Introduction
The WNT signaling pathway is associated with
numerous biological events, including embryonic development and
adult tissue homeostasis. Therefore, abnormal WNT signaling is
associated with diseases, including certain types of cancer
(1,2).
WNTs activate the canonical and non-canonical signaling pathways,
which are mutually exclusive. The canonical WNT signaling pathway
triggers β-catenin-dependent transcriptional regulation, whereas
the non-canonical WNT signaling pathway activates the
β-catenin-independent signaling pathway (1,2).
The canonical WNT signaling pathway regulates normal
breast development, and its deregulation is associated with breast
cancer progression (3,4). WNTs aid in the generation of the
canonical WNT signaling pathway by binding to the coreceptors LDL
receptor-related protein (LRP)5/6 and frizzled on the cell surface,
and activating the β-catenin/t-cell factor (TCF) complex (2). Overexpression of Wnt1, which may be
induced by the integration of the mouse mammary tumor virus,
triggers mammary tumor development (5,6). Autocrine
WNT signaling regulates mammary epithelial cell fate by regulating
renewal and the epithelial-to-mesenchymal transition (EMT), thereby
affecting tumorigenesis and metastasis in a deregulated signaling
state (7,8). Accordingly, the WNT-activated
β-catenin/TCF complex potentiates breast cancer metastasis by
altering the expression of genes associated with EMT (9,10).
Previous studies have revealed multiple inhibitors
of the canonical WNT signaling pathway, including secreted
frizzled-related protein (SFRP), dickkopf (DKK), and WNT inhibitory
factor (WIF) (2,11). In addition, APC downregulated 1
(APCDD1) may inhibit the canonical WNT signaling pathway by
directly binding WNT3A and LRP5 on the cell surface (11,12).
Therefore, APCDD1 represents an inhibitor of the canonical WNT
signaling pathway. While a previous study demonstrated that APCDD1
promoted colorectal cancer growth, its function as the inhibitor of
the canonical WNT signaling pathway was not assessed (13). Furthermore, its function in different
types of cancer, including breast cancer, remains to be fully
understood. The present study evaluated the function of APCDD1 in
breast cancer cells and revealed that APCDD1 regulated breast
cancer cell invasion.
Materials and methods
Cell culture, reagents and
plasmids
The HEK-293T, non-invasive breast cancer MCF-7 and
T-47D, invasive breast cancer SKBR3 and MDA-MB-231 cell lines were
cultured in Dulbecco's modified Eagle's medium supplemented with
10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and 1% penicillin/streptomycin at 37°C in a
humidified atmosphere of 5% CO2 and 95% air. Invasive
breast cancer HCC-1419 and HCC-70 cell lines were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS and 1% penicillin/streptomycin at 37°C in
a humidified atmosphere of 5% CO2 and 95% air. A
concentration of 1×106 MCF-7 or MDA-MB-231 cells were
transfected with the appropriate plasmids for 24 h using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol, and was cultured for
another 24 h at 37°C. Recombinant human WNT3A (catalog no.
5036-GMP; recombinant human WNT3A GMP, carrier free) was purchased
from R&D Systems, Inc. (Minneapolis, MN, USA). To assess the
effect of WNT3A, MCF-7 and MDA-MB-231 cells were treated with 1
µg/ml recombinant human WNT3A. Cyclosporine A, FK506 and BAPTA-AM
were obtained from Tocris Bioscience (Bristol, UK). EGTA-AM was
purchased from EMD Millipore (Billerica, MA, USA). Lentiviral
APCDD1 short hairpin (sh)RNAs [1, TRCN0000413419 (CCG GGA AAG CTA
GGG CCT CTT ATT TCT CGA GAA ATA AGA GGC CCT AGC TTT CTT TTT TG); 2,
TRCN0000136710 (CCG GGA GCT CTT CCT TGG TGA CAT TCT CGA GAA TGT CAC
CAA GGA AGA GCT CTT TTT TG); 3, TRCN0000136596 (CCG GCG GTG CAC AAA
TCC CAC TTA TCT CGA GAT AAG TGG GAT TTG TGC ACC GTT TTT TG); 4,
TRCN0000137317 (CCG GGC CAG AGA ACT GTC CTT CTT TCT CGA GAA AGA AGG
ACA GTT CTC TGG CTT TTT TG); and 5, TRCN0000136748 (CCG GGC TGG AAT
CCA ATG CAG AGT TCT CGA GAA CTC TGC ATT GGA TTC CAG CTT TTT TG)]
and control plasmids pLKO TRC005 (TRCN0000231701; target sequence
TCA GTT CCA GTA CGG CTC CAA) and pLKO TRC001 (TRCN0000072208;
target sequence GCT TCA AGT GGG AGC GCG TGA) were obtained from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). pMD2.G (12259;
Addgene, Inc., Cambridge, MA, USA) and psPAX2 (12260; Addgene,
Inc.) were used for lentiviral packaging. A full length
HindIII/XhoI fragment of APCDD1 was inserted into a
pCMV6-AC-HA plasmid (Origene Technologies, Inc., Rockville, MD,
USA). For the APCDD1-luciferase (luc) plasmid, the APCDD1 promoter
regions between −1,000 bp and +1 bp, −500 bp and +1 bp, and −200 bp
and +1 bp were inserted into a pGL3-Basic vector (Promega
Corporation, Madison, WI, USA). Wild-type β-catenin (β-catenin-WT;
pcDNA3-β-catenin; Addgene ID, 16828), β-catenin-S33Y
(pcDNA3-S33Y-β-catenin; Addgene ID, 19286), β-catenin shRNA (pLKO.1
puro shRNA β-catenin; Addgene ID, 18803), TCF4 mutant lacking
β-catenin interaction domain (∆N-TCF4; pcDNA/MYC
proto-oncogene-∆N-TCF4; Addgene ID, 16513) and TCF/lymphoid
enhancer binding factor (LEF) reporter plasmids (M50 Super 8x
TOPFlash, Addgene ID, 12456; M51 Super 8x FOPFlash, Addgene ID,
12457) were obtained from Addgene, Inc. (Cambridge, MA, USA). All
experiments were performed at least three times.
