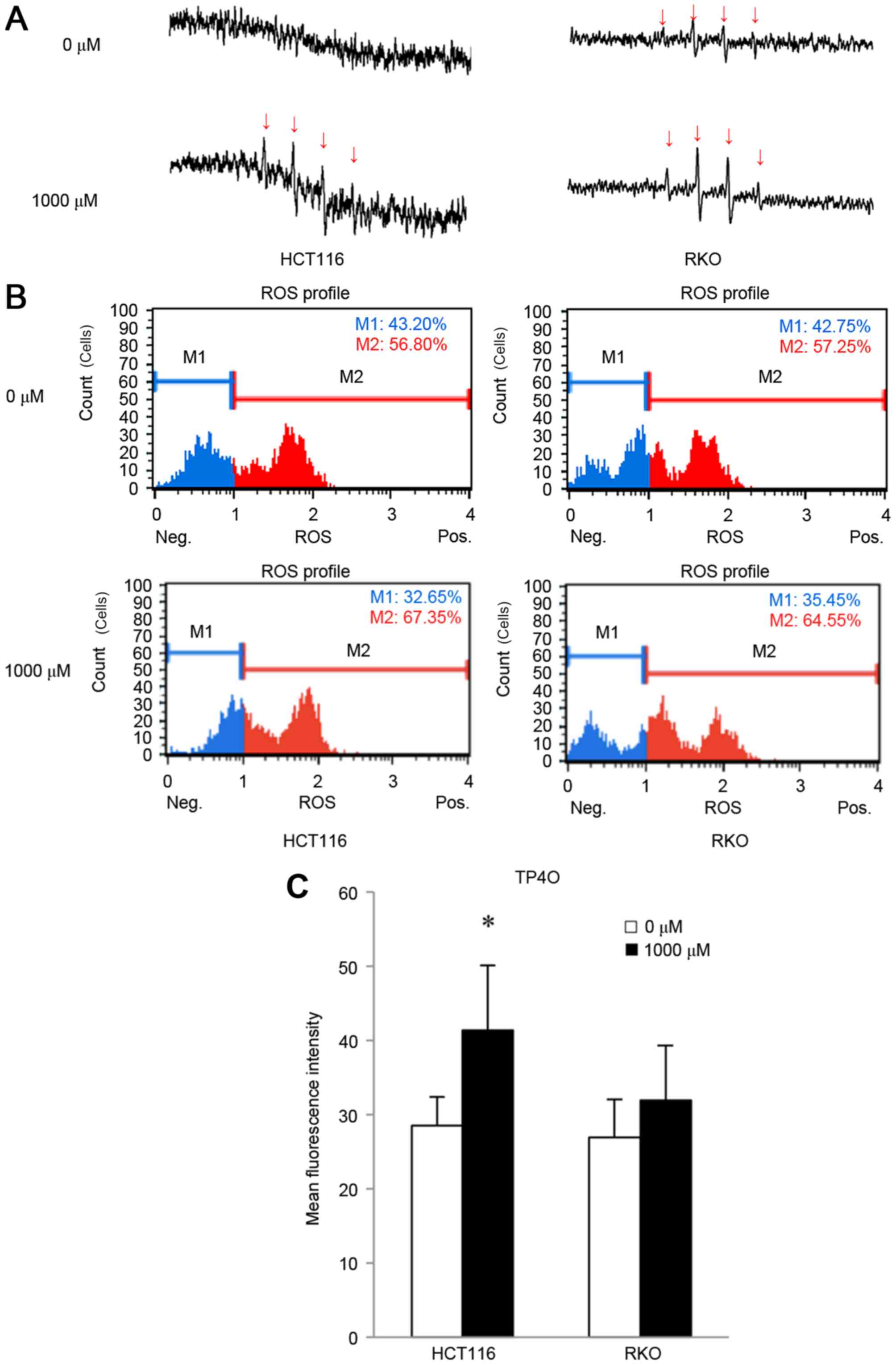 | Figure 3.TP4O induces ROS in human CRC cell
lines. (A) ESR measurement. HCT116 and RKO cells were treated with
0 or 1,000 µM of TP4O for 15 min. ESR was measured in a respiration
buffer containing 5,5-dimethyl-1-pyrroline-N-oxide as the
spin-trapping agent. The production of the hydroxyl radical was
detected with four spikes in ESR. Spikes derived from the hydroxyl
radical (indicated with arrows) became apparent in cells exposed to
1,000 µM TP4O. (B) Quantitative measurements of cellular
populations undergoing oxidative stress, based on the detection of
ROS. HCT116 and RKO cells were untreated or treated with 1,000 µM
TP4O for 24 h. The distribution of cells according to levels of
intracellular ROS was represented as a histogram. Cells were
divided into two groups: The blue area (M1) represented the cells
with low ROS, and the red area (M2) represented the cells with high
ROS. The administration of TP4O led to an increase in the number of
cells in the red area. (C) The mean fluorescence intensity of each
group is presented. The values were compared with those obtained
with 0 µM TP4O. The data are presented as the mean ± SD.
