Introduction
In 2012, breast cancer was the most prevalent type
of malignant tumor and the leading cause of cancer-associated
mortality in females worldwide (1).
It is estimated that there were 231,840 new cases of invasive
tumors, 60,290 new cases of noninvasive, in situ tumors and
40,290 mortalities resulting from breast cancer in 2015 in the
United States (2). The highest
reported prevalence of breast cancer is in economically developed
countries; however, there is an increasing incidence and mortality
in developing countries (3). This
phenomenon is predominantly due to the adoption of a western
lifestyle, the lack of breast cancer awareness and the poor access
to screening and healthcare services (4–7). At
present, the primary methodology for breast cancer therapy is
resective surgery followed by hormonal therapy, chemotherapy,
radiotherapy and/or biological therapy (8,9). Although
great progress has been made in the earlier diagnosis and systemic
therapy of patients with breast cancer in recent years, recurrence
or distant metastasis continue to present major barriers to the
successful treatment of breast cancer (10,11).
Therefore, fully understanding the molecular mechanisms underlying
the progression of breast cancer may be critical for the
development of effective therapeutic strategies against breast
cancer.
MicroRNAs (miRNAs) are a large family of
evolutionarily conserved, 20–24 nucleotide, non-coding and
single-stranded RNA molecules which commonly occur in plant, animal
and viral genomes (12). miRNAs
post-transcriptionally regulate gene expression by base-pairing
with complementary nucleotide sequences in the 3′-untranslated
regions (3′UTRs) of specific target mRNAs, leading to the
transcriptional repression or degradation of the target genes
(13,14). miRNAs have attracted considerable
attention due to their ability to regulate a large number of mRNAs
and influence a wide range of cell functions, including cell
growth, metabolism, development, migration, invasion and survival
(15,16). The importance of miRNAs in tumor
initiation and development has been recognized; the abnormal
expression of miRNAs serves a significant role in human cancer,
caused by a variety of mechanisms, including deletions,
amplifications, epigenetic silencing or mutations in miRNA loci
(17). Emerging evidence has
demonstrated that miRNAs can function as oncogenes or tumor
suppressor genes, depending on the roles of their target genes
(18). The inactivation of oncogenic
miRNAs (19,20) or restoration of tumor-suppressor
miRNAs (21,22) may have potential in cancer
treatment.
In the present study, it was demonstrated that
miR-154 was downregulated in breast cancer; enforced miR-154
expression repressed breast cancer cell proliferation, migration
and invasion. Additionally, ADAM metallopeptidase domain 9 (ADAM9)
was identified as a novel direct target of miR-154 in breast
cancer.
Materials and methods
Tissue samples
A total of 45 samples of human breast cancer and
corresponding non-tumor breast tissue samples (age range, 37–71
years; mean age, 56 years) were obtained during surgery at Binzhou
Medical University Hospital (Binzhou, China) between September 2011
and December 2014. None of the patients had received chemotherapy
or radiotherapy prior to surgery. The study was approved by the
Ethics Committee of Binzhou Medical University Hospital; all
patients provided written, informed consent.
Cell lines, cell culture and
vectors
Breast cancer cell lines, including MCF-7, SKBR3 and
MDA-MB-231, were purchased from the American Type Culture
Collection (Manassas, VA, USA). The MCF-10A normal mammary
epithelial cell line and 293T cells were acquired from the Shanghai
Institute of Biochemistry and Cell Biology (Shanghai, China). All
cells were maintained at 37°C with 5% CO2 in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% fetal bovine
serum (FBS) and 1% penicillin/streptomycin (All Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) throughout the
study.
An miR-154 mimic, a corresponding negative control
(NC) and luciferase reporter vectors [PmirGLO-ADAM9-3′UTR wild-type
(Wt) and mutant (Mut)] were obtained from Shanghai GenePharma Co.,
Ltd. (Shanghai, China). miR-154 potential target genes were
predicted using miRanda (www.microrna.org) and TargetScan (www.targetscan.org) software. The ADAM9 vector and a
corresponding blank vector control were synthesized and purified by
Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was prepared from tissues or cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. To quantify miR-154
expression, TaqMan MicroRNA Reverse Transcription Kit (cat. no.,
4366597, Applied Biosystems; Thermo Fisher Scientific, Inc.) was
used to perform reverse transcription, followed by quantitative
polymerase chain reaction with a TaqMan microRNA assay kit (cat.
no., 4326614, Applied Biosystems; Thermo Fisher Scientific, Inc.)
was applied according to the manufacturer's protocol. To quantify
ADAM9 mRNA expression, cDNA was generated using the ReverTra Ace
qPCR RT Kit (Toyobo Life Science, Osaka, Japan) and qPCR was
performed using SYBR Green Real-time Master mix (Toyobo Life
Science). This reaction includes 2 µl cDNA (100 ng), 2 µl forward
primer, 2 µl reverse primer, 10 µl SYBR Green PCR Master Mix and 4
µl ddH2O. The thermocycling conditions for qPCR were as
follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec
and 60°C for 1 min. The primers were designed as follows: ADAM9
forward, 5′-TGTGGGAACAGTGTGTTCAAGGA-3′; ADAM9 reverse,
5′-CCAATTCATGAGCAACAATGGAAG-3′; GAPDH forward,
5′-CGGAGTCAACGGATTTGGTCGTAT-3′; and GADPH reverse
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′. The relative quantification of
miRNA and mRNA were achieved by normalization to U6 and GADPH,
respectively. Each sample was analysed in triplicate and repeated
three times. The relative expression was calculated by the
2−∆∆Cq method (23).
