Introduction
Esophageal cancer (EC) is associated with an
increased mortality rate globally (1). Despite improving treatments, combining
surgical resection with chemotherapy or radiotherapy remains a
suboptimal treatment option due to early local invasion and
systemic metastasis (2–4). The prognosis for EC remains poor and the
5-year overall survival rate in the USA was 4% in the 1970s and
increased to 14% in the 1990s (5,6). Previous
studies have demonstrated that multiple biological markers may not
only facilitate the diagnosis and treatment of EC but may also help
to predict early recurrence and clinical outcomes following surgery
(7–11).
MACC1 was first identified during a genome-wide
screening of human colon cancer tissues and its abnormal expression
is associated with the metastasis and recurrence of colon cancer
through the regulation of the hepatocyte growth factor-MET
proto-oncogene signaling pathway (12). Furthermore, previous studies have
revealed that the abnormal expression of MACC1 may be associated
with the development and progression of numerous types of solid
tumor, including lung (13,14) and gastric cancer (15–18),
hepatocellular carcinoma (19–21),
breast cancer (22), glioma (23) and colon (12,24) and
esophageal cancer (25). These
studies suggested that MACC1 may serve as a key biomarker for
recurrence, metastasis and patient survival in multiple types of
human cancer. To the best of our knowledge, few studies regarding
the function of MACC1 in EC have been performed. The present study
evaluated the function of MACC1 in EC cells. EC cell viability,
invasion, migration and apoptosis potential were assessed by
regulating the expression of MACC1.
Materials and methods
Cell culture and treatment
The human EC cell lines Eca109 and TE1 were
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China) and cultured in RPMI-1640 medium
containing 10% fetal bovine serum (FBS) (both from Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 100 IU/ml penicillin G
and 100 µg/ml streptomycin at 37°C in a humidified 5%
CO2 incubator. For the analysis of cell viability and
mobility, the Eca109 and TE1 cells were pretreated with 500 nM
phosphatase and tensin homolog (PTEN) inhibitor SF1670 at 37°C for
30 min (Gene Operation LLC, Ann Arbor, MI, USA).
Small interfering (si)RNA
transfection
siRNA duplexes were synthesized and purified by
Suzhou GenePharma LLC (Suzhou, China). siRNA was diluted to 100 nM
with serum-free culture medium. The siRNA sequences for MACC1 were
as follows: Forward, 5′-AAGAGGGGACGGGGACACGGCTT-3′ and reverse,
5′-TTGGCGAACCGGAACAGGGGACG-3′. The transfection of MACC1-siRNA into
the experimental Eca109 and TE1 cells was performed using
Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The transfection of
MACC1-NC (forward, 5′-CCAGTTAAGAACGTCCCCAAGCG-3′ and reverse,
5′-AAGCTTGAGGTCTAGGTAATTTC-3′) into the scramble control Eca109 and
TE1 cells, was conducted using the same protocol.
Western blot analysis
Protein was extracted from the transfected Eca109
and TE1 cells (1×106) cells using
radioimmunoprecipitation assay lysis buffer containing 1/100
phenylmethanesulfonyl fluoride (P0013; Beyotime Institute of
Biotechnology, Haimen, China) at 0–4°C for 30 min. The supernatant
was collected at 3,000 × g in 4°C for 30 min. The total protein
concentration was determined using the bicinchoninic acid method.
Protein (20 µg/lane) was separated using SDS-PAGE on a 10% gel and
subsequently transferred to polyvinylidene fluoride membranes. The
membranes were blocked with 5% nonfat milk at room temperature for
1 h and then incubated overnight at 4°C with primary antibodies.
The membranes were then washed three times in TBS containing 0.05%
Tween-20 and incubated with AP-labeled goat anti-mouse/rabbit IgG
(A0258/A0239; 1:5,000; Beyotime Institute of Biotechnology) for 2 h
at room temperature. The secondary antibodies were detected using
an enhanced chemiluminescence kit according to the manufacturer's
protocol (Pierce; Thermo Fisher Scientific, Inc.). The primary
antibodies were goat polyclonal anti-MACC1 (catalog no. HPA020081;
dilution, 1:1,000; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany),
rabbit monoclonal anti-caspase-3 (catalog no. CST9662), rabbit
monoclonal anti-cleaved caspase-3 (catalog no. 9664), rabbit
monoclonal anti-Bcl-2 (catalog no. 2872) and rabbit monoclonal
anti-Bax (catalog no. 5023) (dilution, 1:1,000; all from Cell
Signaling Technology, Inc., Danvers, MA, USA), rabbit monoclonal
anti-PTEN (catalog no. ab32199), mouse monoclonal anti-PCNA
(catalog no. 29), rabbit monoclonal anti-Akt (pan; catalog no.
85683), rabbit monoclonal anti-Akt (phospho S473; catalog no.
81283) (dilution, 1:1,000; all from Abcam, Cambridge, UK), and
mouse monoclonal anti-GAPDH (catalog no. KC-5G5; dilution, 1:5,000;
Kangcheng Biotechnology Co., Ltd., Nanjing, China). GAPDH protein
was used as the internal control.
Cell viability assay
The viability of Eca109 and TE1 cells under the
aforementioned pretreatments was evaluated using an MTT assay.
