Introduction
Squamous cell carcinoma (SCC) is the most common
skin malignancy, with aggressive behavior and poor prognosis at the
advanced stage. Surgery remains the first choice of SCC treatment,
but the highly invasive tendency and chemoresistance to local
therapy of SCC cells usually lead to in situ occurrence and
distal dissemination (1). Therefore,
exploring a better adjuvant therapy for advanced skin SCCs would
improve patients' life quality and survival rates.
Resveratrol possesses a wide range of biological
activities, including cancer preventive and therapeutic effects
(2). Previous studies performed on
rodent models revealed that resveratrol regulated apoptosis and
cell survival in mouse skin tumors (3), and exerted chemopreventive effects
against ultraviolet-B exposure-mediated damages in SKH-1 hairless
mouse skin (4). Nevertheless, the
impact of resveratrol on human epidermal SCCs has been less
described. The current study therefore aims to investigate i) the
biological effects of resveratrol on human epidermal SCC cells; ii)
the status of Wnt signaling in resveratrol-treated cells; and iii)
the response of normally cultured and resveratrol-treated Colo16
cells when Wnt signal transduction is specifically suppressed by
β-catenin-specific small interfering RNA (siRNA).
Materials and methods
Cells and treatment
Colo16 human cutaneous SCC cells were cultured in
RPMI 1640 medium supplemented with 10% fetal bovine serum (both
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in
5% CO2 (5). Resveratrol
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was dissolved in
dimethylsulfoxide (DMSO) and diluted with culture medium to 100 µM
just prior to use. The treatments lasted for 72 h, and the cells
were collected in 24-h intervals for different experimental
purposes. Normally cultured cells and cells cultured in medium
containing 0.2% DMSO were used as controls. The experiments were
repeated ≥3 times to establish a reliable conclusion.
Cellular and molecular
examinations
Hematoxylin and eosin (H&E) morphological
staining and immunocytochemical (ICC) staining for Ki-67 (sc-23900;
Santa Cruz, Biotechnology, Inc., Dallas, TX, USA), Wnt2 (sc-514382;
Santa Cruz Biotechnology, Inc.), Wnt5a (sc-365376; Santa Cruz
Biotechnology, Inc.), β-catenin (sc-7963; Santa CruzBiotechnology,
Inc.), cyclin D1 (sc-8396; Santa Cruz Biotechnology, Inc.), c-Myc
(sc-40; Santa Cruz Biotechnology, Inc.), vascular endothelial
growth factor (VEGF; sc-7269; Santa Cruz Biotechnology, Inc.),
surviving (sc-17779; Santa Cruz, Biotechnology, Inc.) and Axin2
(BS7417; Bioworld Technology, Inc., St Louis Park, MN, USA) were
performed on cell-bearing coverslips by methods described elsewhere
(6,7).
The proliferation activity and death pattern of resveratrol-treated
Colo16 cells were further analyzed by flow cytometry (BD
Biosciences, San Jose, CA, USA) and terminal deoxynucleotidyl
transferase dUTP nick-end labeling colorimetric apoptotic cell
assay (Promega Corporation, Madison, WI, USA), as previously
described (7). The cell viability was
determined after treatment for 24 or 48 h with different
concentrations of resveratrol using an MTT assay as previously
described (7). The results are
presented as the percentage of cell viability [optical density (OD)
of the experiment samples/OD of the control] or OD values. The 50%
inhibitory concentration (IC50) value was statistically analyzed by
SPSS version 15.0 (SPSS, Inc., Chicago, IL, USA). RNA and protein
samples were prepared from the experimental groups and subjected to
reverse transcription-polymerase chain reaction (RT-PCR) and
western blot analyses for the same parameters evaluated by ICC
staining (Table I)(6,8).