Chromatin immunoprecipitation (ChIP)
and luc assays
TCF4 binding sites at −1,000 bp upstream of the
APCDD1 gene were analyzed in silico using the publicly
available programs PROMO, LASAGNA and JASPAR (14–17). ChIP
assays using a ChIP kit (Abcam, Cambridge, MA, USA) were performed
according to the manufacturer's protocol. A concentration of
3×106 MCF-7 and MDA-MB-231 cells were subjected to the
ChIP assays. As a negative control, rabbit IgG (Abcam) was used.
Quantitative polymerase chain reaction (qPCR) was performed using
SYBR Green real-time PCR master mix (cat no. 4309,155; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Samples were incubated for 10 min at 95°C, denatured for 15 sec at
95°C and annealed and extended for 1 min at 60°C, for 40 cycles.
qPCR reactions were performed using a LightCycler 480 Instrument II
(Roche Diagnostics, Indianapolis, IN, USA), and relative
quantifications were automatically performed using LightCycler 480
software 1.5 (Roche Diagnostics). Primer sequences for ChiP assays
were as follows: forward 5′-TTGGGTCTCAAACGCCCATG-3′, reverse
5′-TTCATATTTCCAGCGCGCGCC-3′. GAPDH was used as a positive
control. Its primer sequences are as follows: Forward,
5′-CGGGATTGTCTGCCCTAATTAT-3′ and reverse, 5′-GCACGGAAGGTCACGATGT-3′
(18). The reporter plasmid
pAPCDD1-luc was subjected to the luc assay. Luc assays were
performed using a Dual-Luciferase Reporter Assay system (Promega,
Madison, WI, USA). In brief, activities of firefly and
Renilla luciferases were sequentially determined and firefly
luciferase activity was divided by Renilla luciferase
activity to obtain a fold activity. All experiments were performed
in triplicate and independently repeated three times.
Western blot analysis and
immunoprecipitation assays
Antibodies against actin (sc-47778; dilution, 1:200)
and lamin (sc-6215; dilution, 1:200) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Antibodies against TCF4
(dilution, 1:1,000; 2569), β-catenin (dilution, 1:1,000; 8480),
snail (dilution, 1:1,000; 3879), twist (dilution 1:1,000; 46702),
vimentin (dilution, 1:1,000; 5741), epithelial (E) -cadherin
(dilution, 1:1,000; 3195) and neural (N)-cadherin (dilution,
1:1,000; 13116) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Antibodies against APCDD1 (dilution, 1:1,000;
ab73063) and hemagglutinin (HA) were purchased from Abcam.
Horseradish peroxidase-conjugated anti-rabbit IgG (#7074) or
anti-mouse IgG (#7076) antibodies (Cell Signaling Technology, Inc.)
were used as secondary antibodies at a dilution of 1:10,000.
Nuclear fraction was achieved using a Cell Fractionation kit (Cell
Signaling Technology, Inc.). Immunoprecipitation assays were
performed using protein A/G Plus agarose beads (Santa Cruz
Biotechnology, Inc.). For western blot analysis, 3×106
cells (all cell types analyzed in this study) were lyzed using
radioimmunoprecipitation buffer for 30 min on ice and centrifuged
at 20,000 × g for 10 min at 4°C. Evaluation of protein
concentrations was performed using Pierce BCA protein assay kit
(Pierce; Thermo Fisher Scientific, Inc.), according to the
manufacture's protocol. A total of 30 µg protein/lane was loaded to
10–12% SDS-PAGE and transferred to a polyvinylidene difluoride
membrane (GE Healthcare Life Sciences, Little Chalfont, UK). For
the immunoprecipitation assays, 300 µg of nuclear protein from
either MCF-7 or MDA-MB-231 cells, 10 µl protein A/G plus agarose
solution (0.5 ml agarose/2.0 ml solution; Santa Cruz Biotechnology,
Inc.) and 1 µg appropriate antibody were mixed and incubated for 12
h at 4°C. Subsequent to blocking with 5% milk for 1 h at room
temperature, the membrane was incubated with the aforementioned
antibodies for another 1 h at room temperature. Actin was detected
as an internal control. The membranes were then incubated with the
aforementioned secondary antibodies for 1 h at room temperature.
Western bands were detected using LumiGLO chemiluminescent reagent
and peroxidase (#7003; Cell Signaling Technology Inc.). Western
blot analyses were performed independently three times. ImageJ
software (version 1.50) was used for relative quantifications
(National Institutes of Health, Bethesda, MA, USA).
Cell proliferation, migration and
invasion assays
A concentration of 3×105
APCDD1-overexpressing or APCDD1-silenced cells together with the
control cells were separately cultured in 6-well plates for 72 h at
37°C, and cell numbers were counted every day. Experiments were
performed in quadruplicate and independently repeated in
triplicate. For cell migration, 3×105
APCDD1-overexpressing or APCDD1-silenced cells together with the
control cells were cultured in 6-well plates and scratched at 37°C
when the confluence reached ~80%, and then the number of migrated
cells was counted 24 h after scratching. Experiments were performed
in triplicate. For the invasion assays, 3×105
APCDD1-overexpressing or APCDD1-silenced cells together with the
control cells were cultured in the upper chambers of
Matrigel-pre-coated Transwell plates and incubated for 16 h at
37°C. The cells in the upper chamber were removed using a swab and
the cells that had invaded through the Matrigel were stained with
0.4% crystal violet for 10 min at room temperature, washed with
water and then counted. Experiments were performed in triplicate.
The cell migration and invasions were determined using a Zeizz
Axiovert inverted microscope, and the images were analyzed using
Zen software version 3.00 (Carl Zeizz, Oberkochen, Germany). A
total of 4 fields were randomly selected and the migrated or
invaded cells were counted.
Data mining from gene expression
dataset
From the Gene Expression Omnibus (GEO) datasets in
NCBI (https://www.ncbi.nlm.nih.gov/geo/), GSE21422 series
was chosen as it featured gene expression profiles of human ductal
carcinoma in situ (DCIS) and invasive ductal breast
carcinoma (IDC). APCDD1 expression levels in DCIS and IDC were then
analyzed using data analysis tools for GDS3853 in dataset browser.
Any event (AE)-free event plot with APCDD1 expression pattern was
obtained from the website, Breast Cancer Gene-Expression Miner v4.0
(http://bcgenex.centregauducheau.fr).
Statistical analysis
Unpaired Student's t-test or one-way analysis of
variance with a post-hoc Tukey's test was performed to calculate
the statistical significance of the results of the present study.
