Introduction
In many developed and developing countries, the
occurrence and mortality from lung cancer rank in first place among
all malignant tumors. In China, deaths caused by lung cancer
account for 20% of total cancer deaths, and this represents the
highest rates of occurrence and mortality in the world (1). Lung cancer can be divided into non-small
cell lung cancer (SCLC) and SCLC according to the different
pathological types. The occurrence of SCLC accounts for 13 to 20%
of the total number of cases of lung cancer, and its occurrence is
closely related to the intensity and duration of smoking (1–3). SCLC is
characterized by rapid development and high invasion ability, and
is often accompanied by secondary tumor syndrome and early
metastasis. Therefore, distant metastases such as dangerous brain
metastases, can often be found upon diagnosis of SCLC, and are
associated with poor prognosis. In some patients, distant
metastases serve as evidence for the initial diagnosis of SCLC
(4). Therefore, the timely use of
more sensitive methods to detect the degree of cancer metastasis is
key to improving the prognosis of SCLC. Among various serological
markers, S100B protein is a nervous system-specific protein.
Generally, S100B protein is highly expressed in glial cells and
Schwann cells (3). The amount of
S100B in serum is very low under physiological conditions. However,
serum S100B protein can increase in response to increased
blood-brain barrier permeability caused by damaged nerve cells or
other pathological conditions (2,3). For
example, increased levels of serum S100B are detectable in patients
with Alzheimer's disease (4).
This study aimed to provide theoretical support for
the early detection of brain metastases by detecting the expression
of serum S100B protein at different stages in patients with
SCLC.
Patients and methods
Patients
A total of 138 patients who underwent single valve
replacement surgery under general anesthesia were selected from
June 2013 to December 2015. The mean age of patients was 66.5±9.8
years. The mean age of healthy controls was 67.3±12.1 years. This
study was approved by the Ethics Committee of People's Hospital of
Rizhao. Signed written informed consents were obtained from the
patients.
Inclusion criteria
i) Patients with SCLC diagnosed by videography and
pathology; and ii) patients who signed the informed consent.
Exclusion criteria
i) Patients treated with immunosuppressive agents;
ii) patients with acute and chronic bacterial and/or viral
infections; iii) patients with autoimmune diseases; iv) patients
with connective tissue diseases; v) patients with malignant tumors;
vi) patients with liver and kidney dysfunction; vii) patients with
chronic muscular diseases; viii) patients with peripheral vascular
diseases, chronic heart failure, thyroid diseases, liver and kidney
dysfunction, liver and kidney tumors, severe trauma within the past
6 months, and those with history of surgery; ix) patients with
diabetes mellitus; x) patients with New York cardiac function grade
III or IV; xi) patients with myocardial infarction, percutaneous
coronary angioplasty, and coronary artery bypass grafting; patients
with recent use of adrenal cortex hormones or other immunomodulator
drugs; and xii) patients and family members who failed in
cooperation, and patients with a history of mental illness.
Sample collection
Fasting elbow vein blood (3 ml) was extracted in the
morning, and collected in ordinary plastic tubes. A total of 1.8 ml
of blood samples was placed in anti-coagulant tubes with 0.2 ml of
3.8% sodium citrate, followed by centrifugation (1116.2 × g) for 10
min at 1 h after collection. Serum was harvested, placed in 0.5 ml
EP tubes, and stored at −30°C. Serum samples were tested within 1
month after extraction.
ELISA
i) Serum pretreatment, EDTA or heparin was used as
anti-coagulant. After collection, samples were centrifuged (1,750 ×
g) for 30 min at 2–8°C; ii) ELISA plates were placed at room
temperature for 20 min; iii) the wells for standards and samples
were established, and 50 µl of standard solution was added to the
standard wells; iv) a total of 50 µl of samples was added to sample
wells, while blank wells were not treated; v) a total of 100 µl of
horseradish peroxidase-labeled antibody was added to each well, and
the samples were sealed and incubated in a thermostatic chamber for
60 min; vi) liquid was discarded, and 350 µl of washing solution
was added and removed after 1 min. The washing step was repeated
4–5 times; vii) a total of 50 µl of solution A and 50 µl of
solution B were added to each well at 37°C in the dark, and
incubated for 15 min; viii) stop solution (50 µl) was added, and
the absorbance (OD value) was measured at 450 nm within 15 min
after adding the stop solution; and ix) a standard curve was
constructed, and the levels of serum S100B were calculated. The
ELISA kit was from Wuhan Boster Biological Engineering Co., Ltd.