Cell proliferation assay
MCF-7 or MDA-MB-231 cells were seeded in 6-well
plates overnight and then transfected with miR-154 mimics or NC,
and/or an ADAM9 or empty vector, using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Cells were trypsinized 24 h after
transfection, counted and seeded in 96-well plates at a density of
3,000 cells/well. A cell proliferation assay was performed at 24,
48, 72 and 96 h after seeding. Briefly, 10 µl Cell Counting kit-8
(CCK8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan)
solution was added to each well. Following incubation at 37°C for a
further 2 h, the absorbance at 450 nm was detected using an ELISA
reader. Each assay was performed in quintuplicate and repeated
three times.
Transwell migration and invasion
assay
Transwell inserts with an 8 µm pore size from
Corning Incorporated (Corning, NY, USA) were used to assess cell
migration and invasion abilities. For transwell migration assays,
MCF-7 or MDA-MB-231 cells were seeded in 6-well plates overnight
and transfected with an miR-154 mimic or NC, and/or an ADAM9 or
empty vector, using Lipofectamine 2000 according to the
manufacturer's protocol. Following incubation at 37°C for 48 h,
cells were trypsinized and counted. Then, 4×104 cells
were resuspended in FBS-free DMEM and seeded in the top chambers. A
total of 500 µl DMEM containing 20% FBS was added to the lower
chamber. At 48 h, cells remaining on the upper membrane were
removed carefully with a cotton swab. The migrated cells attached
to the lower surface of the membrane were fixed with methanol at
room temperature for 10 min, stained with 0.5% crystal violet at
room temperature for 10 min and counted under an inverted
microscope (Olympus Corporation, Tokyo, Japan) in five random
fields. For the transwell invasion assay, the process was the same
as the transwell migration assay, except that the transwell inserts
were coated with pre-coated with Matrigel (BD Biosciences, San
Jose, CA, USA). All assays were repeated three times.
Protein extraction and western
blot
MCF-7 or MDA-MB-231 cells were washed in PBS (Gibco;
Thermo Fisher Scientific, Inc.), and lysed in 1X
radioimmunoprecipitation assay lysis buffer (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at 48 h after transfection
with an miR-154 mimic or NC, or an ADAM9 or empty vector, with
Lipofectamine 2000, according to the manufacturer's protocol. The
protein concentration was determined using a bicinchoninic acid
protein assay kit (Pierce; Thermo Fisher Scientific, Inc.). Equal
quantities of protein (30 µg) were subjected to 10% SDS-PAGE, and
transferred to polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). The membranes were blocked with 5% non-fat
milk in Tris-buffered saline with 0.1% Tween (TBST), followed by
incubation with the primary antibodies overnight at 4°C, including
a monoclonal mouse anti-human ADAM9 antibody (dilution, 1:1,000;
cat. no., ab57934) and a monoclonal mouse anti-human GADPH antibody
(dilution, 1:1,000; cat. no., ab9484; both Abcam, Cambridge, UK).
Subsequent to washing with TBST, a horseradish
peroxidase-conjugated goat anti-mouse secondary antibody (dilution,
1:5,000; cat. no., ab6789; Abcam) was added and incubated for 1 h
at room temperature. The protein bands were visualized using an
enhanced chemiluminescence system (Pierce; Thermo Fisher
Scientific, Inc.). ImageJ 1.49 (National Institutes of Health,
Bethesda, MD, USA) was used to quantify of the western blots. This
assay was repeated three times.
Luciferase reporter assay
293T cells were seeded at 70% confluence into
24-well plates. Cells were co-transfected with PmirGLO-ADAM9-3′ UTR
Wt or Mut, and miR-154 mimics or NC using Lipofectamine 2000
according to the manufacturer's protocol. At 48 h after
transfection, cells were harvested and the luciferase activities
were measured by a Dual-Luciferase Reporter assay system (Promega
Corporation, Madison, WI, USA) according to the manufacturer's
protocol. The experiment was performed independently in
triplicate.
Statistical analysis
All data are presented as mean ± standard deviation.
Statistical analysis was performed with a Student's t-test or a
one-way analysis of variance using SPSS 16.0 software (SPSS, Inc.,
Chicago, IL, USA). The Student-Newman-Keuls test was used as a post
hoc test following ANOVA. P<0.05 was considered to indicate a
statistically significant difference.