Results were presented as the mean ± standard deviation and
P<0.05 was considered to indicate a statistically significant
difference. Calculations were performed using SPSS version 22 (IBM
Corp., Armonk, NY, USA) software.
Results
APCDD1 expression pattern in breast
cancer
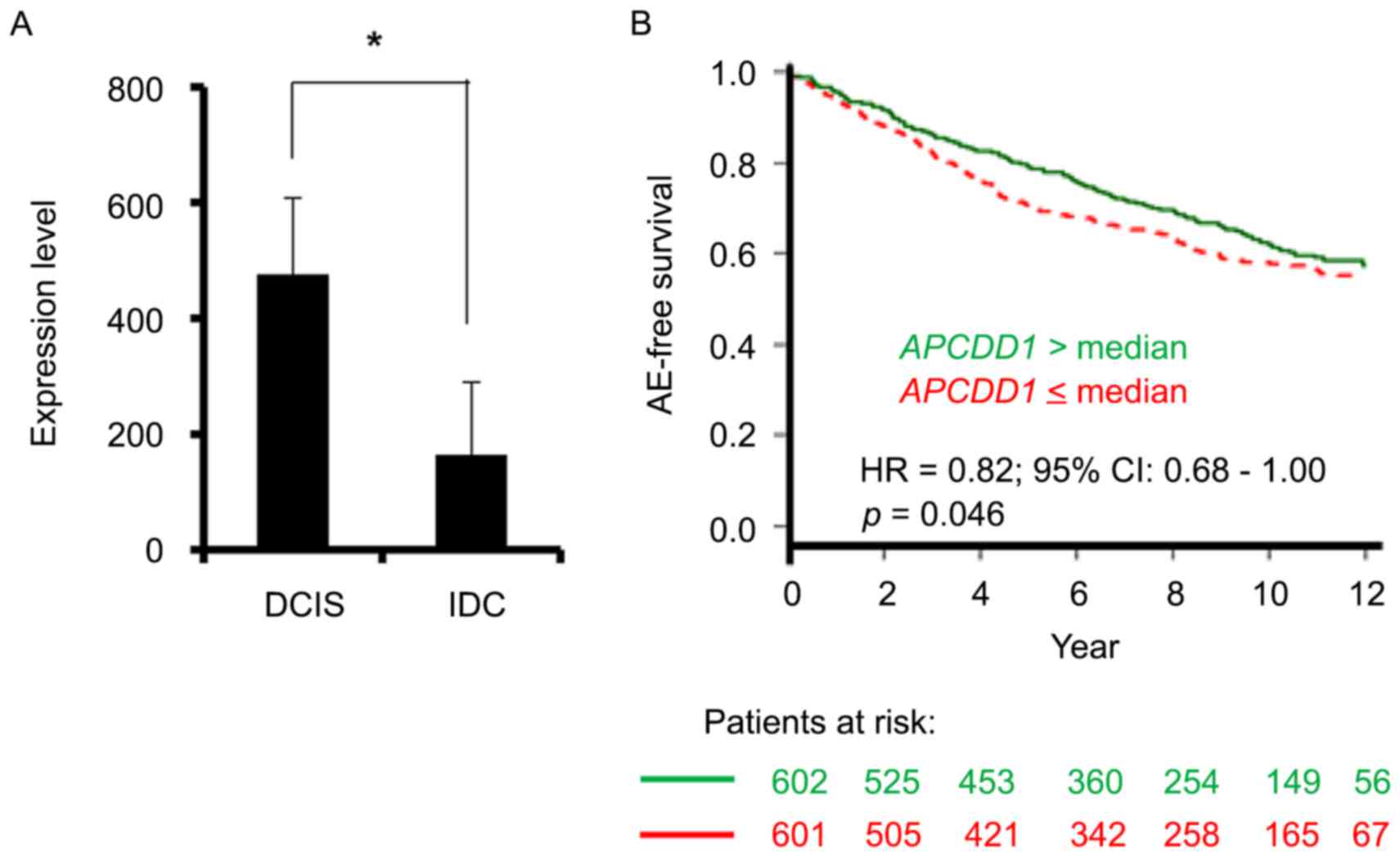
The Gene Expression Omnibus GSE21422 dataset
(19,20) revealed that the level of APCDD1
transcript was significantly increased in ductal carcinoma in
situ (DCIS) compared with that in invasive ductal carcinoma
(IDC) (Fig. 1A). Furthermore, the
GSE1456 and GSE10510 datasets on patients with breast cancer
(20–22) demonstrated that increased APCDD1
expression was associated with a favorable prognosis in terms of
any event-free survival (Fig. 1B).
These datasets suggested that APCDD1 may negatively regulate breast
cancer progression.