Eca109 and TE1 cells were seeded onto 96-well plates (8,000
cells/well) with RPMI-1640 medium and transfected with the siRNA
vectors as aforementioned at 37°C. Following incubation for 48 h at
37°C, 5 mg/ml MTT was added to each well and the cells were
incubated for a further 4 h at 37°C. Following the addition of 150
ml dimethyl sulfoxide to each well, the absorbance was measured at
490 nm (ELX800; BioTek Instruments, Inc., Winooski, VT, USA). The
experiment was repeated three times.
Soft agar colony formation assay
To assess colony formation, Eca109 and TE1 cells
were collected following treatment, and 1×104 cells were
mixed with 0.6% agar solution in RPMI-1640 medium containing 20%
FBS and placed on a 1.2% agar layer in 6-well tissue culture
plates. Following solidification of the gel, the cells were
incubated for 2–3 weeks in a 5% CO2, humidified
incubator at 37°C until colonies formed, during which time,
RPMI-1640 supplemented with 10% FBS was changed every 3 or 4 days.
The colonies containing >50 cells were subsequently counted
using a light microscope. Clones were counted in 10 random fields
at ×100 magnification and the mean ± standard deviation (SD) was
calculated. The experiment was repeated three times.
Cell migration and invasion
assays
Eca109 and TE1 cell migration and invasion were
evaluated using Transwell assays (EMD Millipore, Billerica, MA,
USA), according to the manufacturer's protocol. For the invasion
assay, 5×104 cells in total were seeded onto a Transwell
insert (pore size, 8 mm) coated with extracellular matrix (50 mg/l
Matrigel, 1:6 dilution with serum-free medium; BD Biosciences,
Franklin Lakes, NJ, USA). Subsequently, 100 µl serum-free RPMI-1640
medium containing 10 g/l bovine serum albumin (ST023; Beyotime
Institute of Biotechnology) was added to the upper chamber, and 500
µl complete culture medium was added to the lower chamber. Cells
were incubated at 37°C for 24 h for both the migration and invasion
assays. For the migration assay, 5×104 Eca109 and TE1
cells were seeded onto a Transwell insert, which was not coated
with Matrigel. Following incubation at 37°C for 24 h, the cells
adherent to the upper surface of the filter were removed using a
cotton applicator and stained with 0.5% crystal violet at room
temperature for 15 min. Cell numbers were counted in 10 random
fields at ×100 magnification by light microscope. The values
obtained were calculating the mean ± standard deviation (SD) from
triplicates of each assay.
Apoptosis assays
Eca109 and TE1 cells were seeded onto 6-well plates
(5×105 cells/well) with RPMI-1640 complete culture
medium. Following 48 h of transfection as aforementioned, the cells
were collected, including the supernatant of the culture medium, by
centrifugation at 250 × g for 3 min at room temperature, and the
cells in each well were washed with PBS twice. The cells were
subsequently incubated at room temperature with 5 µl Annexin
V-fluorescein isothiocyanate in 195 µl binding buffer (catalog no.
C1063; Beyotime Institute of Biotechnology) in darkness for 10 min.
Cells were centrifuged at 250 × g for 5 min at room temperature and
resuspended in 190 ml binding buffer and 10 ml propidium iodide.
All samples were analyzed for apoptosis using the BD FACSVerse™
(651156) flow cytometer (BD Biosciences). The BD FACSuite™ software
(11.10; BD Biosciences) was used to analyze the data. The
experiment was repeated three times.
Immunocytochemistry
Eca109 and TE1 cells (2×105) were
cultured on coverslips in 6-well plates with RPMI-1640 complete
culture medium and transfected with MACC1-siRNA as aforementioned.
Following 48 h of culture, the cells were washed with PBS, fixed
with 2% w/v paraformaldehyde for 15 min and permeabilized with 1%
v/v Triton X-100 for 10 min at room temperature. Subsequently,
blocking was achieved through incubation with 10% w/v normal goat
serum (SP Kit-B2; Fuzhou Maixin Biotech. Co., Ltd., Fuzhou, China)
in PBS at room temperature for 1 h. Cells were then incubated with
MACC (catalog no. HPA020081; dilution, 1:100; Sigma-Aldrich; Merck
KGaA), PTEN (catalog no. ab32199; dilution, 1:100) and p-Akt
(phospho S473; catalog no. ab81283; dilution, 1:200) (both from
Abcam) primary antibodies at 4°C overnight. Subsequently, the cells
were washed with PBS, and incubated for 1 h with Cy3-labeled
secondary antibody (catalog no. A0516; dilution, 1:400; Beyotime
Institute of Biotechnology) at room temperature, and subsequently
counterstained with DAPI (1 µg/ml) for 5 min at room temperature
(Sigma-Aldrich; Merck KGaA). Images of the immunostained cells were
obtained using a fluorescent microscope (×200 magnification).
Statistical analysis
All statistical analyses were performed using SPSS
18.0 statistical software (SPSS, Inc., Chicago, IL, USA). The
results are presented as the mean ± SD as appropriate. Student's
t-test was used to compare viability, migration, invasion and
apoptosis between the groups. P<0.05 was considered to indicate
a statistically significant difference.