 | Table I.The primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
The primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primers | Amplicon Size
(bp) | Annealing temperature
(°C) |
|---|
| Wnt2 | F:
5′-GCCACACGCTGCACCTAAAGC-3′ |
|
|
|
| R:
5′-CAATTACCCTAAGGGTGGTAGC-3′ | 379 | 63 |
| Wnt5a | F:
5′-CTAACTTAGCTGTGTGGGACATG-3′ |
|
|
|
| R:
5′-AAATGCAGAAAGCAAGCTAGCAG-3′ | 254 | 60 |
| Axin2 | F:
5′-GGTGTTTGAGGAGATCTGGG-3′ |
|
|
|
| R:
5′-TGCTCACAGCCAAGACAGTT-3′ | 153 | 58 |
| Survivin | F:
5′-GGCATGGGTGCCCCGACGTTG-3′ |
|
|
|
| R:
5′-CAGAGGCCTCAATCCATGGCA-3′ | 439 | 58 |
| c-myc | F:
5′-TGGTCTTCCCCTACCCTCTCAAC-3′ |
|
|
|
| R:
5′-GATCCAGACTCTGACCTTTTGCC-3′ | 265 | 56 |
| Cyclin D1 | F:
5′-CTGTGCTGCGAAGTGGAAACCAT-3′ |
|
|
|
| R:
5′-TTCATGGCCAGCGGGAAGACCTC-3′ | 257 | 57 |
| VEGF | F:
5′-CGAAGTGGTGAAGTTCATGGATG-3′ |
|
|
|
| R:
5′-TTCTGTATCAGTCTTTCCTGGT-3′ | 470 | 60 |
| β-actin | F:
5′-GCATGGAGTCCTGTGGCAT-3′ |
|
|
|
| R:
5′-CATGAAGCATTTGCGGTGG-3′ | 326 | 58 |
Transfection of β-catenin RNA
interference (RNAi)
Since β-catenin is the central player of
Wnt2-mediated signaling (9), the
influence of β-catenin downregulation in the resveratrol
sensitivity of Colo16 cells was evaluated by RNAi transfection
according to the manufacturer's protocol (Roche Diagnostics GmbH,
Mannheim, Germany). Three RNAi candidate sequences for the
β-catenin transcript were transfected into the cells for 24 h at a
final concentration of 0.5 nmol/l. Scrambled oligonucleotides (mock
RNA; sense 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense
5′-ACGUGACACGUUCGGAGA-3′) and p53 siRNAs (sense
5′-CUACUUCCUGAAAACAACGdTdT-3′ and antisense
5′-CGUUGUUUUCAGGAAGUAGdTdT-3′) were used as negative and positive
controls of transfection efficiency, respectively. The siRNAs were