*P<0.05. The results were obtained from four independent
experiments. TP4O, terpinen-4-ol; CRC, colorectal cancer;
IC50, half maximal inhibitory concentration; SD,
standard deviation; ROS, reactive oxygen species; DHE,
dihydroethidium; ESR, electron spin resonance. |
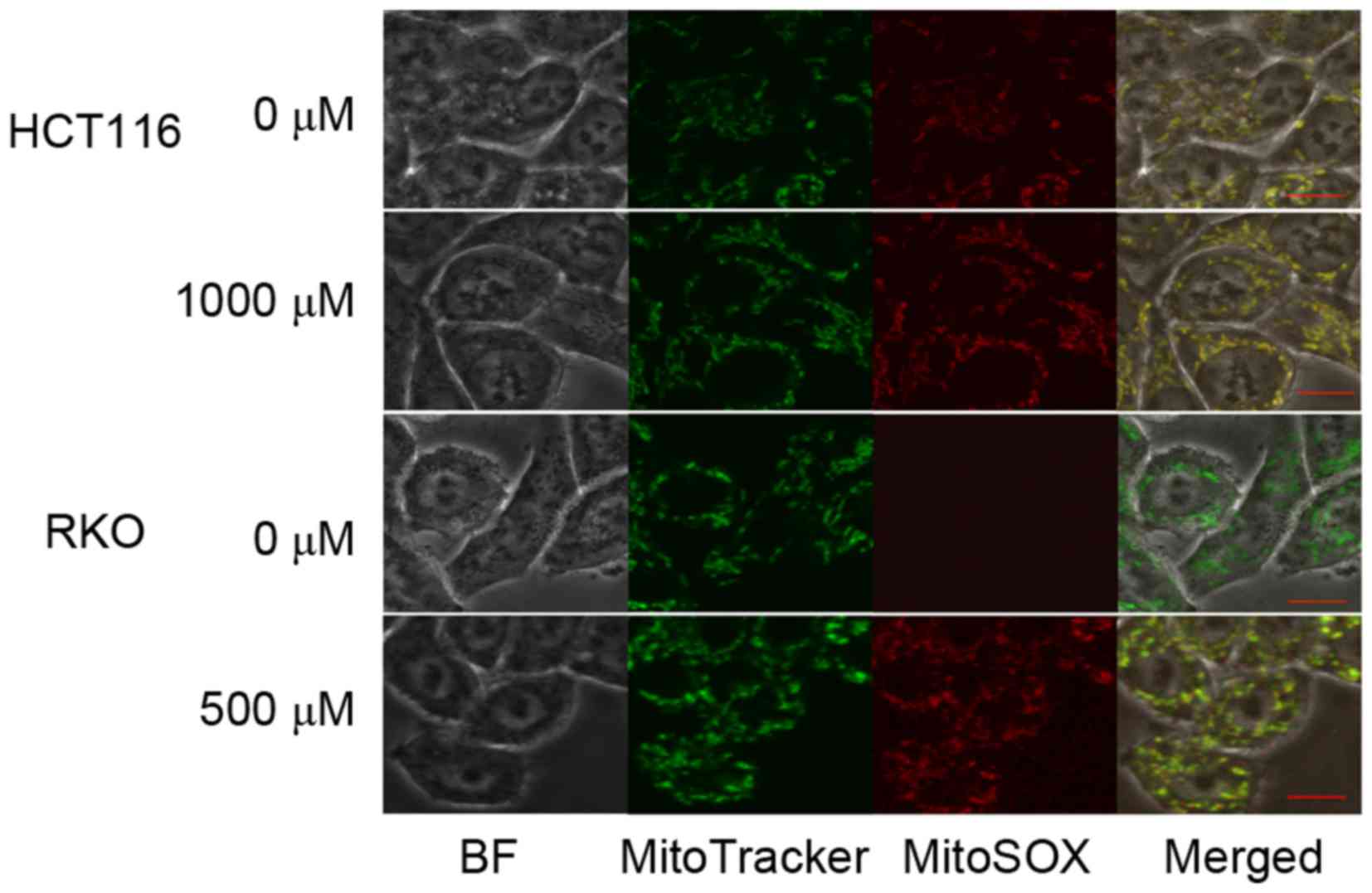
To determine the source of TP4O-induced ROS, CRC
cells were observed by confocal inverted fluorescence microscopy.