(Wuhan, China).
Classification of S100B protein
expression level
Serum S100B protein expression levels were divided
from + to ++++ as follows: +, 0–0.35 ng/µl; ++, 0.36–0.70 ng/µl;
+++, 0.71–1.05 ng/µl; and ++++, 1.06–1.4 ng/µl.
Statistical analysis
SPSS 17.0 statistical software (IBM, Armonk, NY,
USA) was used for data analysis. Numerical data are presented as
mean ± standard deviation (mean ± SD). Repeated measures analysis
of variance (ANOVA) was used to analyze the repeated measurements
of data. The independent-samples t-test was used for comparisons
between groups. The paired t-test was used for comparisons within
groups. Categorical data were analyzed by χ2 test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical staging of enrolled
patients
Among the 138 patients with SCLC, 44 cases with
brain metastases were identified. Other types of metastases were
found in 48 cases. Of the remaining 46 cases, 20 were initially
diagnosed with SCLC and without metastases, and 26 underwent
surgery and postoperative chemotherapy. The remaining 26 cases
underwent surgery and postoperative chemotherapy (Table I).
 | Table I.Clinical staging of enrolled
patients. |
Table I.
Clinical staging of enrolled
patients.
| Cases | Other types of
metastases | Brain metastases | Initial diagnosis
without metastases | Surgery and
postoperative chemotherapy |
|---|
| 138 | 44 (32%) | 48 (35%) | 20 (14%) | 26 (19%) |
The expression of S100B in patients
with SCLC and healthy controls
We found that serum S100B protein level was
significantly higher in patients with SCLC compared with healthy
controls (p<0.05) (Table II).
 | Table II.The expression of S100B in patients
with SCLC and healthy controls. |
Table II.
The expression of S100B in patients
with SCLC and healthy controls.
| Groups | Cases | Age (years) | BMI
(kg/m2) | Course of disease
(months) | S100B (ng/µl) |
|---|
| Patients with
SCLC | 138 | 67.3±12.1 | 21.3±3.4 | – | 0.055±0.001 |
| Healthy controls | 138 | 66.5±9.8 | 20.9±2.1 | 8.2±3.9 | 1.243±0.427 |
| T-value | – | 1.28 | 2.12 | – | 21.87 |
| P-value | – | 0.32 | 0.14 | – | 0.002 |
Subgroup analysis of SCLC
patients
Among the subgroups of patients with SCLC, the
levels of serum S100B in patients with brain metastases were
significantly higher than in other subgroups (p<0.05). There
were no significant differences in serum S100B protein level
between the healthy control group and other subgroups, except the
brain metastases group (p>0.05) (Table III).
 | Table III.The comparison of S100B expression in
SCLC subgroups. |
Table III.
The comparison of S100B expression in
SCLC subgroups.
| Subgroups | Cases | S100B (ng/µl) |
|---|
| Brain metastases | 48 | 2.138±0.174 |
| Other types of
metastases | 44 | 0.203±0.106 |
| No metastases | 20 | 0.128±0.032 |
| Healthy control | 138 | 0.055±0.001 |
| F-value | – | 22.87 |
| P-value | – | 0.001 |
The expression of S100B in patients with brain
metastases from SCLC after exposure to cobalt-60. We found that the
levels of S100B in patients with brain metastases from SCLC were
significantly reduced after 3 weeks of cobalt-60 irradiation
compared with those before treatment (p<0.05) (Table IV).
 | Table IV.The expression of S100B in patients
with brain metastases from SCLC after exposure to cobalt-60. |
Table IV.