Results
Decreased MACC1 expression
significantly inhibits EC cell viability in vitro
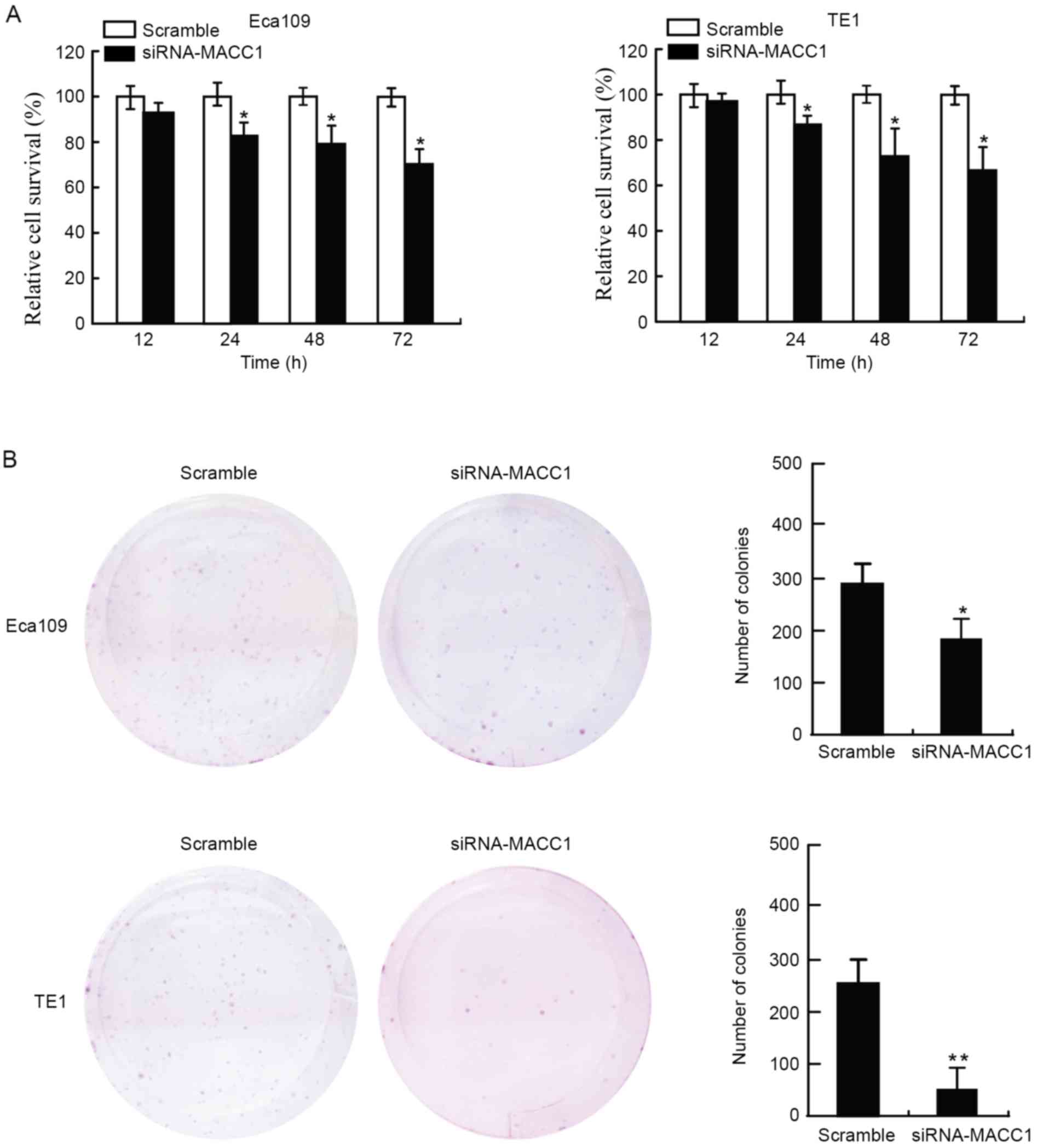
Cell viability was assessed using an MTT assay. The
present study evaluated four time-points (12, 24, 48 and 72 h)
following transfection of the EC cells with siRNA-MACC1. The
results revealed that EC cells transfected with siRNA-MACC1
exhibited significantly decreased cell viability compared with
those transfected with the scramble control (P<0.05; Fig. 1A). The inhibition rate of siRNA-MACC1
on EC cell viability was 5–35%. The present study evaluated the
rate of colony formation of the EC cells 2 weeks following
treatment. The results suggested that the EC cells transfected with
siRNA-MACC1 formed fewer colonies compared with those transfected
with the scramble control (P<0.05, P<0.01; Fig. 1B). The results of the present study
suggested that siRNA-MACC1 inhibited the viability and neoplastic
capacity of EC cells in vitro.