APCDD1 expression pattern in breast
cancer cell lines
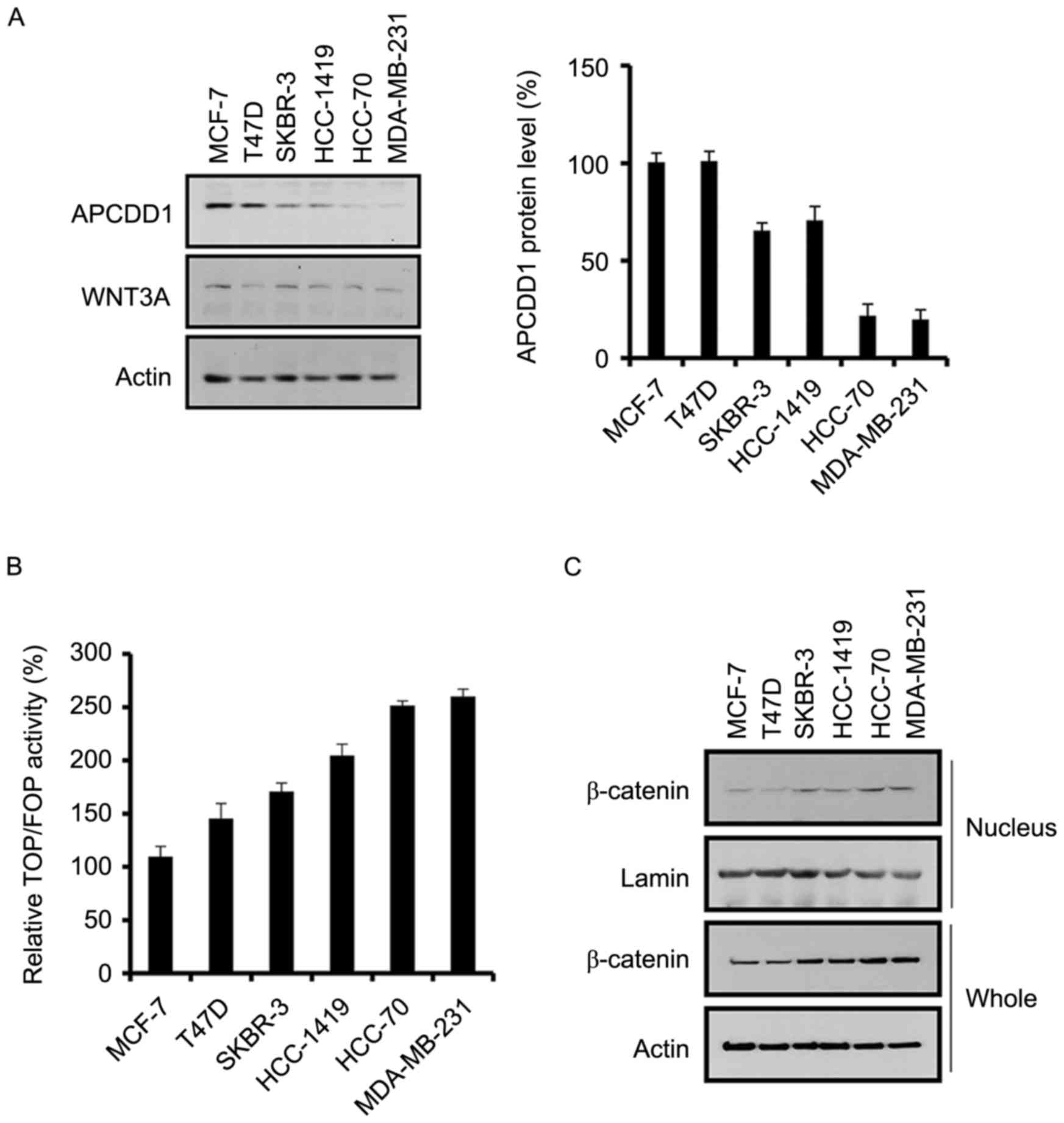
The expression patterns of APCDD1 in multiple breast
cancer cell lines were assessed. Although WNT3A expression did not
differ between the breast cancer cell lines, APCDD1 expression was
increased in non-invasive compared with invasive breast cancer
cells (Fig. 2A). This result was
consistent with data where APCDD1 expression was decreased in IDC
compared with that in DCIS (Fig.
1A).
As APCDD1 inhibits the canonical WNT signaling
pathway (12), TCF/LEF transcription
was assessed in the breast cancer cells. TCF/LEF transcription was
positively associated with the invasive ability of the breast
cancer cells (Fig. 2B), suggesting a
negative association between TCF/LEF transcription and APCDD1
expression.
Since β-catenin is crucial for TCF/LEF transcription
(23,24), β-catenin expression in the breast
cancer cells was assessed. The invasive breast cancer cells
exhibited increased expression of nuclear and total β-catenin
compared with that exhibited by the non-invasive breast cancer
cells (Fig. 2C). Therefore, the
results of the present study demonstrated that the canonical WNT
signaling pathway is positively associated with invasion in breast
cancer cells, which suggests that APCDD1 may regulate breast cancer
cell invasion, as driven by the canonical WNT signaling
pathway.
APCDD1 function in the canonical WNT
signaling pathway in breast cancer cells
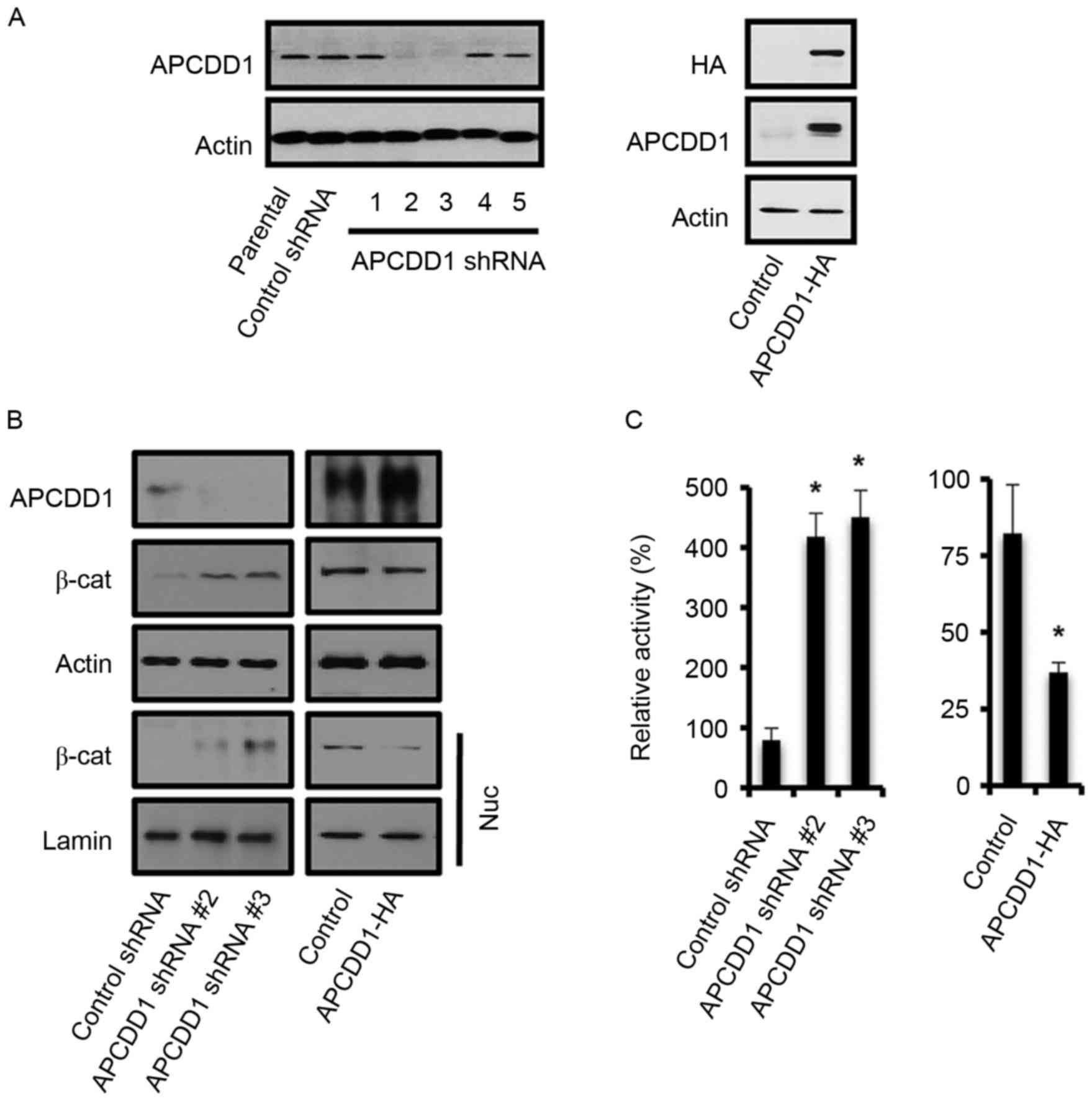
Since APCDD1 expression was associated with the
invasive phenotype of breast cancer cells, the function of APCDD1
in the invasion of breast cancer cells was assessed. Constructs 2
and 3 of the lentiviral APCDD1 shRNA plasmids repressed APCDD1
expression in MCF-7 cells (Fig. 3A).