The expression of S100B in patients
with brain metastases from SCLC after exposure to cobalt-60.
| Parameters | Cases | S100B (ng/µl) |
|---|
| Before treatment | 48 | 2.138±0.174 |
| After treatment | 48 | 1.017±0.212 |
| T-value | – | 31.82 |
| P-value | – | 0.005 |
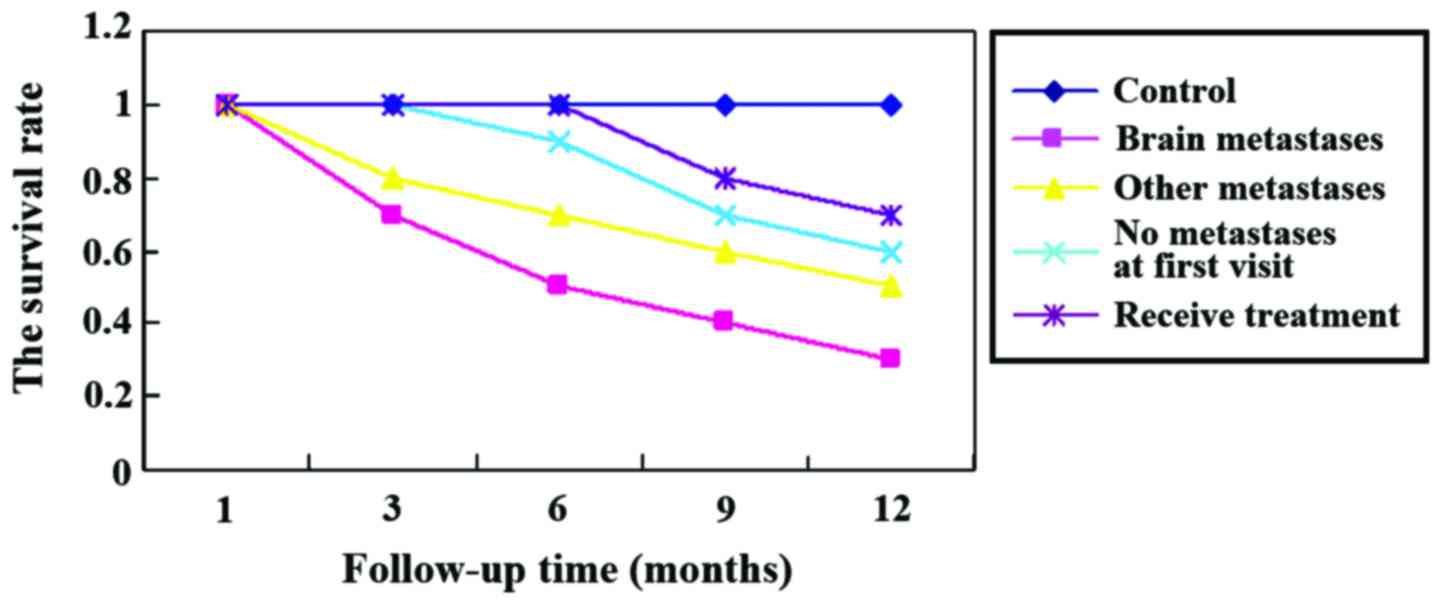
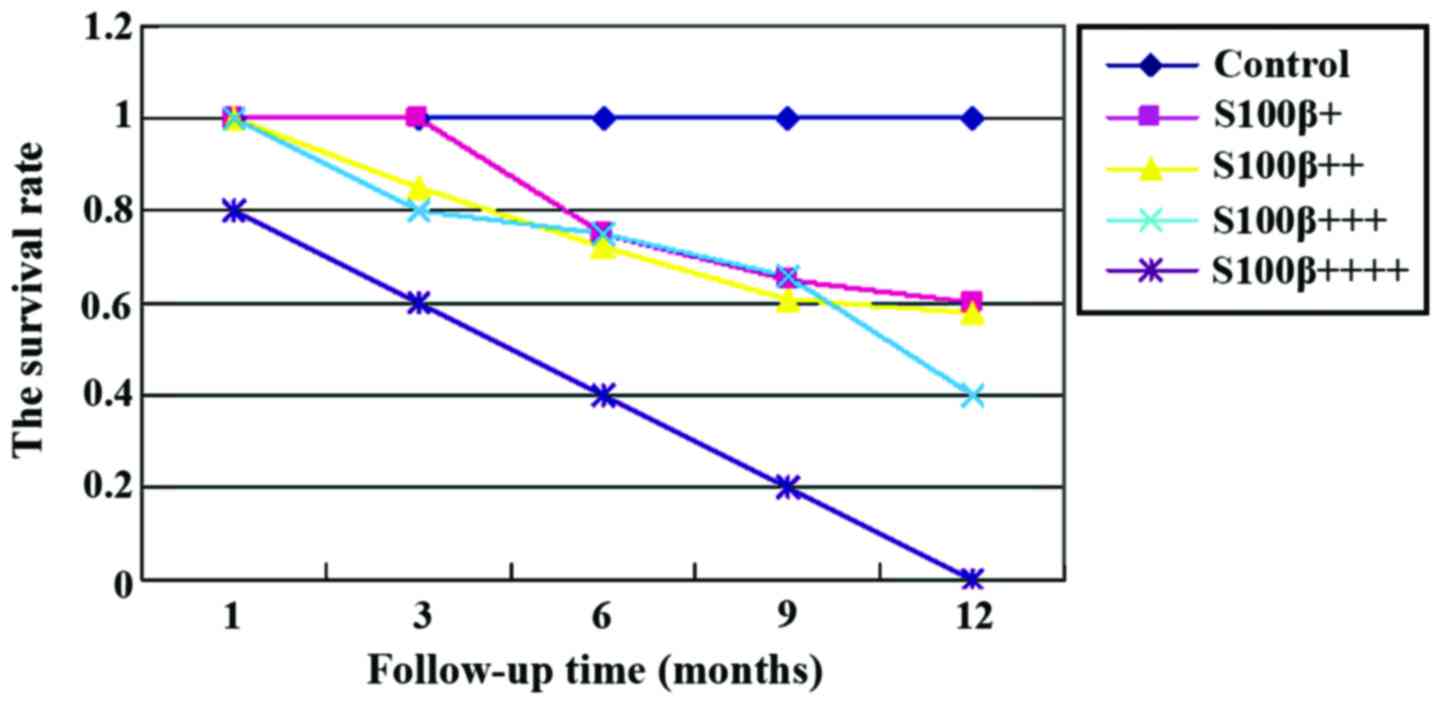
Survival analysis
At the 1 year follow-up, we found that the mortality
rate of patients with brain metastases was significantly higher
than that of other groups (p<0.05). Further statistical analyses
showed that higher expression of S100B protein was usually
associated with poorer prognosis, higher mortality rate at 1 year,
and lower survival rate (Figs. 1 and
2).
Discussion
SCLC accounts for roughly 20% of cases of lung
cancer. SCLC generally presents with a high degree of malignancy,
short doubling time, and early and extensive metastasis. SCLC is
sensitive to chemotherapy and radiotherapy. The early remission
rate is high. However, the drug resistance rate is also high,
leading to a high recurrence rate. Systemic chemotherapy is the
main method of treatment for SCLC. Each year, ~15% of newly
diagnosed cases of lung cancer are SCLC. The incidence of SCLC
increases with age, and ~45% of patients are over the age of 70
(1,2).
Therefore, early diagnosis of SCLC metastasis is an area of intense
research (5–8).
S100B is a 10.4 kDa protein expressed in astrocytes.
Its main synthetic process is accomplished by synaptic nodules in
the brain. It is one of the EF-hand proteins that belongs to the
low molecular weight acidic calcium-binding protein superfamily
(9). This protein is primarily
metabolized by the kidneys, and the waste is excreted with urine.
The expression levels of S100B are consistent among different
races, and no differences in expression were found between males
and females or between day and night (10). Although S100B is not specifically
expressed in the central nervous system, its concentration in brain
tissue is significantly higher than in other tissues (80–90% of
S100B can be found in the brain). Therefore, the protein can serve
as an early marker of brain injury (9). Besides the brain, S100B is synthesized
in adipose tissue (11), skin
melanoma (12,13) and T cells (14). The mechanism of secretion of S100B
protein after activation of astrocytes remains unknown. The
function of the S100B protein secreted by astrocytes depends on its
concentration, it has a neurotrophic effect at low concentration
(nmol), and is neurotoxic at high concentration (mmol) (9). At nanomolar levels, S100B has a
stimulatory effect on astrocytes, and can induce glial hyperplasia
in vitro (15).