Downregulating MACC1 contributes to
the inhibition of migration and invasion of EC cells in vitro
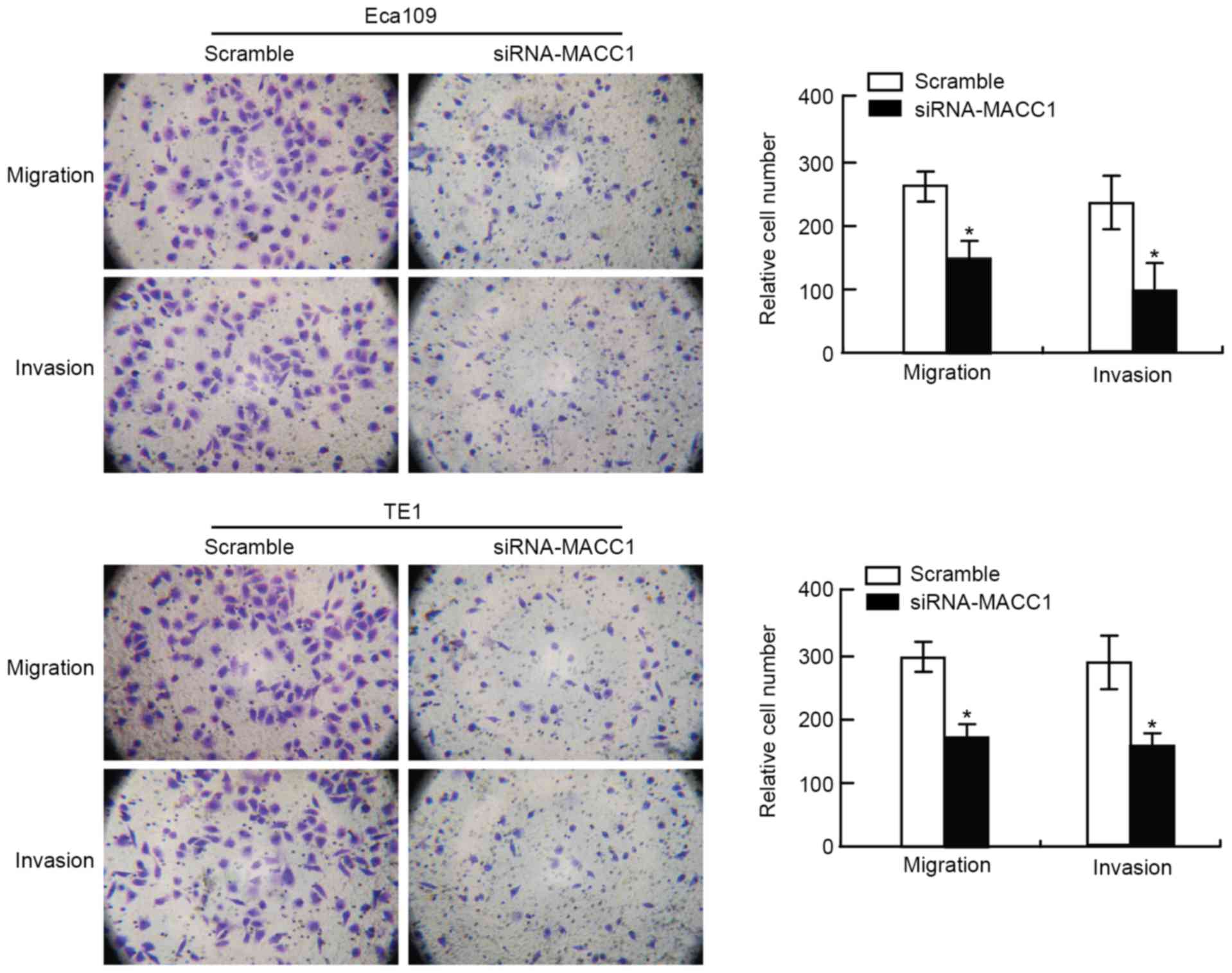
To assess the effect of siRNA-MACC1 on cell
motility, the EC cells underwent migration and invasion assays
following transfection. The results demonstrated that the number of
migratory and invasive cells in the siRNA-MACC1-transfected EC cell
group was significantly decreased compared with that in the
scramble control EC cell group (P<0.05; Fig. 2). These results suggested that the
knockdown of MACC1 contributed to the inhibition of migration and
invasion of EC cells in vitro. The present study suggested
that MACC1 serves a key function in the regulation of EC cell
motility, including invasion and metastasis.
Knockdown of MACC1 markedly induces
apoptosis of EC cells in vitro
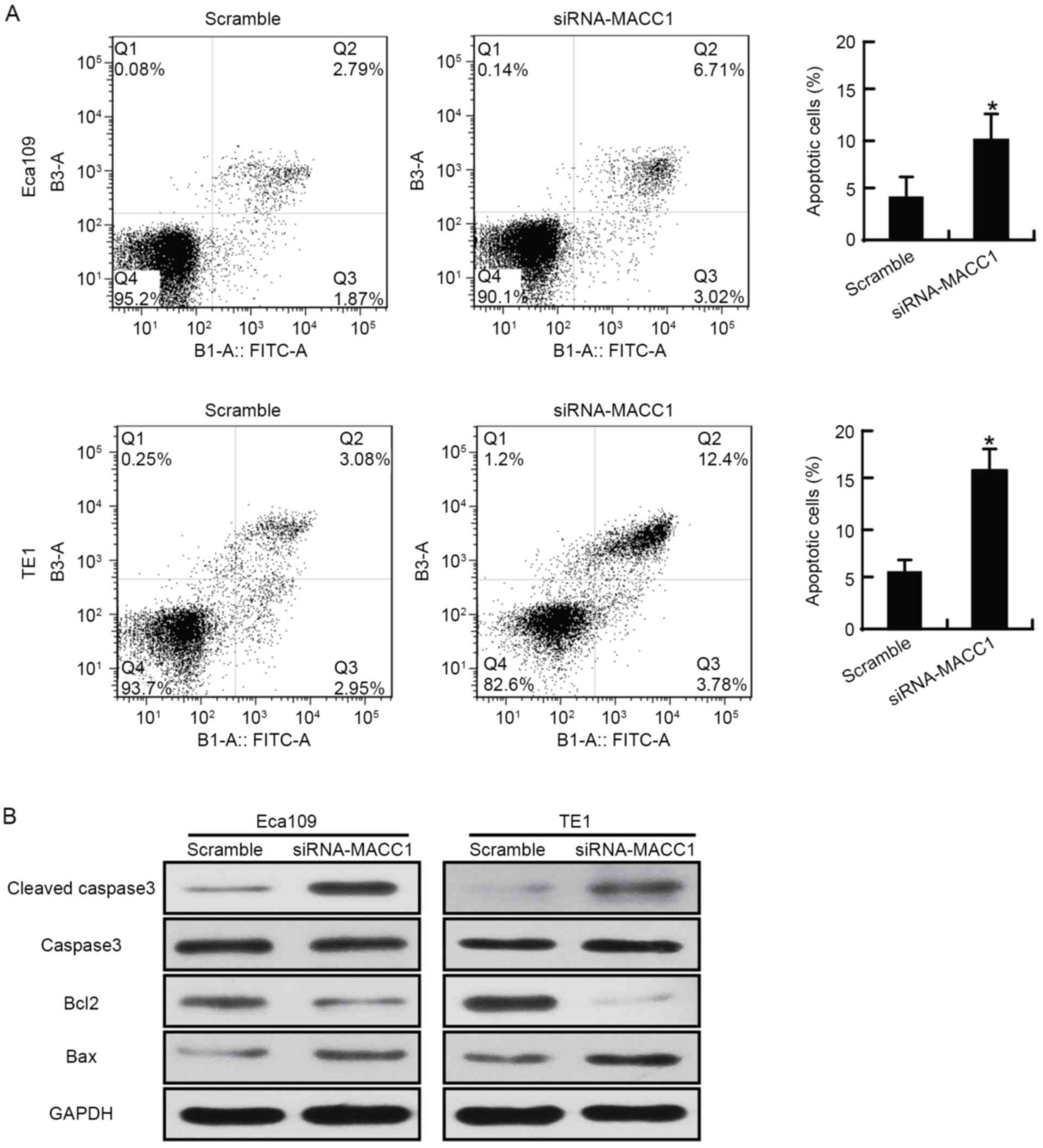
The apoptosis assay revealed that the apoptotic rate
of the siRNA-MACC1-transfected Eca109 cells (9.71±2.89%) was
increased compared with that of the scramble control Eca109 cells
(4.79±1.65%); that of the siRNA-MACC1-transfected TE1 cells
(15.32±2.45%) was increased compared with that of the scramble
control TE1 cells (6.08±0.87%). The difference in apoptotic rate
between these groups was statistically significant in the two cell
lines (P<0.05; Fig. 3A). The
expression of B-cell lymphoma 2 (BCL2), an anti-apoptotic
indicator, was downregulated, and the expression of
apoptosis-associated cleaved caspase-3 and BCL2 associated X was
upregulated, in siRNA-MACC1-transfected EC cells compared with that
in the scramble control EC cells (Fig.
3B). The present study demonstrated that downregulating MACC1