Hemagglutinin (HA)-tagged APCDD1 was overexpressed in MDA-MB-231
cells and detected using an antibody for either HA or APCDD1
(Fig. 3A). Altered APCDD1 expression
negatively affected nuclear β-catenin expression (Fig. 3B) and TCF/LEF functions (Fig. 3C).
APCDD1 function in breast cancer cell
metastasis
Since the canonical WNT signaling pathway regulates
the expression of EMT-associated genes and promotes invasion in
breast cancer cells (9), the present
study assessed whether altering APCDD1 expression in breast cancer
cells affects the expression patterns of EMT-associated genes.
Silencing APCDD1 in MCF-7 cells or inducing APCDD1 overexpression
in MDA-MB-231 cells altered the expression of EMT-associated genes,
including zinc finger E-box binding homeobox 1 (Zeb1), Twist,
Vimentin, E-cadherin and N-cadherin, while altering APCDD1
expression did not affect snail expression (Fig. 4A). APCDD1 silencing increased the
expression levels of Zeb1, Twist, Vimentin and N-cadherin and
reduced E-cadherin expression level. APCDD1 overexpression reversed
expression patterns of those proteins.
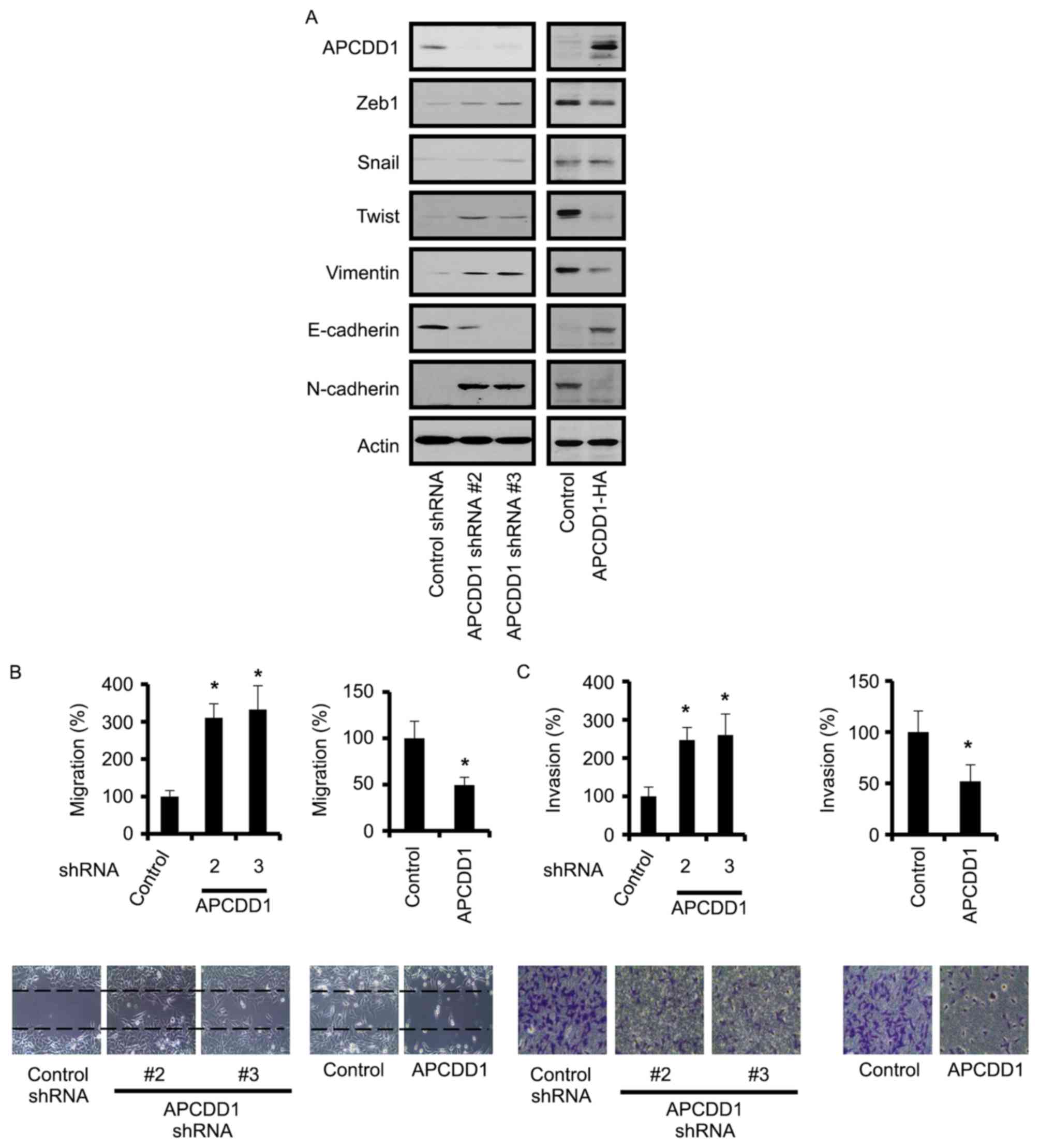 | Figure 4.APCDD1 negatively regulates metastasis
in breast cancer cells. (A) APCDD1 regulated the expression of
EMT-associated proteins in breast cancer cells. The results of
western blot analysis demonstrated the APCDD1-induced alterations
to EMT-associated protein expression. (B) To assess cell migration,
the cells were scratched and subsequently cultured for 24 h. (C) To
evaluate cell invasion, the cells were cultured on the top layer of
the chamber, which was precoated with Matrigel, and incubated for
24 h. Invasive cells were counted following the swabbing of the top
layer. Cell migration and invasion assays were performed in
triplicate and independently repeated three times. *P<0.05 vs.