synthesized by Shanghai Genepharma, Co., Ltd., Shanghai, China.
Upon ascertaining the efficiency of β-catenin inhibition according
to the gray analysis, Colo16 transfectants were further treated
with 100 µM resveratrol, and the cellular responses were evaluated
by H&E staining, flow cytometry and ICC staining (7).
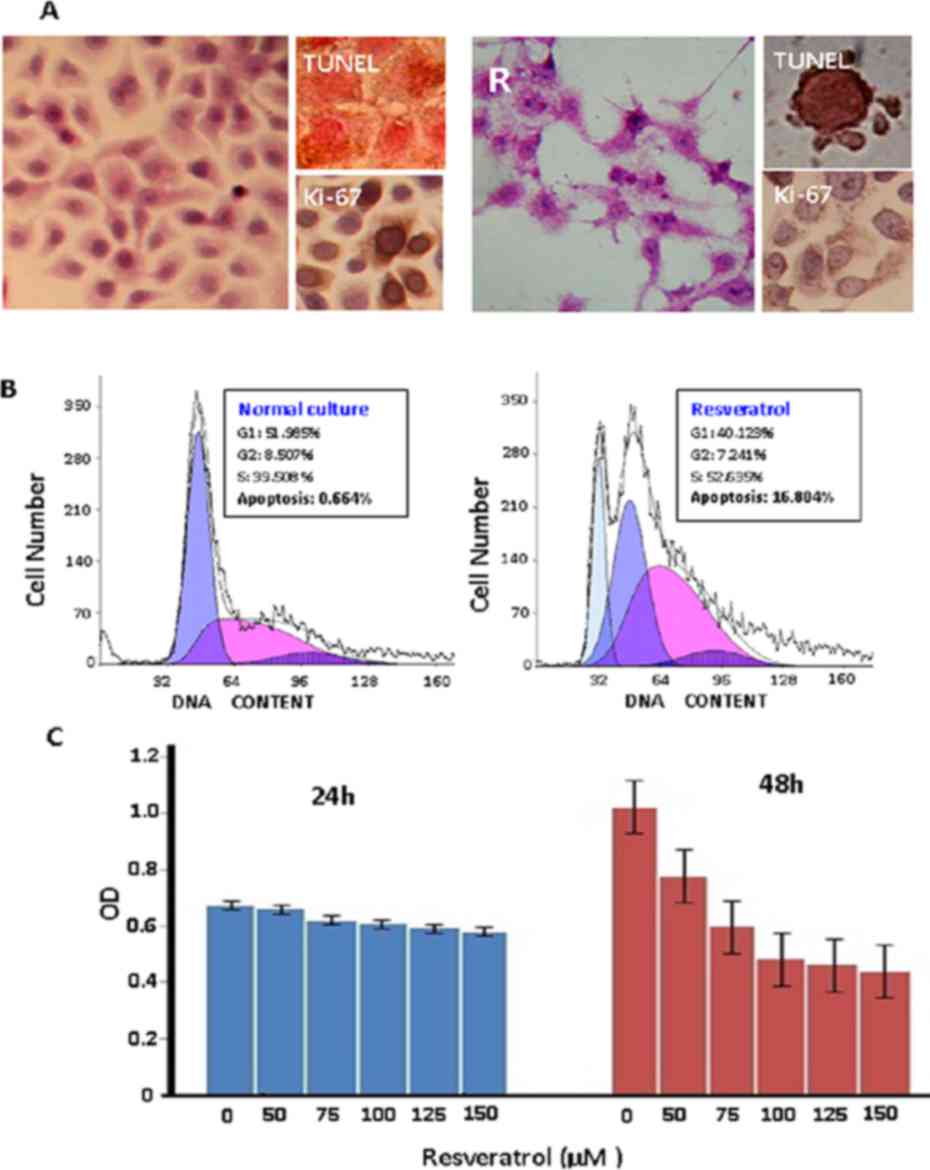
Results
Resveratrol inhibits the growth of
Colo16 cells
Colo16 SCC cells were treated with 0, 50, 75, 100,
125 and 150 µM resveratrol for 72 h. The IC50 value was determined
to be 113.79 µM (data not shown); therefore, 100 µM resveratrol was
selected to treat Colo16 SCC cells in subsequent experiments. After
36 h of treatment, decreased Ki-67 production and apoptotic
phenotypes could be observed in the Colo16 cell population, which
became particularly distinct at 48 h. The majority of treated cells
died at 72 h (Fig. 1A). Flow
cytometry analysis further demonstrated accumulation of S-phase
cells and increased apoptotic fraction (16.804%) in
resveratrol-treated cells at 48 h (Fig.
1B) and MTT assay showed growth suppression (Fig 1C).
Resveratrol inhibits Wnt
activation
The expression of Wnt2, Wnt5a and four target genes
of the classical Wnt signaling pathway in Colo16 cells with and
without resveratrol treatment was examined by RT-PCR and western
blot analyses. As shown in Fig. 2,
constitutive expression of Wnt2, Wnt5a, survivin, VEGF, cyclin D1
and c-Myc was observed in Colo16 cells under normal culture
conditions. Upon resveratrol treatment, Wnt2, survivin, cyclin D1,
c-Myc and VEGF transcription was downregulated, while the levels of
Wnt5a expression were almost unchanged. As presented in Table II, the expression of Axin2, a
negative regulator of the Wnt signaling pathway, was upregulated in
resveratrol-treated Colo16 cells (10).