The location of the mitochondria was represented by MitoTracker
Green FM™ fluorescence, while accumulated ROS were represented by
MitoSOX Red™ Mitochondrial Superoxide Indicator fluorescence. The
merged image demonstrated that the ROS induced by TP4O were
generated by mitochondria (Fig.
4).
Protein levels of intrinsic oxidant scavengers were
analysed by western blotting. Protein levels of SOD2 and GPX1
tended to increase following exposure to TP4O (Fig. 5A). However, there was no significant
difference in comparison with that of 0 µM TP4O (Fig. 5B). The results suggested that ROS
accumulation was not due to suppression of ROS scavengers, but due
to increased ROS production. CRC cells were exposed to specific
antioxidants (NAC, EB and MnTBAP), which prevented significant
TP4O-derived cell death (Fig. 5C),
demonstrating that ROS accumulation was the cause for cell
death.
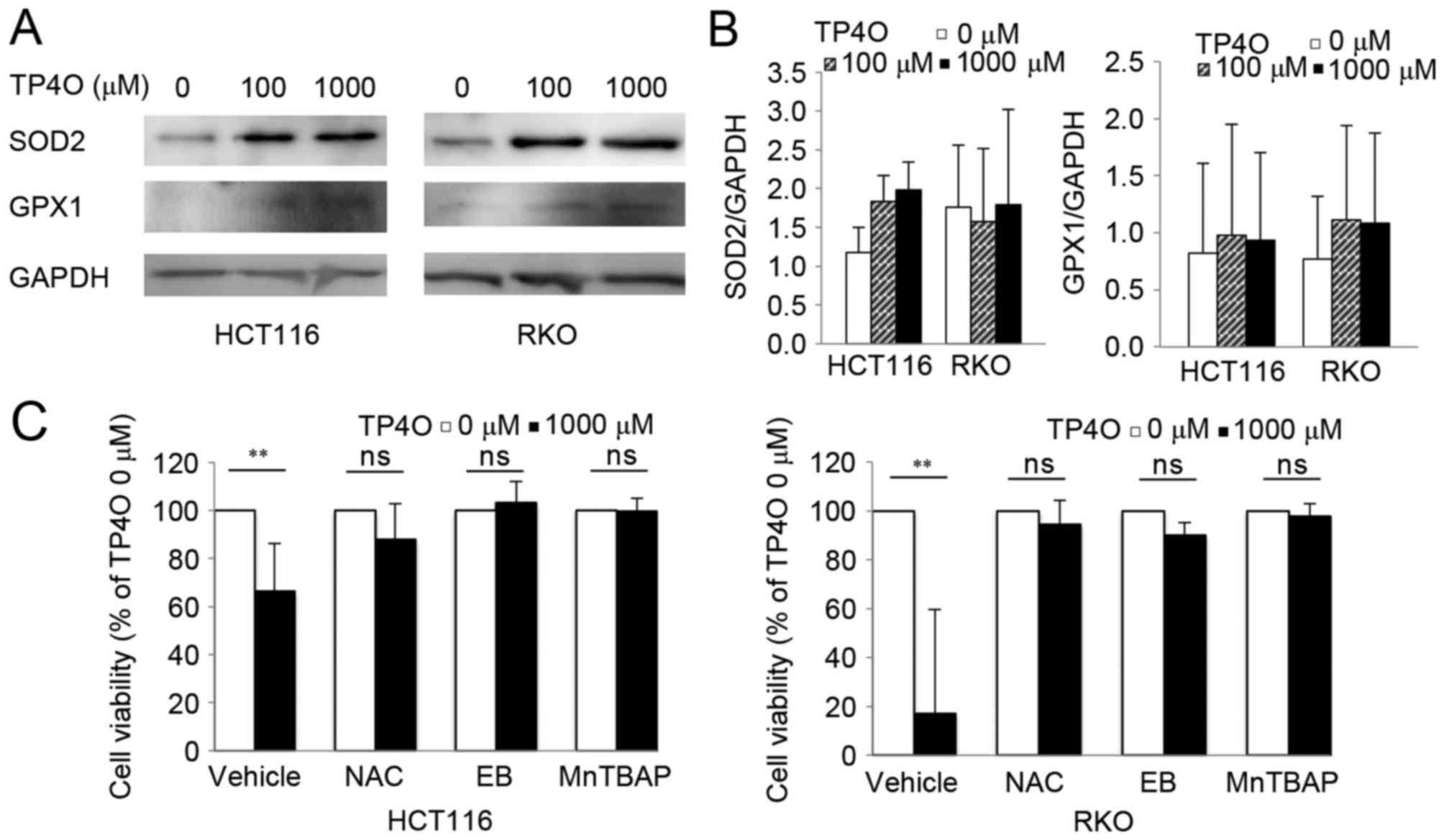 | Figure 5.TP4O induces the activation of
antioxidants, and antioxidant reagents rescue TP4O cytotoxicity.