significantly induced apoptosis in EC cells in vitro.
Decreasing MACC1 expression suppresses
the PTEN/phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)
signaling pathway of EC cells
The present study evaluated the effect of
downregulating MACC1 expression on PTEN and the PI3K/Akt signaling
pathway in EC cells (Fig. 4). The
siRNA-MACC1-transfected EC cells revealed a significant decrease in
MACC1 protein expression compared with that in the scramble control
EC cells (Fig. 4B). In the EC cells
in which MACC1 expression was downregulated, compared with the
scramble control EC cells, the expression of PTEN was increased,
the expression of total Akt remained unaltered, and the expression
of phosphorylated (p)Akt and proliferating cell nuclear antigen was
decreased (Fig. 4B). The present
study obtained similar results from immunofluorescence experiments
(Fig. 4A). Furthermore, the present
study used the PTEN inhibitor SF1670 to assess the effect of PTEN
on signaling pathways in the EC cells. The results revealed that
treatment with 500 nM SF1670 decreased the inhibition of viability,
mobility, and phosphorylation of Akt in EC cells (Fig. 5). The results of the present study
suggested that decreasing MACC1 expression affected the expression
of PTEN and the phosphorylation of Akt in the EC cells, which
suggested that MACC1 represents a potential oncoprotein and a
promoting factor for the activation of the PTEN/PI3K/Akt signaling
pathway in these cells. Furthermore, decreasing MACC1 expression in
the EC cells may have facilitated the regulation of the PI3K/Akt
signaling pathway by PTEN and helped PTEN function as a tumor
suppressor.