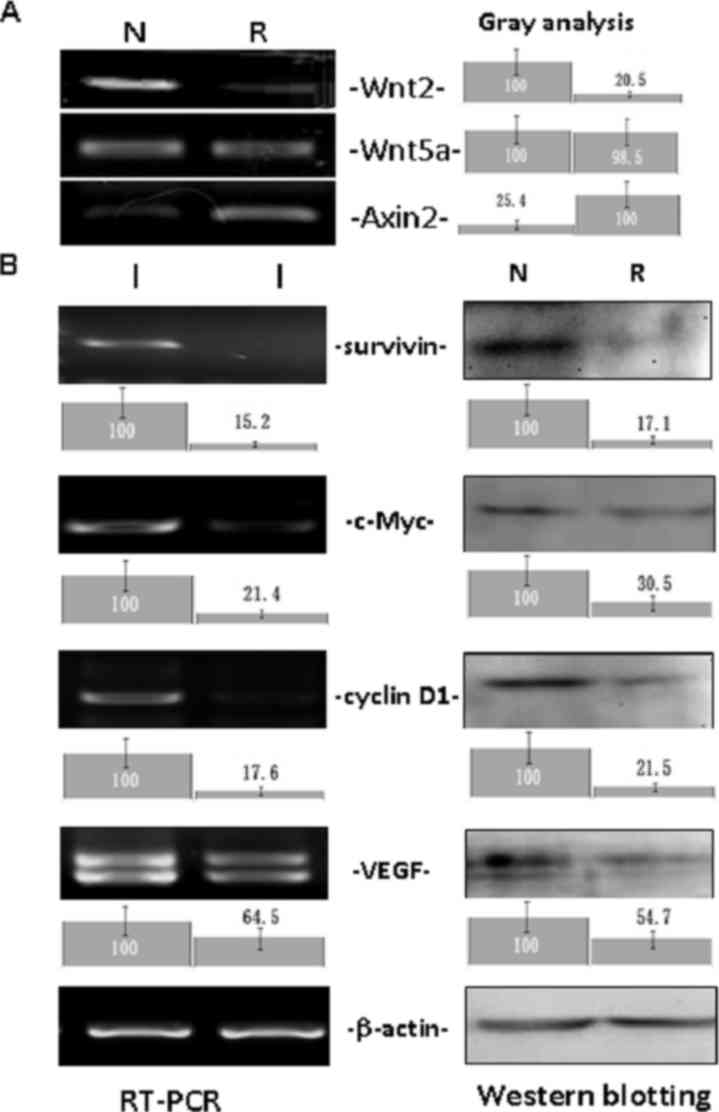 | Figure 2.Analyses of Wnt2, Wnt5a, Axin2 and Wnt
target gene (survivin, c-Myc, cyclin D1 and VEGF) expression in
Colo16 cells without and with 100 µM resveratrol treatment for 48 h
by RT-PCR and western blotting. β-actin was used as a quantitative
control. Gray density analysis was conducted on the Wnt2, Wnt5a,
Axin2 and Wnt target gene data. N, normal culture; R, 100 µM
resveratrol treatment; VEGF, vascular endothelial growth factor;
RT-PCR, reverse transcription-polymerase chain reaction. |
 | Table II.Resveratrol-regulated gene expression
in Colo16 cells. |
Table II.
Resveratrol-regulated gene expression
in Colo16 cells.