(A) Western blot analysis of antioxidant proteins. Lysates of
HCT116 and RKO cells were examined following treatment with the
indicated concentrations of TP4O for 24 h. GAPDH was used as a
loading control. (B) HCT116 and RKO cells were treated with 0 or
1,000 µM TP4O in the presence of vehicle, 20 mM NAC, 20 µM EB or
100 µM MnTBAP for 24 h, and cell viability was measured by WST-8
assay. The values for each group were compared with those obtained
with 0 µM TP4O. The data are represented as the mean ± standard
deviation. **P<0.01. The results were obtained from three
independent experiments. TP4O, terpinen-4-ol; MnTBAP, manganese
(III) tetrakis (4-benzoic acid) porphyrin chloride; SOD2,
superoxide dismutase 2; GPX1, glutathione peroxidase 1; NAC,
N-acetyl-L-cysteine; EB, ebselen; ns, not significant. |
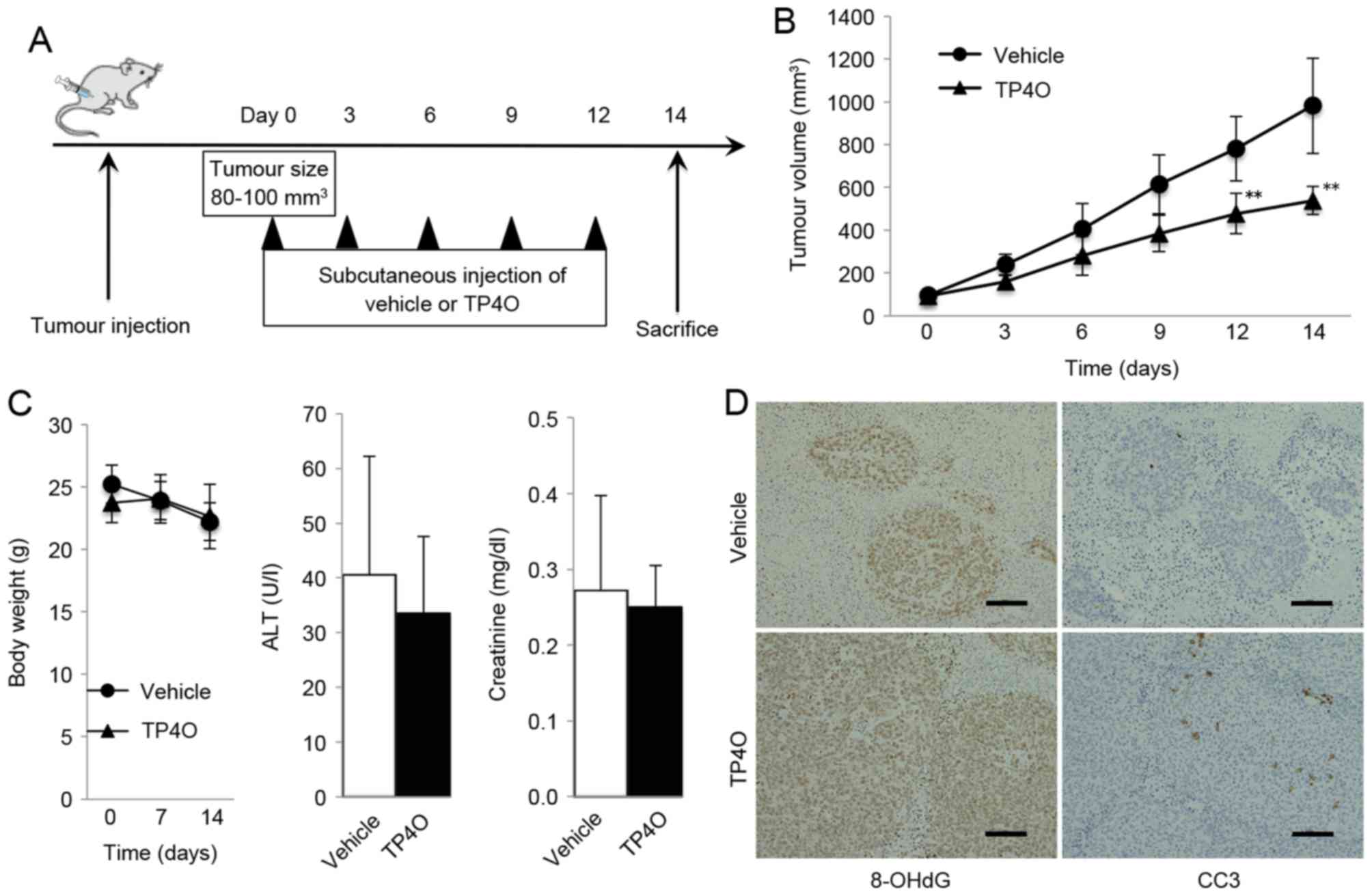
TP4O treatment inhibits tumor growth
in a xenograft mouse model
A schematic representation of the experimental
design is provided in Fig. 6A. Mice
treated with TP4O developed significantly smaller tumors compared
with the group treated with vehicle (P<0.05; Fig. 6B). TP4O administration did not cause
marked complications or body weight loss, and the serum levels of
ALT and creatinine were not different in the TP4O group compared
with those in the control group (Fig.
6C). For the histological analysis, tumors were stained using
antibodies against the oxidative stress marker 8-OHdG. The areas of
anti-8-OH-dG staining were larger in tissues from the TP4O group
compared with those from the control group (Fig. 6D). In addition, areas stained by
anti-cleaved caspase-3 antibody were larger in the TP4O group than
in the control group (Fig. 6D).
Discussion
TP4O is an essential oil extracted from aromatic
plants and is a type of monoterpene (10). Monoterpenes, the C10 class of
isoprenoids, are a diverse family of natural products, ranging in
structure from linear to polycyclic (24). Menthol, limonene, eucalyptol and
citral are familiar monoterpenes that are used as food and cosmetic
additives (8,24).
The anticancer effects of essential oils have
previously been investigated by biological approaches focusing on
pharmaceutical and therapeutic potentials of essential oils