 | Figure 4.Downregulating MACC1 expression
suppresses the PTEN/PI3K/Akt signaling pathway in esophageal cancer
cells. (A) MACC1, PTEN and pAkt expression was detected using
immunofluorescence (magnification ×200). (B) The protein expression
of MACC1, PTEN, tAkt, pAkt and PCNA in Eca109 and TE1 cells with
downregulated MACC1 expression were detected using western blot
analysis. PTEN, phosphatase and tensin homolog; PI3K,
phosphoinositide 3-kinase; Akt, protein kinase B; p,
phosphorylated; t, total; PCNA, proliferating cell nuclear antigen;
si, small interfering. |
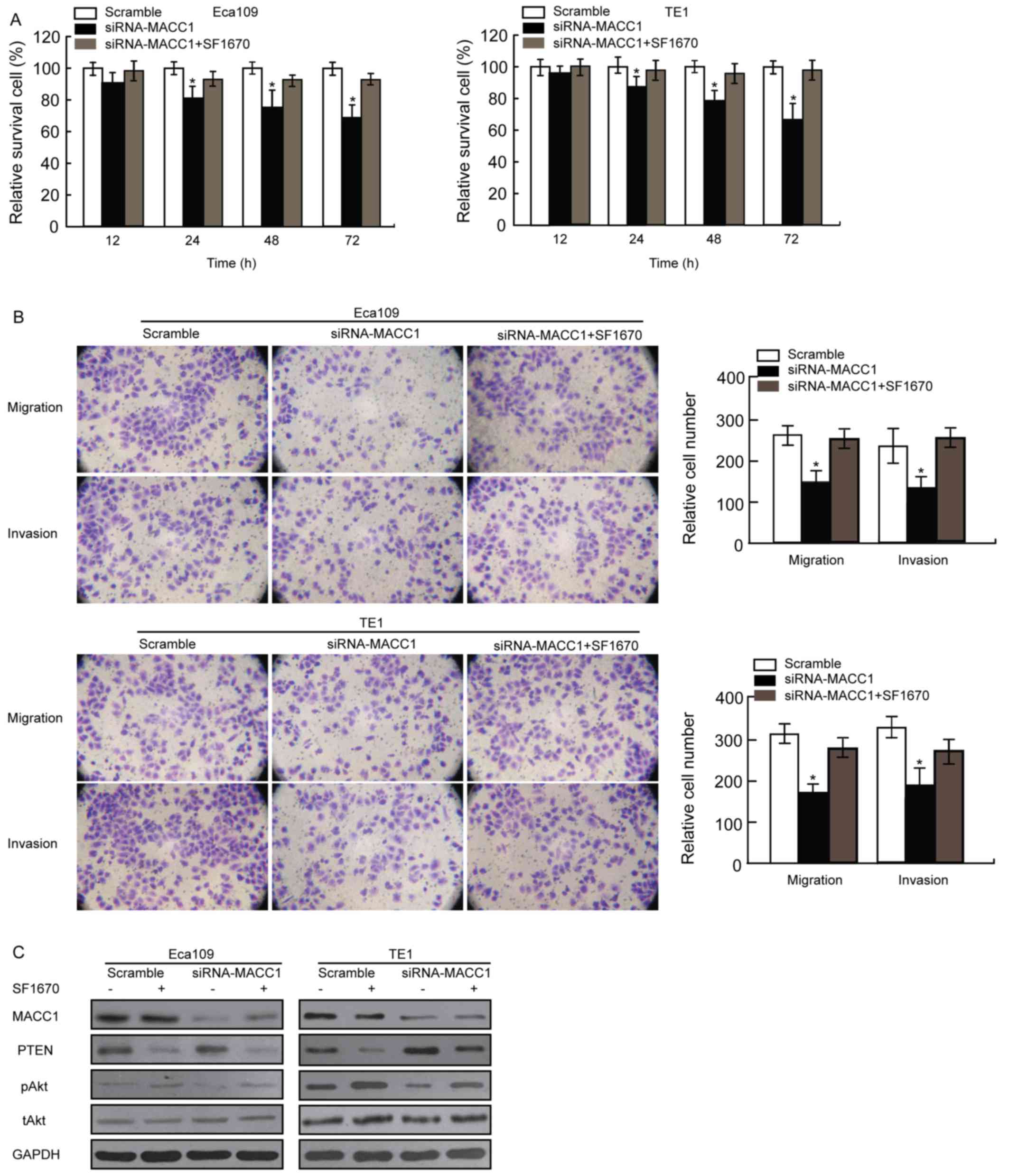 | Figure 5.PTEN inhibitor SF1670 affects
viability, mobility and the PTEN/PI3 K/Akt signaling pathway in EC
cells. (A) EC cell viability was assessed using an MTT assay.
Eca109 and TE1 cells were pretreated with the scramble control
siRNA and siRNA-MACC1 with or without 500 nM SF1670 for 12, 24, 48
or 72 h. (B) The migration and invasion of Eca109 and TE1 cells
with downregulated MACC1 expression with or without 500 nM SF1670
were evaluated using a Matrigel or Transwell assay. Data were
presented as the mean ± standard deviation, as derived from three
independent experiments. (C) The expression of MACC1, PTEN, tAkt
and pAkt proteins in Eca109 and TE1 cells with downregulated MACC1
expression with or without 500 nM SF1670 was detected using western
blot analysis. *P<0.05 vs. scramble control. PTEN, phosphatase
and tensin homolog; PI3K, phosphoinositide 3-kinase; Akt, protein
kinase B; EC, esophageal cancer; si, small interfering; t, total;
p, phosphorylated. Magnification, ×100. |
Discussion
Among types of cancer, EC is associated with the
sixth highest global mortality rate in 1990s (3). Although research and novel molecular
targets have improved early diagnosis and therapeutic options, and
provided less invasive surgery options, the 5-year survival rate
for EC remains low due to increased rates of metastasis and
recurrence (5).