the control group. EMT, epithelial-to-mesenchymal transition; Zeb1,
zinc finger E-box binding homeobox 1; E, epithelial; N, neural; sh,
short hairpin; APCDD1, APC downregulated 1. |
Furthermore, silencing APCDD1 in MCF-7 cells
promoted migration and invasion, and its overexpression in
MDA-MB-231 cells attenuated migration and invasion (Fig. 4B and C). However, altering APCDD1
expression did not significantly affect growth in these cell lines
(data not shown). Therefore, these results suggested that APCDD1
inhibited the invasion of the breast cancer cells.
Canonical WNT signaling pathway
regulates APCDD1 expression via the TCF4/β-catenin complex
Since β-catenin is crucial for TCF4 transcription
(23–25), β-catenin interaction with TCF4 in the
breast cancer cells was further assessed. The interaction rate
between β-catenin and TCF4 was increased in highly invasive
MDA-MB-231 cells compared with that in less invasive MCF-7 cells
(Fig. 5A). Subsequently, TCF4
interaction with APCDD1 promoter regions was evaluated in MCF-7 and
MDA-MB-231 breast cancer cell lines using ChIP assays with
anti-TCF4 antibodies. The interaction rate of TCF4 with APCDD1
promoter regions was increased in MDA-MB-231 cells compared with
that in MCF-7 cells (Fig. 5B).
Therefore, the results of the present study suggested that the
canonical WNT signaling pathway was more active in the invasive
than in the non-invasive breast cancer cells.
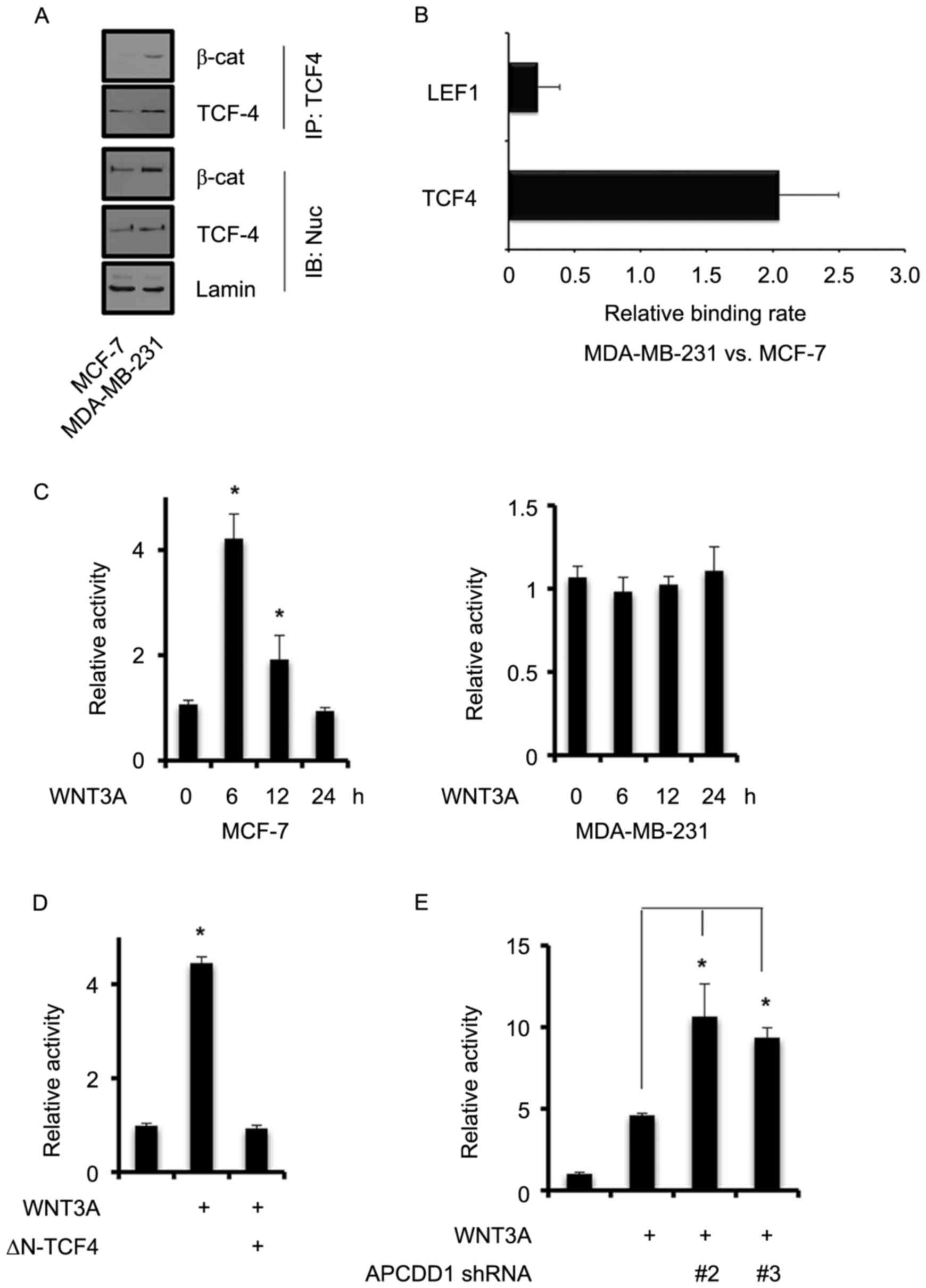 | Figure 5.Canonical WNT signaling pathway
regulates APCDD1 expression via the β-catenin/TCF4 complex. (A)
Interaction between β-catenin and TCF4 in the MCF-7 and MDA-MB-231
breast cancer cells. Nuclear TCF4 was immunoprecipitated with an
appropriate antibody and subsequently immunoblotted with the
indicated antibodies. (B) TCF4 interaction with APCDD1 promoter
regions in MCF-7 and MDA-MB-231 cells. TCF4 binding sites of the
APCDD1 promoter region (between −2,000 and +1 bp). Chromatin
immunoprecipitation assays were performed using anti-LEF1 or
anti-TCF4 antibodies. Interaction in the MCF-7 and MDA-MB-231
breast cancer cells was measured using quantitative polymerase
chain reaction analysis. (C) APCDD1 promoter activity was measured
using reporter assays. The cells were transfected with pAPCDD1-luc
construct for 48 h and the medium was subsequently replaced with
serum-depleted medium. The cells were treated with recombinant
WNT3A for 6–24 h and luc activity was subsequently measured. (D)
MCF-7 cells were transfected with pAPCDD1-luc and ∆N-TCF4 for 30 h,
with WNT3A for a further 6 h, and subsequently subjected to luc
assays. (E) MCF-7 cells were infected with lentiviral APCDD1 shRNA
for 48 h and subsequently treated with WNT3A for a further 12 h.