(7,9,10,25). Previous studies have indicated that
the anticancer effects of TPO4 are due to the induction of
apoptosis in human melanoma cells (14), human non-small cell lung cancer
(15), human leukaemia cells
(16) and CRC cells (17). However, Greay et al (26) reported that TP4O induces necrosis and
cell cycle arrest in murine mesothelioma and melanoma cells. In the
present study, TP4O elevated caspase-3/7 activity and increased the
number of Annexin V-positive cells without inducing necrotic LDH
release in CRC cells. In the in vivo experiments, the number
of cleaved caspase-3-positive cells increased significantly in the
TP4O group compared with that in the control group. These results
indicated that TP4O induced cell death in human CRC cell lines by
apoptosis in vitro and in vivo.
In addition, the data from the present study
revealed the source of ROS generation in the in vitro and
in vivo TP4O treatments. Previous studies have demonstrated
the antioxidant activity of essential oils [reviewed in (7)], including TTO, which contains TP4O
(27). Kim et al (28) investigated the antioxidant activity of
TTO, and determined that three compounds of TTO serve important
roles in the antioxidant mechanism, whereas TP4O did not possess
antioxidant activity. In the current study, the hydroxyl radical
was directly detected by ESR. Moreover, the protein levels of the
major ROS scavengers and GPX1 were not decreased, which indicates
that the increase in ROS following TP4O treatment is not due to
inhibition of the redox system but is the result of an increase in
ROS production. The addition of antioxidant reagents inhibited
TP4O-induced cell death. Taken together, these results suggested
that the ROS induced following TP4O exposure serve a role in the
anticancer effect of TP4O.
To maintain active proliferation, cancer cells
produce more oxidative metabolites than normal cells (29). The redox system is activated in
cancer, but cancer cells are not able to adapt to excess oxidative
stress due to a decreased capacity for ROS metabolism compared with
that of normal cells (29). However,
normal cells produce lower levels of ROS than cancer cells; thus,
they can adapt to increases in ROS (30,31).