Results
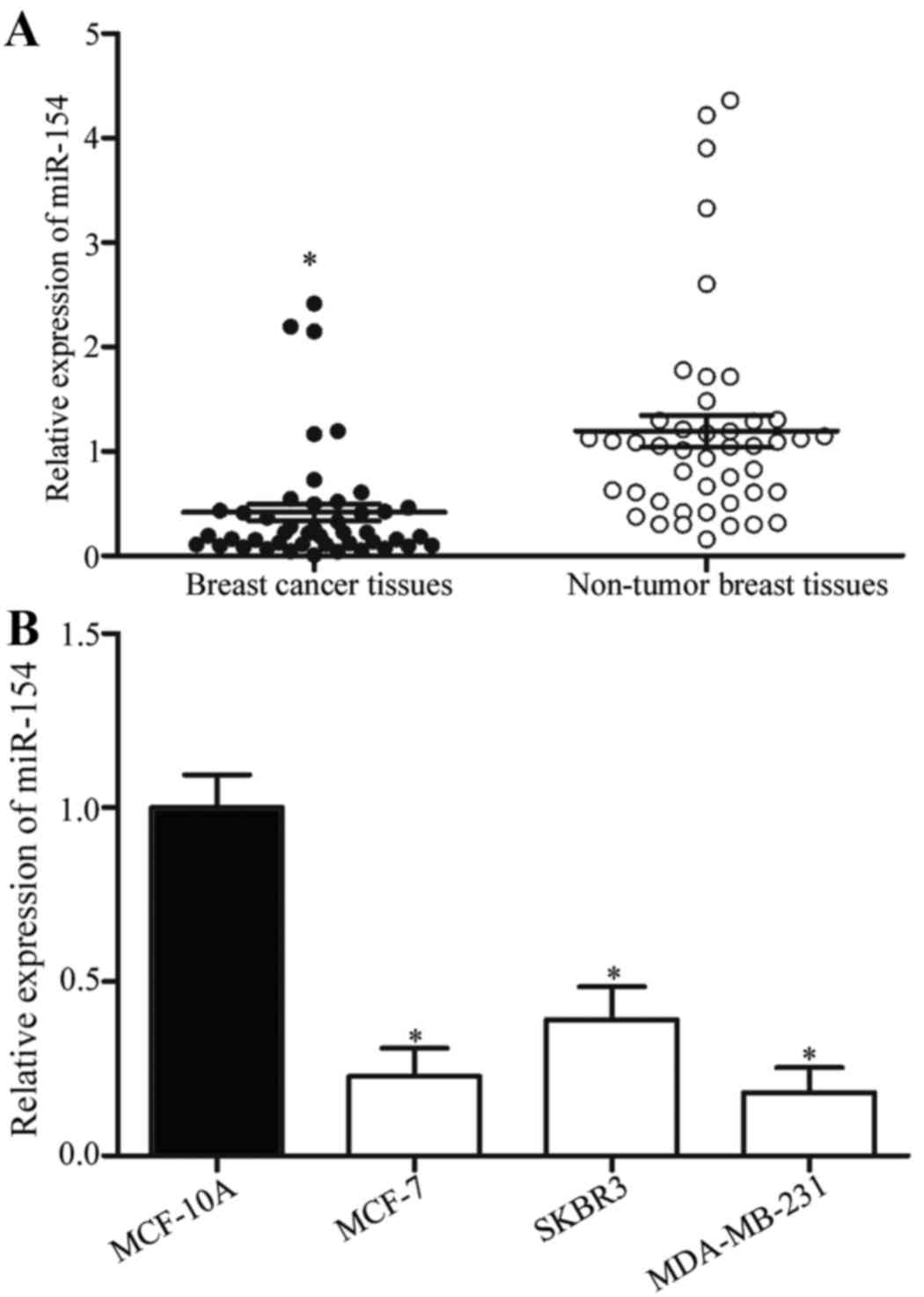
miR-154 is downregulated in breast
cancer tissues and cell lines
To investigate the role of miR-154 in human breast
cancer, its expression was analyzed in breast cancer and
corresponding non-tumor breast tissue samples. Using RT-qPCR, it
was identified that miR-154 expression levels were significantly
reduced in breast cancer tissues compared with the corresponding
non-tumor breast tissues (Fig. 1A;
P<0.05). miR-154 expression levels were then analyzed in the
breast cancer cell lines MCF-7, SKBR3 and MDA-MB-231, and in the
MCF-10A normal mammary epithelial cell line. Compared with MCF-10A,
miR-154 was downregulated in the breast cancer cell lines (Fig. 1B; P<0.05). These results suggested
that the downregulation of miR-154 may be associated with the
initiation and progression of breast cancer.
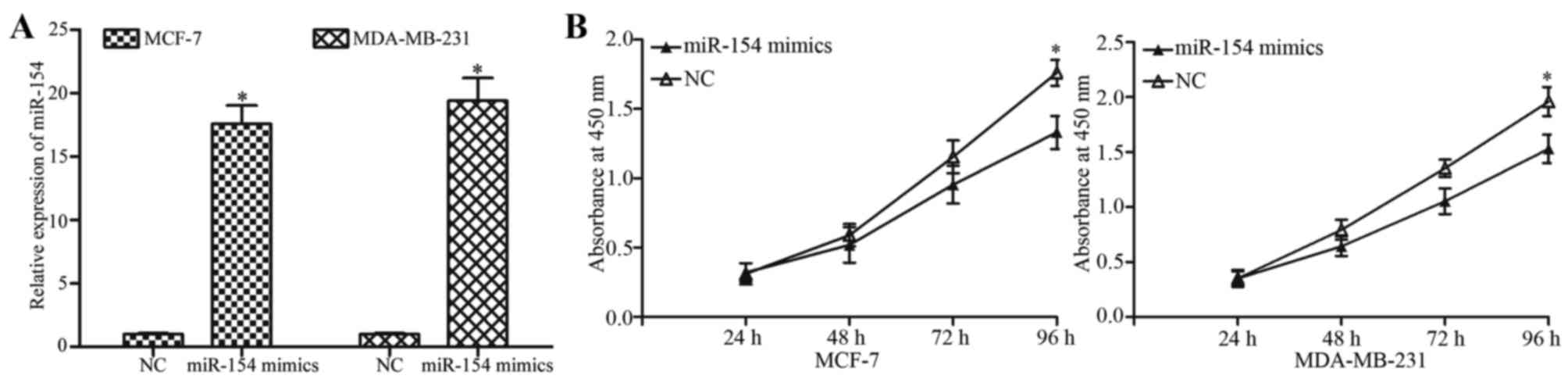
miR-154 inhibits the proliferation of
breast cancer cells
To investigate the effect of miR-154 on breast
cancer cell proliferation, MCF-7 and MDA-MB-231 cells were
transfected with an miR-154 mimic or NC. The overexpression of
miR-154 in the cells transfected with the mimic was confirmed by
RT-qPCR (Fig. 2A; P<0.05). The
extent of proliferation in the miR-154 mimic- or NC-treated cells
was detected by with a CCK8 assay. Transfection with the miR-154
mimic significantly decreased the proliferation of MCF-7 and
MDA-MB-231 cells compared with NC-transfected cells (Fig. 2B; P<0.05) at 96 h.