| Gene | ICC | Western blotting | RT-PCR |
|---|
| Wnt2 | ↓ | ― | ↓ |
| Wnt5a | ― | ― | ― |
| Axin2 | ↑ | ↑ | ↑ |
| Survivin | ↓ | ↓ | ↓ |
| c-Myc | ↓ | ↓ | ↓ |
| cyclin D1 | ↓ | ↓ | ↓ |
| VEGF | ↓ | ↓ | ↓ |
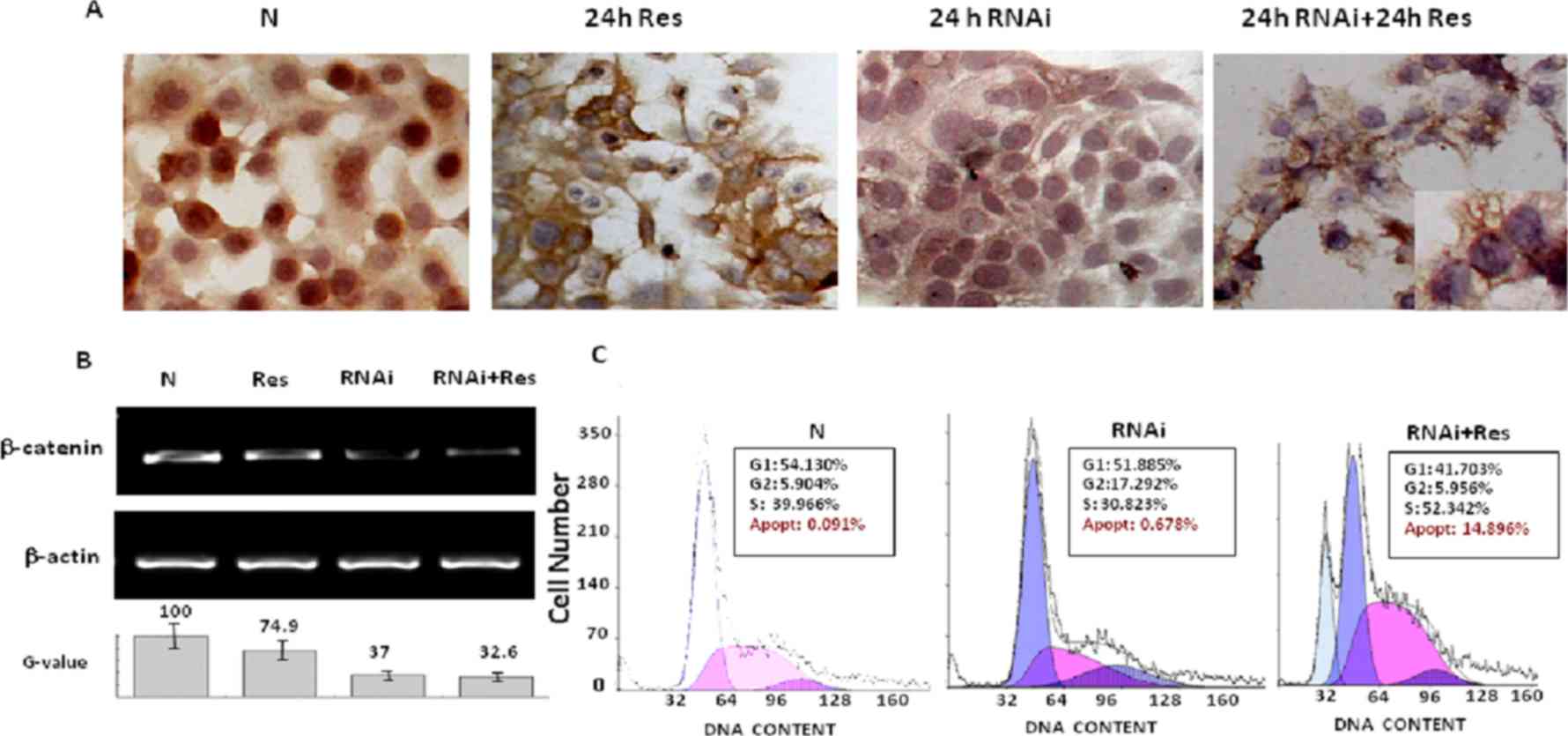
Enhanced resveratrol sensitivity by
β-catenin siRNA transfection
RT-PCR analysis revealed that β-catenin expression
was inhibited by siRNA approach. According to the results of gray
analysis, the siRNA candidates in the sequences of sense
5′-GCUUUAUUCUCCCAUUGAATT-3′ and antisense
5′-UUCAAUGGGAGAAUAAAGCAG-3′ exhibited a marked inhibitory effect
(63%) on β-catenin expression (Fig.
3B), which was accompanied with less frequent β-catenin nuclear
translocation in the transfectants (Fig.