Previous reports have demonstrated that the differences in cellular
metabolism of ROS could be a target for cancer treatment (31–37). There
are three strategies to amplify intracellular oxidative stress. The
first one is the inhibition of redox enzymes, i.e., GPX (35) or SOD (31). The second one is facilitating ROS
production (36); the use of TP4O is
included in this category. The third strategy is a combination of
the two strategies mentioned above (37). These previous studies have revealed
IC50 discrepancies between cancer cells and normal
cells. The results of the present study support those from previous
reports suggesting that ROS-mediated compounds have therapeutic
potential against malignant neoplasms.
A number of limitations of the present study should
be acknowledged. First, the underlying molecular mechanisms of ROS
production by TP4O or the apoptotic pathway involved have not been
detailed. As indicated by previous reports, excessive production
and accumulation of ROS affects several mitogen-activated protein
kinase signalling pathways, e.g., p38 mitogen-activated protein
kinase, extracellular signal-regulated kinase and c-Jun N-terminal
kinase signalling pathways, which may be associated with ROS
production (31,34,38,39). Thus,
further studies are required to elucidate the association between
TP4O and ROS generation or signalling pathways. Second, the
activities of ROS scavengers were estimated using western blot
analysis of SOD2 and GPX1; however, the actual activities of these
enzymes or other redox systems have not been measured. The
possibility that TP4O suppresses other redox systems cannot be
ruled out. Third, ROS cause DNA damage and can promote tumorigenic
signalling pathways (40). ROS are
also known to lead to Alzheimer's disease (41), ischaemic vascular disease (42), non-alcoholic steatohepatitis (43) and other diseases (44). Although the data from the current
study demonstrated that TP4O inhibited CRC xenograft growth without
marked liver or renal injury in vivo, more precise
assessment of the risk of the use of ROS for cancer therapy is
required. The therapeutic concentration in the present study of
TPO4 was similar to that of a previous report (17), which is considered high in clinical
use. Therefore, consideration of a practical dose for future
clinical use is crucial. As Shapira et al (17) reported, combination therapy may reduce
the dose of TP4O and existing cancer drugs. Additionally,
Berndtsson et al (45)
reported the ROS accumulation effect of oxaliplatin, which is
typically used for the treatment of CRC (4). Thus, it is expected that combination
therapy with TP4O and oxaliplatin would result in a synergistic
effect. Upon sufficiently addressing the aforementioned issues,
this new therapeutic strategy could be applicable in a clinical
setting.
In conclusion, the results from the present study
suggest that TP4O induces apoptosis in CRC cells through the
generation of ROS in vitro and in vivo without
damaging normal cells. Furthermore, TP4O is potentially useful for
the future development of therapies against CRC.
Acknowledgements
The authors thank Dr Yumiko N. Nagano (Department of
Gastroenterology) for her technical advice on evaluating ROS and
Mrs. Ako Takahashi (Department of Surgery, Division of
Gastroenterological and Hepatobiliary Surgery and Organ
Transplantation, Faculty of Medicine, University of Tsukuba) for
her technical assistance. The authors also thank the American
Journal Experts (Durham, NC, USA) for proofreading their English
writing. The present study was supported in part by a grant from
the Ministry of Education, Culture, Sports, Science and Technology
of Japan (Grants-in-Aid for Scientific Research; grant no.
25462069).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scott AM, Wolchok JD and Old LJ: Antibody
therapy of cancer. Nat Rev Cancer. 12:278–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gustavsson B, Carlsson G, Machover D,
Petrelli N, Roth A, Schmoll HJ, Tveit KM and Gibson F: A review of
the evolution of systemic chemotherapy in the management of
colorectal cancer. Clin Colorectal Cancer. 14:1–10. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mann J: Natural products in cancer
chemotherapy: Past, present and future. Nat Rev Cancer. 2:143–148.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the 30 years from 1981 to 2010. J Nat
Prod. 75:311–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Edris AE: Pharmaceutical and therapeutic
potentials of essential oils and their individual volatile
constituents: A review. Phytother Res. 21:308–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aggarwal BB, Prasad S, Reuter S, Kannappan
R, Yadev VR, Park B, Kim JH, Gupta SC, Phromnoi K, Sundaram VR, et
al: Identification of novel anti-inflammatory agents from Ayurvedic
medicine for prevention of chronic diseases: ‘Reverse pharmacology’
and ‘bedside to bench’ approach. Curr Drug Targets. 12:1595–1653.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gautam N, Mantha AK and Mittal S:
Essential oils and their constituents as anticancer agents: A
mechanistic view. Biomed Res Int. 2014:1541062014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sobral MV, Xavier AL, Lima TC and de Sousa
DP: Antitumor activity of monoterpenes found in essential oils.