Luc assays were then performed to measure APCDD1 promoter activity.
Experiments were performed in triplicate and repeated independently
three times. *P<0.05 vs. the control. TCF, transcription factor;
LEF, lymphoid enhancer binding factor; luc, luciferase; β-cat,
β-catenin; ∆N-TCF4, TCF4 mutant lacking β-cat interaction domain;
sh, short hairpin; APCDD1, APC downregulated 1. |
The results of the present study revealed that
APCDD1 expression was negatively associated with TCF4/β-catenin
activity. Therefore, the present study assessed how the
β-catenin/TCF4 complex regulates APCDD1 promoter activity. In MCF-7
cells, the recombinant human WNT3A significantly increased APCDD1
promoter activity 6 h after treatment, but reduced it again 12 h
after treatment (Fig. 5C). However,
WNT3A did not significantly increase APCDD1 promoter activity in
MDA-MB-231 cells at any time after treatment (Fig. 5C).
The present study found that, following the
transfection of MCF-7 cells with ∆N-TCF4 and treatment with WNT3A
for 6 h, APCDD1 promoter activity was not altered as compared with
the control (Fig. 5D), indicating
that WNT3A requires TCF4 for APCDD1 promoter activation.
The present study demonstrated that the canonical
WNT signaling pathway positively regulated APCDD1 expression in the
non-invasive breast cancer cells, whereas the activity of the
canonical WNT signaling pathway was negatively associated with
APCDD1 expression. Therefore, this suggested that APCDD1 repressed
the induction of APCDD1 expression by WNT3A through a negative
feedback mechanism. Silencing APCDD1 in MCF-7 cells using
lentiviral APCDD1 shRNA and subsequently treating the cells with
WNT3A for 12 h increased APCDD1 promoter activity (Fig. 5E). Therefore, the results of the
present study indicated that the canonical WNT signaling pathway in
the non-invasive breast cancer cells generated APCDD1-mediated
negative feedback signaling.
Discussion
The canonical WNT signaling pathway is crucial for
breast cancer progression, including distant metastasis (1,2,4,6). APCDD1
represents an inhibitor of the canonical WNT signaling pathway
(12). Although APCDD1 has been
revealed to promote colorectal cancer cell proliferation (13), its functions in other types of cancer
are yet to be fully understood. The present study demonstrated that
APCDD1 suppressed the canonical WNT signaling pathway-driven
invasion of breast cancer cells.
APCDD1 expression was decreased in the invasive
compared with the non-invasive breast cancer cells. Furthermore,
the results of the present study suggested that APCDD1, via its
inhibitory function in the canonical WNT signaling pathway,
suppressed the invasion of the breast cancer cells. When
considering autocrine WNT signaling in breast cancer cells
(8), APCDD1 may determine an input
level of autocrine WNT signaling and thereby facilitate
invasion.
The results of the present study demonstrated that
the β-catenin/TCF4 complex regulated APCDD1 expression in the
breast cancer cells, which is consistent with the results of a
previous study that demonstrated that the β-catenin/TCF4 complex
positively regulated APCDD1 expression in colorectal cancer cells
(13). However, APCDD1 functioned as
a negative regulator of the canonical WNT signaling pathway in the
breast cancer cells of the present study, which is consistent with
a previous study that revealed that APCDD1 binds to WNT3A and LRP5
to inhibit the canonical WNT signaling pathway in hair follicles
(12). Although APCDD1 has been
demonstrated to promote colorectal cancer cell proliferation
(13), in the present study it
suppressed breast cancer cell invasion without affecting
proliferation. Therefore, APCDD1 may balance the canonical WNT
signaling pathway via the autocrine signaling pathway. However, the
mechanisms that repress APCDD1 expression to potentiate the
canonical WNT signaling pathway, which is crucial for invasion,
since invasion depends on the altered expression of EMT-associated
genes, is yet to be fully understood.
Inhibitors of WNT signaling pathways, including WIF,
DKK and SFRP, have been demonstrated to repress breast cancer
development and metastasis (11,26,27).
However, the manner in which WNT signaling pathways and their
inhibitors function together during cancer progression remains
unclear. Whereas APCDD1 expression is negatively associated with
invasion, WIF, DKK and SFRP are epigenetically inactivated in
breast cancer cells, independent of invasion (28,29).
Therefore, different WNT signaling pathway inhibitors may serve
crucial functions in the multiple roles of the canonical WNT
signaling pathway in breast cancer progression. In conclusion,
inhibitors against WNT signaling pathway are numerous and their
functions overlap. Thus, future research should investigate the
specific roles of each inhibitor, which will be useful to design
drugs and treatment schedules for breast cancer.
Acknowledgements
The present study was supported by the Korea
National University of Transportation in 2016 and by the Basic
Science Research Program through the National Research Foundation
of Korea funded by the Ministry of Science, ICT and Future Planning
(grant no. NRF-2014R1A1A1035831).
References
|
1
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Incassati A, Chandramouli A, Eelkema R and
Cowin P: Key signaling nodes in mammary gland development and
cancer:β-catenin. Breast Cancer Res. 12:2132010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smalley MJ and Dale TC: Wnt signalling in
mammalian development and cancer. Cancer Metastasis Rev.