EC cells migrate from the primary site to distant
sites through the bloodstream or the lymphatic system, a crucial
factor in the prognosis of EC. MACC1, first identified as a colon
cancer oncogene, potentially functions as an early diagnosis
biomarker and is associated with the progression of multiple solid
tumors (12–28). Stein et al (12) demonstrated that MACC1 upregulation was
associated with benign-malignant tumor transition. Wang et
al (29) revealed that inhibiting
MACC1 significantly inhibited the proliferation and migration of
pancreatic cancer cells. However, the effect of MACC1 expression in
EC cells remains unclear. Using MACC1-siRNA, the present study
assessed the expression of MACC1 in EC cells and its effect on the
viability, migration, invasion and apoptosis of the EC cells. The
results revealed that inhibiting MACC1 expression in EC cells
decreased viability and affected migration and invasion, which are
key processes in metastasis. Furthermore, downregulating MACC1
contributed to the induction of EC cell apoptosis. These results
suggested that increased MACC1 expression may be associated with
the survival and aggressiveness of EC cells and may serve an
important function in their metastasis.
Previous studies have suggested that MACC1 is
associated with the Akt and Ras/EPH receptor B2 signaling pathways
(28–30). Zhang et al (28) demonstrated that MACC1 expression may
affect the PI3K/Akt signaling pathway and the protein expression of
matrix metallopeptidase (MMP)2 and MMP9. Meng et al
(30) revealed that pAkt was a key
target gene of MACC1, and that pAkt expression was significantly
suppressed by MACC1 downregulation in nasopharyngeal carcinoma
cells. In the present study, downregulating MACC1 expression
increased the expression of PTEN and decreased the phosphorylation
of Akt in EC cells. PTEN, a tumor suppressor gene with numerous
functions in multiple tissues, serves as a central negative
regulator of the PI3K/Akt signaling pathway by dephosphorylating
the PI (31) and P3 of PI3K and
regulating downstream signals (32).
The present study demonstrated that downregulating MACC1 decreased
EC cell viability and mobility. The present study also demonstrated
that the PTEN inhibitor SF1670 not only decreased the inhibitory
effect of siRNA-MACC1 on viability and mobility, but also affected
the PTEN/PI3K/Akt signaling pathway in EC cells. These results
suggested that MACC1 represents a potential oncoprotein, and a
promoting factor for the activation of the PTEN/PI3K/Akt signaling
pathway in EC cells. Furthermore, decreasing MACC1 expression in
the EC cells may have facilitated the regulation of the PI3K/Akt
signaling pathway by PTEN and helped PTEN function as a tumor
suppressor.
The present study suggested that MACC1 abnormal
expression may affect the PTEN/PI3K/Akt signaling pathway and is
thereby crucial for the viability, migration, invasion and
apoptosis of EC cells. These results suggested that MACC1 may
potentially serve as a therapeutic target for EC. However, the
molecular mechanisms underlying the function of MACC1 in the
PTEN/PI3K/Akt signaling pathway remain to be fully understood.
Further studies are required to clarify the genetic features of
MACC1 and its potential as a target in antitumor therapy for
EC.
References
|
1
|
Zhang YQ, Zhang JJ, Song HJ and Li DW:
Expression and prognostic influence of NF-κB and EGFR in esophageal
cancer. Genet Mol Res. 14:16819–16826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pisani P, Parkin DM, Bray F and Ferlay J:
Erratum: Estimates of the worldwide mortality from 25 cancers in
1990. Int. J. Cancer, 83, 18–29 (1999). Int J Cancer. 83:870–873.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Collard JM, Otte JB, Fiasse R, Laterre PF,
De Kock M, Longueville J, Glineur D, Romagnoli R, Reynaert M and
Kestens PJ: Skeletonizing en bloc esophagectomy for cancer. Ann
Surg. 234:25–32. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blanchard P, Quero L and Hennequin C:
Prognostic and predictive factors of oesophageal carcinoma. Bull
Cancer. 96:379–389. 2009.(In French). PubMed/NCBI
|
|
8
|
Li C, Li Z, Zhu M, Zhao T, Chen L, Ji W,
Chen H and Su C: Clinicopathological and prognostic significance of
survivin over-expression in patients with esophageal squamous cell
carcinoma: A meta-analysis. PLoS One. 7:e447642012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin YC, Wu MY, Li DR, Wu XY and Zheng RM:
Prognostic and clinicopathological features of E-cadherin,
alpha-catenin, beta-catenin, gamma-catenin and cyclin D1 expression
in human esophageal squamous cell carcinoma. World J Gastroenterol.
10:3235–3239. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Michaylira CZ, Wong GS, Miller CG,
Gutierrez CM, Nakagawa H, Hammond R, Klein-Szanto AJ, Lee JS, Kim
SB, Herlyn M, et al: Periostin, a cell adhesion molecule,
facilitates invasion in the tumor microenvironment and annotates a
novel tumor-invasive signature in esophageal cancer. Cancer Res.
70:5281–5292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen M, Cai E, Huang J, Yu P and Li K:
Prognostic value of vascular endothelial growth factor expression
in patients with esophageal cancer: A systematic review and
meta-analysis. Cancer Epidemiol Biomarkers Prev. 21:1126–1134.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stein U, Walther W, Arlt F, Schwabe H,
Smith J, Fichtner I, Birchmeier W and Schlag PM: MACC1, a newly
identified key regulator of HGF-MET signaling, predicts colon
cancer metastasis. Nat Med. 15:59–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Z, Cai M, Weng Y, Zhang F, Meng D,
Song J, Zhou H and Xie Z: Circulating MACC1 as a novel diagnostic
and prognostic biomarker for nonsmall cell lung cancer. J Cancer
Res Clin Oncol. 141:1353–1361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chundong G, Uramoto H, Onitsuka T,
Shimokawa H, Iwanami T, Nakagawa M, Oyama T and Tanaka F: Molecular
diagnosis of MACC1 status in lung adenocarcinoma by
immunohistochemical analysis. Anticancer Res. 31:1141–1145.