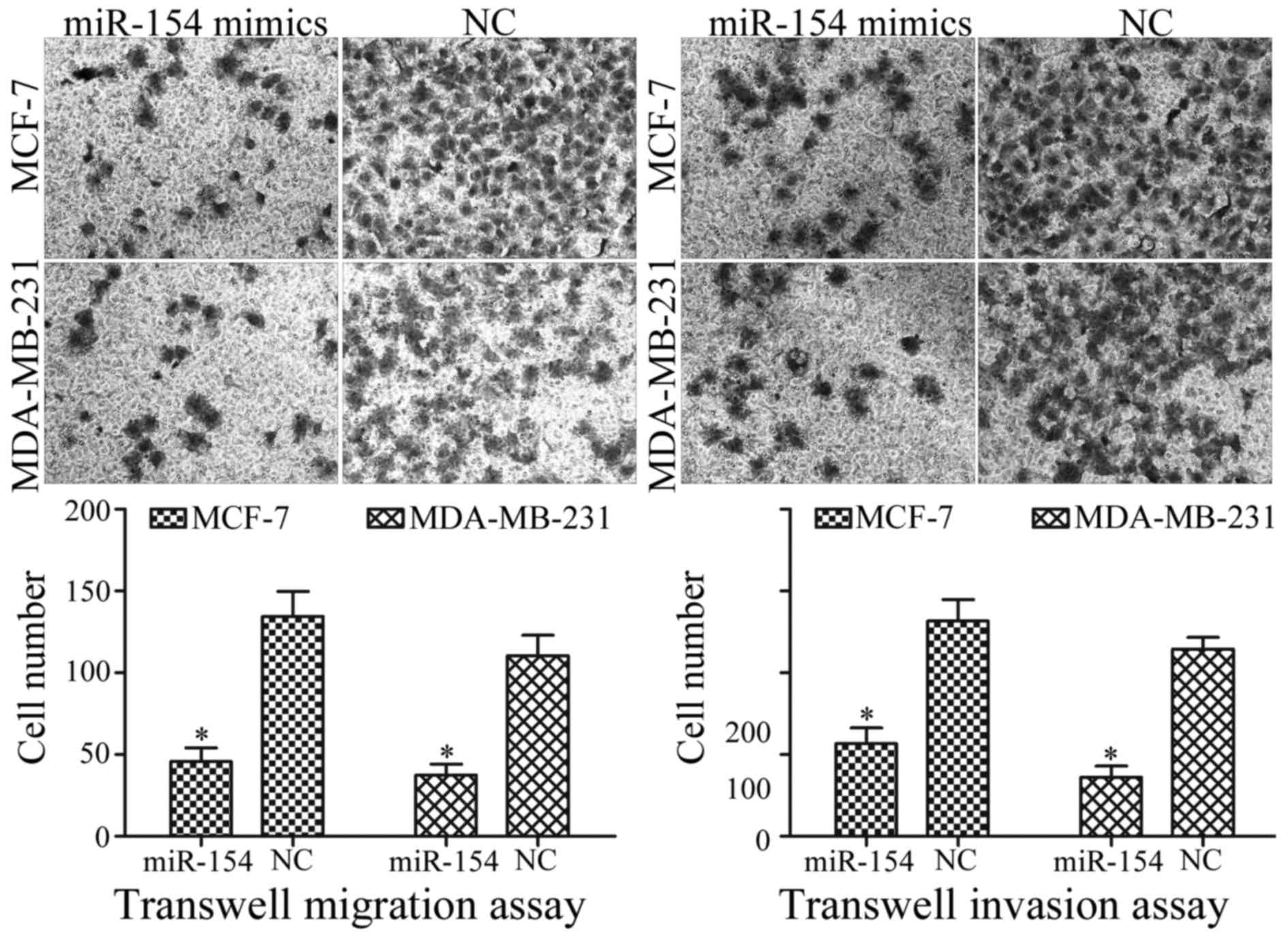
miR-154 inhibits the migration and
invasion of breast cancer cells
To determine whether miR-154 was associated with the
regulation of breast cancer metastasis, transwell migration and
invasion assays were performed. Compared with the NC, transfection
with the miR-154 mimic significantly decreased the migration
ability of MCF-7 and MDA-MB-231 cells (Fig. 3; P<0.05). Furthermore, miR-154
overexpression significantly repressed the capacity for invasion of
MCF-7 and MDA-MB-231 cells, compared with the NC (Fig. 3; P<0.05). Taken together, the
results indicated that miR-154 acted as a tumor suppressor in
breast cancer cells.