18:215–230. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nusse R and Varmus HE: Many tumors induced
by the mouse mammary tumor virus contain a provirus integrated in
the same region of the host genome. Cell. 31:99–109. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Y, Hively WP and Varmus HE: Use of
MMTV-Wnt-1 transgenic mice for studying the genetic basis of breast
cancer. Oncogene. 19:1002–1009. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scheel C, Eaton EN, Li SH, Chaffer CL,
Reinhardt F, Kah KJ, Bell G, Guo W, Rubin J, Richardson AL and
Weinberg RA: Paracrine and autocrine signals induce and maintain
mesenchymal and stem cell states in the breast. Cell. 145:926–940.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bafico A, Liu G, Goldin L, Harris V and
Aaronson SA: An autocrine mechanism for constitutive Wnt pathway
activation in human cancer cells. Cancer Cell. 6:497–506. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yook JI, Li XY, Ota I, Hu C, Kim HS, Kim
NH, Cha SY, Ryu JK, Choi YJ, Kim J, et al: A Wnt-Axin2-GSK3beta
cascade regulates Snail1 activity in breast cancer cells. Nat Cell
Biol. 8:1398–1406. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu ZQ, Li XY, Hu CY, Ford M, Kleer CG and
Weiss SJ: Canonical Wnt signaling regulates Slug activity and links
epithelial-mesenchymal transition with epigenetic breast cancer 1,
early onset (BRCA1) repression. P Natl Acad Sci USA.
109:16654–16659. 2012. View Article : Google Scholar
|
|
11
|
Cruciat CM and Niehrs C: Secreted and
transmembrane wnt inhibitors and activators. Cold Spring Harb
Perspect Biol. 5:a0150812013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shimomura Y, Agalliu D, Vonica A, Luria V,
Wajid M, Baumer A, Belli S, Petukhova L, Schinzel A, Brivanlou AH,
et al: APCDD1 is a novel Wnt inhibitor mutated in hereditary
hypotrichosis simplex. Nature. 464:1043–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takahashi M, Fujita M, Furukawa Y,
Hamamoto R, Shimokawa T, Miwa N, Ogawa M and Nakamura Y: Isolation
of a novel human gene, APCDD1, as a direct target of the
beta-Catenin/T-cell factor 4 complex with probable involvement in
colorectal carcinogenesis. Cancer Res. 62:5651–5656.
2002.PubMed/NCBI
|
|
14
|
Mathelier A, Zhao X, Zhang AW, Parcy F,
Worsley-Hunt R, Arenillas DJ, Buchman S, Chen CY, Chou A, Ienasescu
H, et al: JASPAR 2014: An extensively expanded and updated
open-access database of transcription factor binding profiles.
Nucleic Acids Res. 42:(Database issue). D142–D147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Messeguer X, Escudero R, Farré D, Núñez O,
Martinez J and Albà MM: PROMO: Detection of known transcription
regulatory elements using species-tailored searches.
Bioinformatics. 18:333–334. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Farré D, Roset R, Huerta M, Adsuara JE,
Roselló L, Albà MM and Messeguer X: Identification of patterns in
biological sequences at the ALGGEN server: PROMO and MALGEN.
Nucleic Acids Res. 31:3651–3653. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee C and Huang CH: LASAGNA-Search: An
integrated web tool for transcription factor binding site search
and visualization. Biotechniques. 54:141–153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kretschmer C, Sterner-Kock A, Siedentopf
F, Schoenegg W, Schlag PM and Kemmner W: Identification of early
molecular markers for breast cancer. Mol Cancer. 10:152011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Edgar R, Domrachev M and Lash AE: Gene
expression omnibus: NCBI gene expression and hybridization array
data repository. Nucleic Acids Res. 30:207–210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pawitan Y, Bjöhle J, Amler L, Borg AL,
Egyhazi S, Hall P, Han X, Holmberg L, Huang F, Klaar S, et al: Gene
expression profiling spares early breast cancer patients from
adjuvant therapy: Derived and validated in two population-based
cohorts. Breast Cancer Res. 7:R953–R964. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Calabrò A, Beissbarth T, Kuner R, Stojanov
M, Benner A, Asslaber M, Ploner F, Zatloukal K, Samonigg H, Poustka
A and Sültmann H: Effects of infiltrating lymphocytes and estrogen
receptor on gene expression and prognosis in breast cancer. Breast
Cancer Res Treat. 116:69–77. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Graham TA, Ferkey DM, Mao F, Kimelman D
and Xu W: Tcf4 can specifically recognize beta-catenin using
alternative conformations. Nat Struct Biol. 8:1048–1052. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morin PJ, Sparks AB, Korinek V, Barker N,
Clevers H, Vogelstein B and Kinzler KW: Activation of
beta-catenin-Tcf signaling in colon cancer by mutations in
beta-catenin or APC. Science. 275:1787–1790. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Poy F, Lepourcelet M, Shivdasani RA and
Eck MJ: Structure of a human Tcf4-beta-catenin complex. Nat Struct
Biol. 8:1053–1057. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matsuda Y, Schlange T, Oakeley EJ, Boulay
A and Hynes NE: WNT signaling enhances breast cancer cell motility
and blockade of the WNT pathway by sFRP1 suppresses MDA-MB-231
xenograft growth. Breast Cancer Res. 11:R322009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mikheev AM, Mikheeva SA, Maxwell JP, Rivo
JV, Rostomily R, Swisshelm K and Zarbl H: Dickkopf-1 mediated tumor
suppression in human breast carcinoma cells. Breast Cancer Res
Treat. 112:263–273. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Trifa F, Karray-Chouayekh S, Jmal E, Jmaa
ZB, Khabir A, Sellami-Boudawara T, Frikha M, Daoud J and
Mokdad-Gargouri R: Loss of WIF-1 and Wnt5a expression is related to
aggressiveness of sporadic breast cancer in Tunisian patients.
Tumor Biol. 34:1625–1633. 2013. View Article : Google Scholar
|
|
29
|
Suzuki H, Toyota M, Caraway H, Gabrielson
E, Ohmura T, Fujikane T, Nishikawa N, Sogabe Y, Nojima M, Sonoda T,
et al: Frequent epigenetic inactivation of Wnt antagonist genes in
breast cancer. Br J Cancer. 98:1147–1156. 2008. View Article : Google Scholar : PubMed/NCBI
|