3A). Distinct apoptosis (14.896%) appeared much earlier in
resveratrol-treated transfectants than in Colo16 cells treated by
resveratrol only (Fig. 3A and C).
Almost all cells in the Res + RNAi group died at the 48-h time
point.
Discussion
Resveratrol exerts therapeutic effects on different
types of human malignancies at a concentration of 100 µM (11–13), while
its influence in the growth and survival of human cutaneous SCCs
remains obscure. To shed light on this issue, Colo16 cutaneous SCC
cells were treated with 100 µM resveratrol in the present study.
Decreased Ki-67 production and apoptotic phenotypes in the Colo16
cell population were markedly distinct at 48 h, and the majority of
treated cells died at 72 h. Accumulation of S-phase cells and
increased apoptotic fraction (16.804%) in resveratrol-treated cells
were observed at 48 h. These results suggest that Colo16 is a
resveratrol-sensitive human SCC cell line.
Frequent activation of Wnt signaling has been
observed in human cutaneous SCC cells (6), but its biological importance in these
cells is less known. Therefore, RT-PCR and western blot analyses
were performed in the present study to detect the expression of
Wnt2, Wnt5a and target genes of the classical Wnt signaling pathway
in Colo16 cells. The results suggest that resveratrol can lead
Colo16 cells to growth arrest and apoptosis, probably due to
inhibition of the classical Wnt signaling pathway and
downregulation of certain Wnt target genes that are critical for
human SCCs (6,14,15). This
notion was further supported by the remarkable upregulation of
Axin2 expression in resveratrol-treated Colo16 cells, since Axin2
acts as a negative regulator of the Wnt signaling pathway by
promoting glycogen synthase kinase 3β-dependent phosphorylation of
β-catenin (16). The unchanged Wnt5a
expression in resveratrol-treated Colo16 cells observed in the
present study was not unexpected, as Wnt5a mediates non-canonical
(i.e. β-catenin-independent) signaling and functions as a negative
regulator of Wnt/β-catenin activity (17) or as a tumor-suppressor gene (18).
β-catenin serves a central role in the classical Wnt
signaling pathway through nuclear translocation to initiate Wnt
target gene transcription (9). To
ascertain the importance of Wnt activation in Colo16 cells,
β-catenin expression was inhibited by siRNA approach in the present
study. The results revealed that β-catenin siRNA treatment by
itself failed to cause cell death, suggesting that the suppressed
biological activities of Wnt signaling may be compensated by other
activated signaling pathways to maintain the survival of Colo16
cells. Furthermore, distinct apoptosis (14.896%) appeared much
earlier in resveratrol-treated transfectants than in Colo16 cells
treated with resveratrol only. This phenomenon further suggested
multiple molecular targeting features of resveratrol in cancer
cells (13,19). In this context, resveratrol treatment
rather than a signaling-specific strategy may achieve better
therapeutic effects on human epidermal SCCs.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81450016,
81272786, 81071971, 81072063 and 30971038), Research Fund for PhD
supervisors from the National Education Department of China (grant
no. 20122105110005), Program Fund for Liaoning Excellent Talents in
University (grant no. LJQ2012078) and Program Fund for Liaoning
Natural Science Foundation (grant nos. 2013023050 and
2013023040).
References
|
1
|
Kim RH and Armstrong AW: Nonmelanoma skin
cancer. Dermatol Clin. 30125–139. (ix)2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singh CK, Ndiaye MA and Ahmad N:
Resveratrol and cancer: Challenges for clinical translation.
Biochim Biophys Acta. 1852:1178–1185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
George J, Singh M, Srivastava AK, Bhui K,
Roy P, Chaturvedi PK and Shukla Y: Resveratrol and black tea
polyphenol combination synergistically suppress mouse skin tumors
growth by inhibition of activated MAPKs and p53. PLoS One.
6:e233952011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aziz MH, Reagan-Shaw S, Wu J, Longley BJ
and Ahmad N: Chemoprevention of skin cancer by grape constituent
resveratrol: Relevance to human disease? FASEB J. 19:1193–1195.