ScientificWorldJournal. 2014:9534512014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pazyar N, Yaghoobi R, Bagherani N and
Kazerouni A: A review of applications of tea tree oil in
dermatology. Int J Dermatol. 52:784–790. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carson CF and Riley TV: Antimicrobial
activity of the major components of the essential oil of Melaleuca
alternifolia. J Appl Bacteriol. 78:264–269. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hart PH, Brand C, Carson CF, Riley TV,
Prager RH and Finlay-Jones JJ: Terpinen-4-ol, the main component of
the essential oil of Melaleuca alternifolia (tea tree oil),
suppresses inflammatory mediator production by activated human
monocytes. Inflamm Res. 49:619–626. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calcabrini A, Stringaro A, Toccacieli L,
Meschini S, Marra M, Colone M, Salvatore G, Mondello F, Arancia G
and Molinari A: Terpinen-4-ol, the main component of Melaleuca
alternifolia (tea tree) oil inhibits the in vitro growth of human
melanoma cells. J Invest Dermatol. 122:349–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu CS, Chen YJ, Chen JJ, Shieh JJ, Huang
CH, Lin PS, Chang GC, Chang JT and Lin CC: Terpinen-4-ol induces
apoptosis in human nonsmall cell lung cancer in vitro and in vivo.
Evid Based Complement Alternat Med. 2012:8182612012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Banjerdpongchai R and Khaw-On P:
Terpinen-4-ol induces autophagic and apoptotic cell death in human
leukemic HL-60 cells. Asian Pac J Cancer Prev. 14:7537–7542. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shapira S, Pleban S, Kazanov D, Tirosh P
and Arber N: Terpinen-4-ol: A novel and promising therapeutic agent
for human gastrointestinal cancers. PLoS One. 11:e01565402016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Legrand C, Bour JM, Jacob C, Capiaumont J,
Martial A, Marc A, Wudtke M, Kretzmer G, Demangel C, Duval D, et
al: Lactate dehydrogenase (LDH) activity of the cultured eukaryotic
cells as marker of the number of dead cells in the medium
[corrected]. J Biotechnol. 25:231–243. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tamura M, Matsui H, Kaneko T and Hyodo I:
Alcohol is an oxidative stressor for gastric epithelial cells:
Detection of superoxide in living cells. J Clin Biochem Nutr.
53:75–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bindokas VP, Jordán J, Lee CC and Miller
RJ: Superoxide production in rat hippocampal neurons: Selective
imaging with hydroethidine. J Neurosci. 16:1324–36. 1996.PubMed/NCBI
|
|
21
|
Toyokuni S, Tanaka T, Hattori Y, Nishiyama
Y, Yoshida A, Uchida K, Hiai A, Ochi A and Osawa T: Quantitative
immunohistochemical determination of 8-hydroxy-2′deoxyguanosine by
a monoclonal antibody N45.1: Its application to ferric
nitrilotriacetate-induced renal carcinogenesis model. Lab Invest.
76:365–374. 1997.PubMed/NCBI
|
|
22
|
Vermes I, Haanen C, Steffens-Nakken H and
Reutelingsperger C: A novel assay for apoptosis. Flow cytometric
detection of phosphatidylserine expression on early apoptotic cells
using fluorescein labelled Annexin V. J Immunol Methods. 17:39–51.
1995. View Article : Google Scholar
|
|
23
|
Britigan BE, Cohen MS and Rosen GM:
Detection of the production of oxygen-centered free radicals by
human neutrophils using spin trapping techniques: A critical
perspective. J Leukoc Biol. 41:349–362. 1987.PubMed/NCBI
|
|
24
|
Mahmoud SS and Croteau RB: Strategies for
transgenic manipulation of monoterpene biosynthesis in plants.
Trends Plant Sci. 7:366–373. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gupta SC, Prasad S, Sethumadhavan DR, Nair
MS, Mo YY and Aggarwal BB: Nimbolide, a limonoid triterpene,
inhibits growth of human colorectal cancer xenografts by
suppressing the proinflammatory microenvironment. Clin Cancer Res.
19:4465–4476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Greay SJ, Ireland DJ, Kissick HT, Levy A,
Beilharz MW, Riley TV and Carson CF: Induction of necrosis and cell
cycle arrest in murine cancer cell lines by Melaleuca alternifolia
(tea tree) oil and terpinen-4-ol. Cancer Chemother Pharmacol.