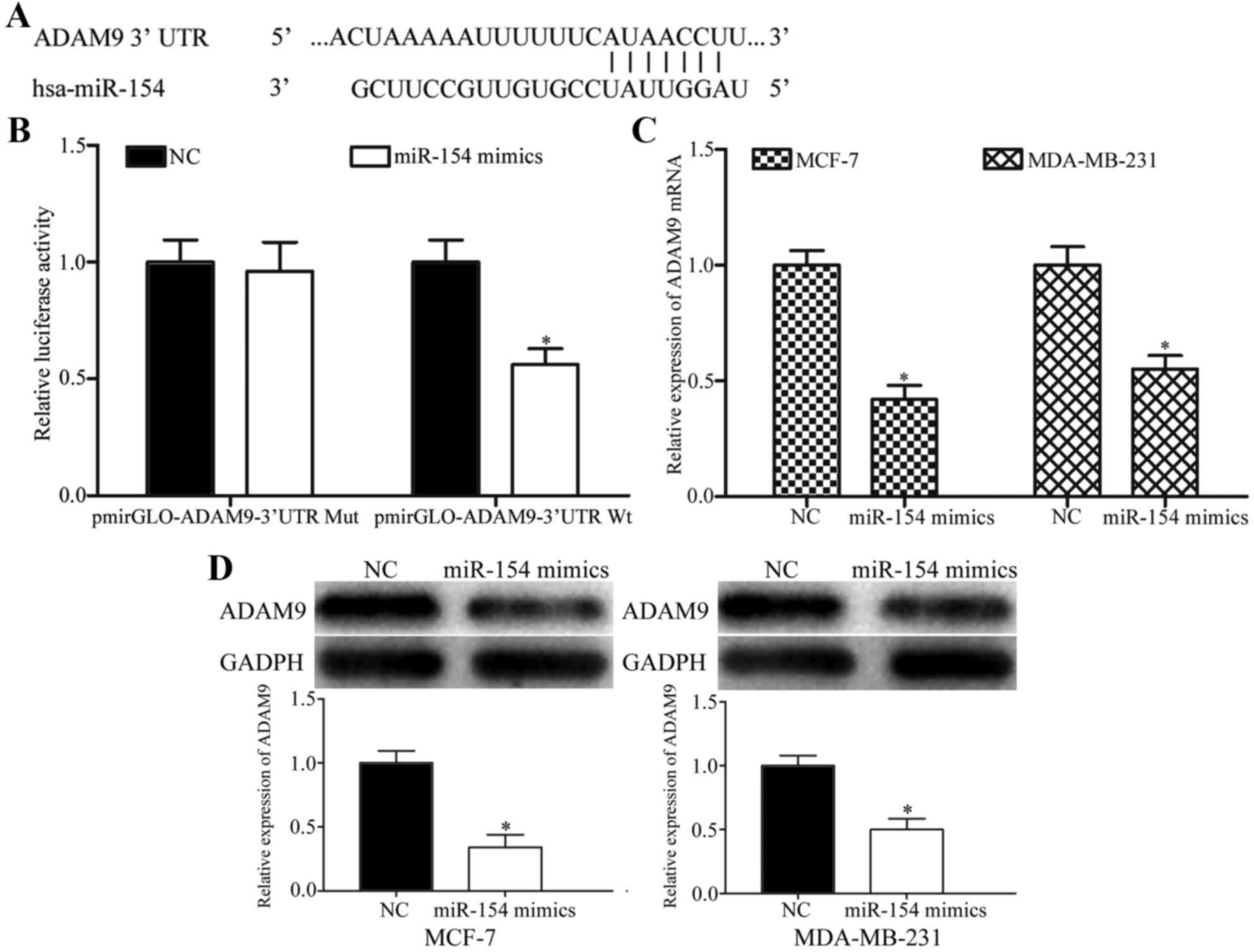
ADAM9 is a direct target of
miR-154
To investigate the molecular mechanism by which
miR-154 inhibited breast cancer cell proliferation, migration and
invasion, potential target genes of miR-154 were predicted with
miRanda and TargetScan. As shown in Fig.
4A, the 3′UTR of ADAM9 contained a putative miR-154 binding
site.
Luciferase reporter assays were performed to explore
whether ADAM9 was a direct target of miR-154. 293T cells were
transfected with luciferase reporter vectors, along with an miR-154
mimic or NC. The miR-154 mimic significantly decreased the
luciferase activities of PmirGLO-ADAM9-3′ UTR Wt compared with the
NC (Fig. 4B; P<0.05), but had no
effect on the PmirGLO-ADAM9-3′UTR Mut, suggesting that miR-154
could directly bind to the 3′UTR of ADAM9.
To determine whether ADAM9 expression was regulated
by miR-154, RT-qPCR and western blotting were performed to detect
the ADAM9 expression levels in MCF-7 and MDA-MB-231 cells
transfected with the miR-154 mimic or NC. Restoration of miR-154
expression suppressed the ADAM9 mRNA expression levels in MCF-7 and
MDA-MB-231 cells transfected with miR-154 mimics, compared with the
NC (Fig. 4C; P<0.05). Western
blotting demonstrated that the ectopic expression of miR-154
resulted in a significant decrease in ADAM9 protein expression
(Fig. 4D; P<0.05). Taken together,
ADAM9 was identified as a novel direct target of miR-154 in breast
cancer.