2011.PubMed/NCBI
|
|
15
|
Shirahata A, Sakata M, Kitamura Y,
Sakuraba K, Yokomizo K, Goto T, Mizukami H, Saito M, Ishibashi K,
Kigawa G, et al: MACC 1 as a marker for peritoneal-disseminated
gastric carcinoma. Anticancer Res. 30:3441–3444. 2010.PubMed/NCBI
|
|
16
|
Burock S, Herrmann P, Wendler I,
Niederstrasser M, Wernecke KD and Stein U: Circulating metastasis
associated in colon cancer 1 transcripts in gastric cancer patient
plasma as diagnostic and prognostic biomarker. World J
Gastroenterol. 21:333–341. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang T, He W, Cui F, Xia J, Zhou R, Wu Z,
Zhao Y and Shi M: MACC1 mediates acetylcholine-induced invasion and
migration by human gastric cancer cells. Oncotarget. 7:18085–18094.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun L, Duan J, Jiang Y, Wang L, Huang N,
Lin L, Liao Y and Liao W: Metastasis-associated in colon cancer-1
upregulates vascular endothelial growth factor-C/D to promote
lymphangiogenesis in human gastric cancer. Cancer Lett.
357:242–253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qu JH, Chang XJ, Lu YY, Bai WL, Chen Y,
Zhou L, Zeng Z, Wang CP, An LJ, Hao LY, et al: Overexpression of
metastasis-associated in colon cancer 1 predicts a poor outcome of
hepatitis B virus-related hepatocellular carcinoma. World J
Gastroenterol. 18:2995–3003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shirahata A, Fan W, Sakuraba K, Yokomizo
K, Goto T, Mizukami H, Saito M, Ishibashi K, Kigawa G, Nemoto H, et
al: MACC 1 as a marker for vascular invasive hepatocellular
carcinoma. Anticancer Res. 31:777–780. 2011.PubMed/NCBI
|
|
21
|
Yao Y, Dou C, Lu Z, Zheng X and Liu Q:
MACC1 suppresses cell apoptosis in hepatocellular carcinoma by
targeting the HGF/c-MET/AKT pathway. Cell Physiol Biochem.
35:983–996. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang Y, Zhang H, Cai J, Fang L, Wu J, Ye
C, Zhu X and Li M: Overexpression of MACC1 and Its significance in
human breast cancer progression. Cell Biosci. 3:162013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shang C, Hong Y, Guo Y, Liu YH and Xue YX:
Influence of the MACC1 gene on sensitivity to chemotherapy in human
U251 glioblastoma cells. Asian Pac J Cancer Prev. 16:195–199. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Isella C, Mellano A, Galimi F, Petti C,
Capussotti L, De Simone M, Bertotti A, Medico E and Muratore A:
MACC1 mRNA levels predict cancer recurrence after resection of
colorectal cancer liver metastases. Ann Surg. 257:1089–1095. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu M, Xu Y, Mao X, Gao Y, Shao L and Yan
F: Overexpression of metastasis-associated in colon cancer-1
associated with poor prognosis in patients with esophageal cancer.
Pathol Oncol Res. 19:749–753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rikitake Y, Kawashima S, Yamashita T,
Ueyama T, Ishido S, Hotta H, Hirata Ki and Yokoyama M:
Lysophosphatidylcholine inhibits endothelial cell migration and
proliferation via inhibition of the extracellular signal-regulated
kinase pathway. Arterioscler Thromb Vasc Biol. 20:1006–1012. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang L, Wu Y, Lin L, Liu P, Huang H, Liao
W, Zheng D, Zuo Q, Sun L, Huang N, et al: Metastasis associated in
colon cancer-1 upregulation predicts a poor prognosis of gastric
cancer, and promotes tumor cell proliferation and invasion. Int J
Cancer. 133:1419–1430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang K, Tian F, Zhang Y, Zhu Q, Xue N,
Zhu H, Wang H and Guo X: MACC1 is involved in the regulation of
proliferation, colony formation, invasion ability, cell cycle
distribution, apoptosis and tumorigenicity by altering Akt
signaling pathway in human osteosarcoma. Tumour Biol. 35:2537–2548.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang G, Kang MX, Lu WJ, Chen Y, Zhang B
and Wu YL: MACC1: A potential molecule associated with pancreatic
cancer metastasis and chemoresistance. Oncol Lett. 4:783–791.
2012.PubMed/NCBI
|
|
30
|
Meng F, Li H, Shi H, Yang Q, Zhang F, Yang
Y, Kang L, Zhen T, Dai S, Dong Y and Han A: MACC1 downregulation
inhibits proliferation and tumourigenicity of nasopharyngeal
carcinoma cells through Akt/β-catenin signaling pathway. PLoS One.
8:e608212013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aravalli RN, Steer CJ and Cressman EN:
Molecular mechanisms of hepatocellular carcinoma. Hepatology.
48:2047–2063. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baker SJ: PTEN enters the nuclear age.
Cell. 128:25–28. 2007. View Article : Google Scholar : PubMed/NCBI
|