65:877–888. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brand C, Ferrante A, Prager RH, Riley TV,
Carson CF, Finlay-Jones JJ and Hart PH: The water-soluble
components of the essential oil of Melaleuca alternifolia (tea tree
oil) suppress the production of superoxide by human monocytes, but
not neutrophils, activated in vitro. Inflamm Res. 50:213–219. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim HJ, Chen F, Wu C, Wang X, Chung HY and
Jin Z: Evaluation of antioxidant activity of Australian tea tree
(Melaleuca alternifolia) oil and its components. J Agric Food Chem.
52:2849–2854. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schumacker PT: Reactive oxygen species in
cancer cells: live by the sword, die by the sword. Cancer Cell.
10:175–176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nogueira V and Hay N: Molecular pathways:
Reactive oxygen species homeostasis in cancer cells and
implications for cancer therapy. Clin Cancer Res. 19:4309–4314.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Glasauer A, Sena LA, Diebold LP, Mazar AP
and Chandel NS: Targeting SOD1 reduces experimental non-small-cell
lung cancer. J Clin Invest. 124:117–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Provinciali M, Donnini A, Argentati K, Di
Stasio G, Bartozzi B and Bernardini G: Reactive oxygen species
modulate Zn(2+)-induced apoptosis in cancer cells. Free Radic Biol
Med. 32:431–445. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuo PL, Chen CY and Hsu YL:
Isoobtusilactone A induces cell cycle arrest and apoptosis through
reactive oxygen species/apoptosis signal-regulating kinase 1
signaling pathway in human breast cancer cells. Cancer Res.
67:7406–7420. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Whibley CE, McPhail KL, Keyzers RA, Maritz
MF, Leaner VD, Birrer MJ, Davies-Coleman MT and Hendricks DT:
Reactive oxygen species mediated apoptosis of esophageal cancer
cells induced by marine triprenyl toluquinones and
toluhydroquinones. Mol Cancer Ther. 6:2535–2543. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Trachootham D, Zhou Y, Zhang H, Demizu Y,
Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J and
Huang P: Selective killing of oncogenically transformed cells
through a ROS-mediated mechanism by beta-phenylethyl
isothiocyanate. Cancer Cell. 10:241–252. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ka H, Park HJ, Jung HJ, Choi JW, Cho KS,
Ha J and Lee KT: Cinnamaldehyde induces apoptosis by ROS-mediated
mitochondrial permeability transition in human promyelocytic
leukemia HL-60 cells. Cancer Lett. 196:143–152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Noh J, Kwon B, Han E, Park M, Yang W, Cho
W, Yoo W, Khang G and Lee D: Amplification of oxidative stress by a
dual stimuli-responsive hybrid drug enhances cancer cell death. Nat
Commun. 6:69072015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Avisetti DR, Babu KS and Kalivendi SV:
Activation of p38/JNK pathway is responsible for embelin induced
apoptosis in lung cancer cells: Transitional role of reactive
oxygen species. PLoS One. 9:e870502014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ichijo H, Nishida E, Irie K, ten Dijke P,
Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K and Gotoh
Y: Induction of apoptosis by ASK1, a mammalian MAPKKK that
activates SAPK/JNK and p38 signaling pathways. Science. 275:90–94.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Toyokuni T and Akatsuka S: What has been
Learned from the Studies of Oxidative Stress-induced
Carcinogenesis: Proposal of the Concept of Oxygenomics. J Clin
Biochem Nutr. 39:3–10. 2006. View Article : Google Scholar
|
|
41
|
Perry G, Castellani RJ, Hirai K and Smith
MA: Reactive oxygen species mediate cellular damage in alzheimer
disease. J Alzheimers Dis. 1:45–55. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim YW and Byzova TV: Oxidative stress in
angiogenesis and vascular disease. Blood. 123:625–631. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rolo AP, Teodoro JS and Palmeira CM: Role
of oxidative stress in the pathogenesis of nonalcoholic
steatohepatitis. Free Radic Biol Med. 52:59–69. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Forbes JM, Coughlan MT and Cooper ME:
Oxidative stress as a major culprit in kidney disease in diabetes.
Diabetes. 57:1446–1454. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Berndtsson M, Hägg M, Panaretakis T,
Havelka AM, Shoshan MC and Linder S: Acute apoptosis by cisplatin
requires induction of reactive oxygen species but is not associated
with damage to nuclear DNA. Int J Cancer. 120:175–180. 2007.
View Article : Google Scholar : PubMed/NCBI
|