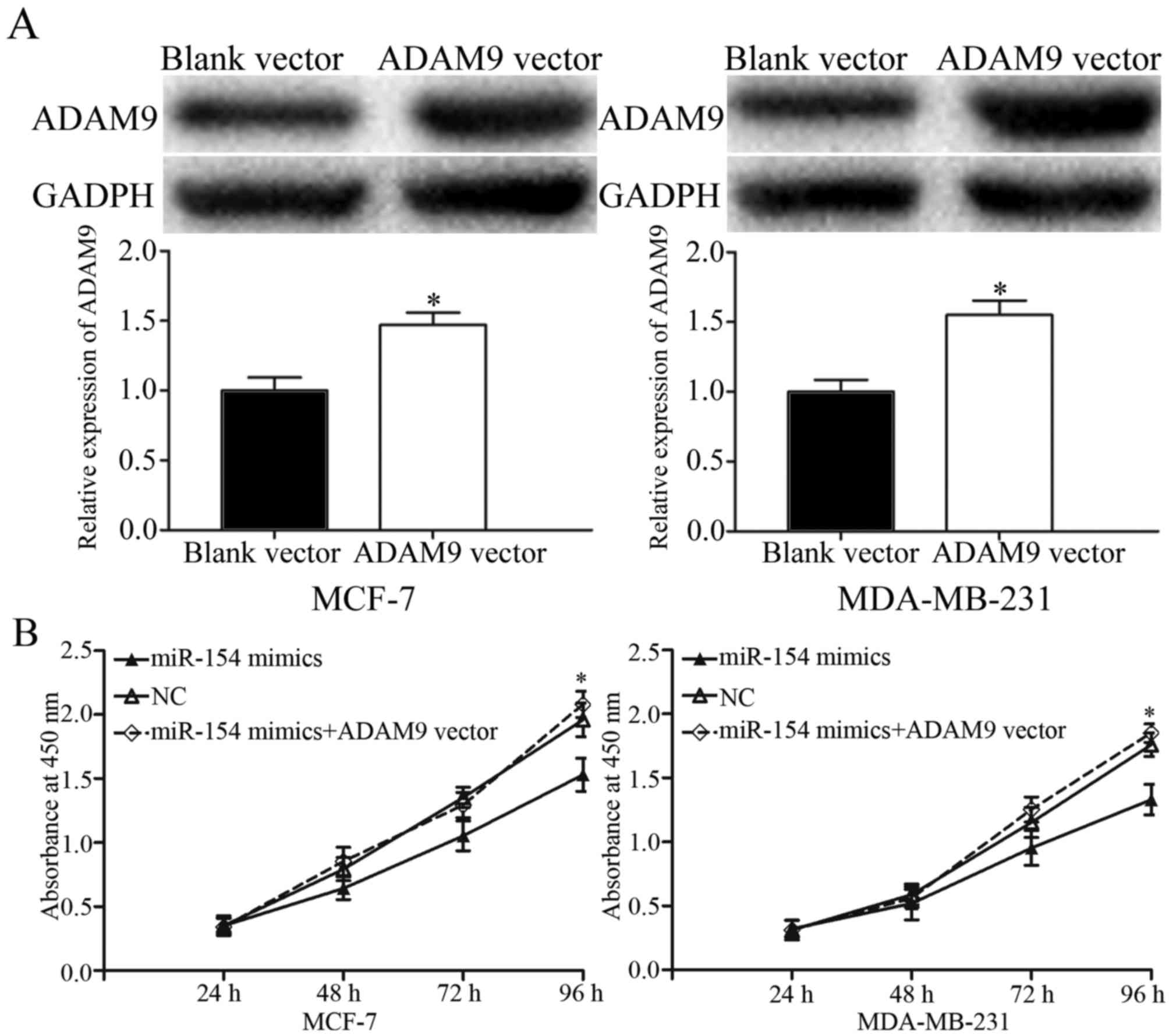
Restoration of ADAM9 abrogates tumor
suppression by miR-154 in breast cancer cells
To determine whether ADAM9 acted as a functional
target for miR-154, a gain-of-function analysis was performed by
the transfection of an ADAM9 vector or control into the
miR-154-overexpressing MCF-7 and MDA-MB-231 cells. Western blotting
revealed that ADAM9 was significantly upregulated in MCF-7 and
MDA-MB-231 cells transfected with the ADAM9 vector compared with
the control (Fig. 5A; P<0.05).
Proliferation assays, and transwell migration and invasion assays,
were performed. The data showed that ADAM9 overexpression
significantly abrogated the tumor suppressive effects of miR-154 on
breast cancer cell proliferation (Fig.
5B; P<0.05), migration and invasion (Fig. 5C). These results provided further
support that ADAM9 was a downstream functional target of
miR-154.
Discussion
Preventing tumor growth and metastasis is the
central problem in the treatment of breast cancer (24). The abnormal expression of miRNAs has
been identified in various types of human cancer; miRNAs have been
demonstrated to serve a significant role in the progression and
carcinogenesis of cancer (25). In
breast cancer, a number of miRNAs have been reported to affect
growth and metastasis. For example, miR-362-5p may target CYLD
lysine 63 deubiquitinase to inhibit the proliferation, migration
and invasion of breast cancer cells (26). Zhang et al (27) reported that miR-147 repressed the
growth and metastasis of breast cancer through the Akt/mechanistic
target of rapamycin pathway. Therefore, miRNAs may be suitable for
development as therapeutic targets to prevent breast cancer growth
and metastasis.
In the present study, the expression and role of
miR-154 in breast cancer was investigated. It was identified that
miR-154 was significantly downregulated in breast cancer tissues
and cell lines. Functional studies demonstrated that miR-154
overexpression suppressed the proliferation, migration and invasion
of breast cancer cells. These results indicated that miR-154 acted
as a tumor suppressor, and therefore, the low expression of miR-154
may contribute to breast cancer initiation and development.
miR-154 was previously identified as dysregulated in
a number of cancer types, and its expression has been associated
with clinical outcomes. For example, in colorectal cancer, miR-154
was previously identified as downregulated in tumor tissue, and its
low expression was associated with an increased tumor size, a
positive lymph node metastasis status and an advanced clinical
stage (28). In addition,
multivariate analysis identified low miR-154 expression as an
independent predictor of reduced survival time (28). Lin et al (29) reported that miR-154 expression levels
were lower in non-small-cell lung cancer relative to normal lung
tissue, and that low miR-154 expression was associated with
metastasis, a larger tumor size and an advanced TNM stage. The
results of these studies collectively indicated that miR-154 may be
a prognostic marker in a number of types of cancer.