2005.PubMed/NCBI
|
|
5
|
Moore GE, Merrick SB, Woods LK and Arabasz
NM: A human squamous cell carcinoma cell line. Cancer Res.
35:2684–2688. 1975.PubMed/NCBI
|
|
6
|
Li Y, Liu ZL, Zhang KL, Chen XY, Kong QY,
Wu ML, Sun Y, Liu J and Li H: Methylation-associated silencing of
S100A4 expression in human epidermal cancers. Exp Dermatol.
18:842–848. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xia SL, Wu ML, Li H, Wang JH, Chen NN,
Chen XY, Kong QY, Sun Z and Liu J: CRABP-II- and FABP5-independent
responsiveness of human glioblastoma cells to all-trans retinoic
acid. Oncotarget. 6:5889–5902. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu ZL, Li Y, Kong QY, Zhan C, Wang Q,
Chen XY, Sun Y, Wen S, Tu CX, Liu J and Li H: Immunohistochemical
profiling of Wnt, NF-kappaB, Stat3 and Notch signaling in human
epidermal tumors. J Dermatol Sci. 52:133–136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Skalka N, Caspi M, Caspi E, Loh YP and
Rosin-Arbesfeld R: Carboxypeptidase E: A negative regulator of the
canonical Wnt signaling pathway. Oncogene. 32:2836–2847. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu ZQ, Brabletz T, Fearon E, Willis AL, Hu
CY, Li XY and Weiss SJ: Canonical Wnt suppressor, Axin2, promotes
colon carcinoma oncogenic activity. Proc Natl Acad Sci USA. 109:pp.
11312–11317. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mikuła-Pietrasik J, Sosińska P and Książek
K: Resveratrol inhibits ovarian cancer cell adhesion to peritoneal
mesothelium in vitro by modulating the production of α5β1 integrins
and hyaluronic acid. Gynecol Oncol. 134:624–630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Q, Li H, Wang XW, Wu DC, Chen XY and
Liu J: Resveratrol promotes differentiation and induces
Fas-independent apoptosis of human medulloblastoma cells. Neurosci
Lett. 351:83–86. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wen S, Li H, Wu ML, Fan SH, Wang Q, Shu
XH, Kong QY, Chen XY and Liu J: Inhibition of NF-κB signaling
commits resveratrol-treated medulloblastoma cells to apoptosis
without neuronal differentiation. J Neurooncol. 104:169–177. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Klaus A and Birchmeier W: Wnt signalling
and its impact on development and cancer. Nat Rev Cancer.
8:387–398. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Malanchi I, Peinado H, Kassen D, Hussenet
T, Metzger D, Chambon P, Huber M, Hohl D, Cano A, Birchmeier W and
Huelsken J: Cutaneous cancer stem cell maintenance is dependent on
beta-catenin signalling. Nature. 452:650–653. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bernkopf DB, Hadjihannas MV and Behrens J:
Negative-feedback regulation of the Wnt pathway by conductin/axin2
involves insensitivity to upstream signalling. J Cell Sci.
128:33–39. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bisson JA, Mills B, Helt JC Paul, Zwaka TP
and Cohen ED: Wnt5a and Wnt11 inhibit the canonical Wnt pathway and
promote cardiac progenitor development via the Caspase-dependent
degradation of AKT. Dev Biol. 398:80–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abdelmaksoud-Dammak R, Miladi-Abdennadher
I, Saadallah-Kallel A, Khabir A, Sellami-Boudawara T, Frikha M,
Daoud J and Mokdad-Gargouri R: Downregulation of WIF-1 and Wnt5a in
patients with colorectal carcinoma: Clinical significance. Tumour
Biol. 35:7975–7982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu LJ, Wu ML, Li H, Chen XY, Wang Q, Sun
Y, Kong QY and Liu J: Inhibition of STAT3 expression and signaling
in resveratrol-differentiated medulloblastoma cells. Neoplasia.
10:736–744. 2008. View Article : Google Scholar : PubMed/NCBI
|