Alterations in miR-154 expression have been
demonstrated to contribute to the initiation and progression of
various types of cancer. Lin et al (29) identified that miR-154 overexpression
suppressed non-small-cell lung cancer cell proliferation, colony
formation, invasion and migration, and induced apoptosis and G0/G1
cell cycle arrest in vitro. Restoration of miR-154
expression also decreased the growth of non-small-cell lung cancer
cell xenografts in vivo (29).
Xin et al (30) reported that
miR-154 inhibited the proliferation, colony formation, migration
and invasion of colorectal cancer cells. In prostate cancer,
enforced miR-154 expression decreased the proliferation of prostate
cancer cells in vitro (31).
In accord with the present study, these studies suggest that
miR-154 acts as a tumor suppressor and may be a suitable
therapeutic target in these types of cancer.
miRNAs serve major roles in a wide range of
physiological and pathological processes through negative
regulation of their target mRNAs (13,14).
Cyclin D2 (31), high mobility group
AT-hook 2 (32) and toll-like
receptor 2 (30) were previously
validated as target genes of miR-154. In the present study, ADAM9
was identified as a direct and functional target of miR-154 in
breast cancer. Bioinformatics analysis identified a putative
miR-154 binding site in the 3′UTR of ADAM9. miR-154 mimic
transfection markedly decreased the luciferase activity of a
luciferase reporter vector containing the ADAM9 3′UTR sequence.
miR-154 mimic transfection also suppressed ADAM9 mRNA and protein
levels in breast cancer cells. The restoration of ADAM9 expression
abrogated the suppression of cell proliferation, invasion and
migration by miR-154 in breast cancer cells. These data
demonstrated that miR-154 inhibited breast cancer cell
proliferation, migration and invasion through the miR-154/ADAM9
axis.
A disintegrin and metalloproteinases (ADAMs),
members of the metzincin superfamily of matrix metalloproteinases,
have been demonstrated to contribute to a range of biological
functions, including fertilization, adhesion, migration and
proteolysis (33,34). ADAM9, a member of the ADAM family, has
been demonstrated to be upregulated in a range of types of human
cancer, including renal cell cancer (35), prostate cancer (36), breast cancer (37), hepatocellular carcinoma (38), and pancreatic cancer (39). Previous studies have also identified
miRNAs that may interact with ADAM9. Wang et al (40) identified that miR-126 targeted ADAM9
to inhibit osteosarcoma proliferation and invasion. Zhang et
al (41) reported that miR-33a
repressed breast cancer cell growth and metastasis through the
negative regulation of ADAM9. Further studies that explore
additional novel targets for miR-154 and other miRNAs that can
regulate ADAM9 in breast cancer will facilitate a complete
understanding of the molecular mechanisms underlying the initiation
and progression of the disease.
In conclusion, the data presented in the present
study indicated that miR-154 may be a tumor-suppressing gene in
breast cancer. Transfection with an miR-154 mimic inhibited the
proliferation, migration and invasion of breast cancer cells.
Therefore, the restoration of miR-154 expression may be a effective
therapeutic strategy for the treatment of breast cancer in the
future.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fu SW, Lee W, Coffey C, Lean A, Wu X, Tan
X, Man YG and Brem RF: miRNAs as potential biomarkers in early
breast cancer detection following mammography. Cell Biosci.
6:62016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Porter P: ‘Westernizing’ women's risks?
Breast cancer in lower-income countries. N Engl J Med. 358:213–216.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beaglehole R and Yach D: Globalisation and
the prevention and control of non-communicable disease: The
neglected chronic diseases of adults. Lancet. 362:903–908. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parkin DM and Fernández LM: Use of
statistics to assess the global burden of breast cancer. Breast J.
12 Suppl 1:S70–S80. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Badar F, Faruqui ZS, Ashraf A and Uddin N:
Third world issues in breast cancer detection. J Pak Med Assoc.
57:137–140. 2007.PubMed/NCBI
|
|
7
|
Bray F, Ren JS, Masuyer E and Ferlay J:
Global estimates of cancer prevalence for 27 sites in the adult
population in 2008. Int J Cancer. 132:1133–1145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakamura S, Yagata H, Ohno S, Yamaguchi H,
Iwata H, Tsunoda N, Ito Y, Tokudome N, Toi M, Kuroi K and Suzuki E:
Multi-center study evaluating circulating tumor cells as a
surrogate for response to treatment and overall survival in
metastatic breast cancer. Breast Cancer. 17:199–204. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gong Y, He T, Yang L, Yang G, Chen Y and
Zhang X: The role of miR-100 in regulating apoptosis of breast
cancer cells. Sci Rep. 5:116502015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weigelt B, Peterse JL and van 't Veer LJ:
Breast cancer metastasis: Markers and models. Nat Rev Cancer.
5:591–602. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alonso DF, Ripoll GV, Garona J, Iannucci
NB and Gomez DE: Metastasis: Recent discoveries and novel
perioperative treatment strategies with particular interest in the
hemostatic compound desmopressin. Curr Pharm Biotechnol.
12:1974–1980. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nilsson I and Hoffmann I: Cell cycle
regulation by the Cdc25 phosphatase family. Prog Cell Cycle Res.
4:107–114. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hydbring P and Badalian-Very G: Clinical
applications of microRNAs. F1000Res. 2:1362013.PubMed/NCBI
|
|
14
|
Gromak N: Intronic microRNAs: A crossroad
in gene regulation. Biochem Soc Trans. 40:759–761. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iorio MV, Ferracin M, Liu CG, Veronese A,
Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M,
et al: MicroRNA gene expression deregulation in human breast
cancer. Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kosaka N, Iguchi H and Ochiya T:
Circulating microRNA in body fluid: A new potential biomarker for
cancer diagnosis and prognosis. Cancer Sci. 101:2087–2092. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pichler M and Calin GA: MicroRNAs in
cancer: From developmental genes in worms to their clinical
application in patients. Br J Cancer. 113:569–573. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Medina PP, Nolde M and Slack FJ: OncomiR
addiction in an in vivo model of microRNA-21-induced pre-B-cell
lymphoma. Nature. 467:86–90. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Obad S, dos Santos CO, Petri A, Heidenblad
M, Broom O, Ruse C, Fu C, Lindow M, Stenvang J, Straarup EM, et al:
Silencing of microRNA families by seed-targeting tiny LNAs. Nat
Genet. 43:371–378. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saito Y, Liang G, Egger G, Friedman JM,
Chuang JC, Coetzee GA and Jones PA: Specific activation of
microRNA-127 with downregulation of the proto-oncogene BCL6 by
chromatin-modifying drugs in human cancer cells. Cancer Cell.
9:435–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lujambio A, Calin GA, Villanueva A, Ropero
S, Sánchez-Céspedes M, Blanco D, Montuenga LM, Rossi S, Nicoloso
MS, Faller WJ, et al: A microRNA DNA methylation signature for
human cancer metastasis. Proc Natl Acad Sci USA. 105:pp.
13556–13561. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma L, Ma S, Zhao G, Yang L, Zhang P, Yi Q
and Cheng S: miR-708/LSD1 axis regulates the proliferation and
invasion of breast cancer cells. Cancer Med. 5:684–692. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van Jaarsveld MT, Helleman J, Berns EM and
Wiemer EA: MicroRNAs in ovarian cancer biology and therapy
resistance. Int J Biochem Cell Biol. 42:1282–1290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ni F, Gui Z, Guo Q, Hu Z, Wang X, Chen D
and Wang S: Downregulation of miR-362-5p inhibits proliferation,
migration and invasion of human breast cancer MCF7 cells. Oncol
Lett. 11:1155–1160. 2016.PubMed/NCBI
|
|
27
|
Zhang Y, Zhang HE and Liu Z: MicroRNA-147
suppresses proliferation, invasion and migration through the
AKT/mTOR signaling pathway in breast cancer. Oncol Lett.
11:405–410. 2016.PubMed/NCBI
|
|
28
|
Kai Y, Qiang C, Xinxin P, Miaomiao Z and
Kuailu L: Decreased miR-154 expression and its clinical
significance in human colorectal cancer. World J Surg Oncol.
13:1952015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin X, Yang Z, Zhang P and Shao G: miR-154
suppresses non-small cell lung cancer growth in vitro and in vivo.
Oncol Rep. 33:3053–3060. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xin C, Zhang H and Liu Z: miR-154
suppresses colorectal cancer cell growth and motility by targeting
TLR2. Mol Cell Biochem. 387:271–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu C, Shao P, Bao M, Li P, Zhou H, Cai H,
Cao Q, Tao L, Meng X, Ju X, et al: miR-154 inhibits prostate cancer
cell proliferation by targeting CCND2. Urol Oncol. 32:31.e9–e16.
2014. View Article : Google Scholar
|
|
32
|
Zhu C, Li J, Cheng G, Zhou H, Tao L, Cai
H, Li P, Cao Q, Ju X, Meng X, et al: miR-154 inhibits EMT by
targeting HMGA2 in prostate cancer cells. Mol Cell Biochem.
379:69–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Blobel CP: ADAMs: Key components in EGFR
signalling and development. Nat Rev Mol Cell Biol. 6:32–43. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Edwards DR, Handsley MM and Pennington CJ:
The ADAM metalloproteinases. Mol Aspects Med. 29:258–289. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fritzsche FR, Wassermann K, Jung M, Tölle
A, Kristiansen I, Lein M, Johannsen M, Dietel M, Jung K and
Kristiansen G: ADAM9 is highly expressed in renal cell cancer and
is associated with tumour progression. BMC Cancer. 8:1792008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fritzsche FR, Jung M, Tölle A, Wild P,
Hartmann A, Wassermann K, Rabien A, Lein M, Dietel M, Pilarsky C,
et al: ADAM9 expression is a significant and independent prognostic
marker of PSA relapse in prostate cancer. Eur Urol. 54:1097–1106.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
O'Shea C, McKie N, Buggy Y, Duggan C, Hill
AD, McDermott E, O'Higgins N and Duffy MJ: Expression of ADAM-9
mRNA and protein in human breast cancer. Int J Cancer. 105:754–761.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tao K, Qian N, Tang Y, Ti Z, Song W, Cao D
and Dou K: Increased expression of a disintegrin and
metalloprotease-9 in hepatocellular carcinoma: Implications for
tumor progression and prognosis. Jpn J Clin Oncol. 40:645–651.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Grützmann R, Lüttges J, Sipos B, Ammerpohl
O, Dobrowolski F, Alldinger I, Kersting S, Ockert D, Koch R,
Kalthoff H, et al: ADAM9 expression in pancreatic cancer is
associated with tumour type and is a prognostic factor in ductal
adenocarcinoma. Br J Cancer. 90:1053–1058. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang S, Wang X, Guo Q, Wang G, Han X, Li
X, Shi ZW and He W: MicroRNA-126 overexpression inhibits
proliferation and invasion in osteosarcoma cells. Technol Cancer
Res Treat. 15:NP49–NP59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang C, Zhang Y, Ding W, Lin Y, Huang Z
and Luo Q: MiR-33a suppresses breast cancer cell proliferation and
metastasis by targeting ADAM9 and ROS1. Protein Cell. 6:881–889.
2015. View Article : Google Scholar : PubMed/NCBI
|