Kawata et al reported that the S100B protein
cooperates with other serological markers such as neuron-specific
enolase, glial fibril acidic protein, and tau protein, and has good
clinical value for the accurate diagnosis of mild traumatic brain
injury (16). Duarte-Rojo et
al found that serum S100B concentrations were significantly
higher in patients with cirrhosis compared with those in healthy
volunteers, and S100B concentrations were further increased with
hepatic encephalopathy (17,18). Therefore, S100B, as a serological
biomarker, may have important clinical significance for the early
diagnosis of hepatic encephalopathy (17–20). We
found that patients with brain metastases from SCLC were usually
accompanied by varying degrees of increase of S100B protein
expression. Moreover, the increased S100B protein was found not to
be a marker for the early detection of other types of metastasis
from SCLC. There were no differences in the levels of S100B between
patients with non-brain metastases from SCLC and the healthy
control group. This suggests that the increase of S100B protein
level is more specific in brain metastases. In general, the
sensitivities of imaging methods such as PET-CT are usually poor
for the diagnosis of brain metastases, leading to misdiagnosis of
early brain metastases, which in turn affect the quality of life of
patients. In addition, we found that the level of serum S100B was
closely related to prognosis, higher expression was associated with
shorter survival time. Previous studies have shown that S100B can
accumulate in the extracellular matrix after the death of
astrocytes, or cell disintegration caused by substantial damage.
Under these conditions, the S100B concentration is approximately at
the micromolar level, and causes neurotoxicity (20). Therefore, large amounts of S100B
protein aggregation not only cause neurotoxic damage, but also
greatly reduce patient survival time. However, we found that S100B
protein expression levels were reduced along with reduced nerve
damage caused by cancer cells after radiotherapy. Therefore, we
believe that serum S100B protein has important clinical
significance for the early detection of brain metastases from
SCLC.
In conclusion, S100B protein can be used as a
serological marker for brain metastases from SCLC, and has
important clinical value for the early detection of brain
metastases.
References
|
1
|
Charpidou A, Tsagouli S, Gkiozos I, Grapsa
D, Moutsos M, Kiagia M and Syrigos K: Bone metastases in patients
with small cell lung carcinoma: Rate of development, early versus
late onset, modality of treatment, and their impact on survival. A
single-institution retrospective cohort study. Clin Exp Metastasis.
33:453–460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Higgins E, Edwards G, Tanguay J and Button
M: 93P: Chemoradiotherapy with radical intent for small cell lung
cancer (SCLC): A 5 year retrospective review. J Thorac Oncol. 11
Suppl 4:S972016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peng L, Xu L and Ouyang W: Role of
peripheral inflammatory markers in postoperative cognitive
dysfunction (POCD): A meta-analysis. PLoS One. 8:e796242013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scott DA, Evered LA and Silbert BS:
Cardiac surgery, the brain, and inflammation. J Extra Corpor
Technol. 46:15–22. 2014.PubMed/NCBI
|
|
5
|
Potter DS, Galvin M, Brown S, Lallo A,
Hodgkinson CL, Blackhall F, Morrow CJ and Dive C: Inhibition of
PI3K/BMX cell survival pathway sensitizes to BH3 mimetics in SCLC.
Mol Cancer Ther. 15:1248–1260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gridelli C, Casaluce F, Sgambato A, Monaco
F and Guida C: Treatment of limited-stage small cell lung cancer in
the elderly, chemotherapy vs. sequential chemoradiotherapy vs.
concurrent chemoradiotherapy: That's the question. Transl Lung
Cancer Res. 5:150–154. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi X, Duan H, Liu X, Zhou L and Liang Z:
Genetic alterations and protein expression in combined small cell
lung cancers and small cell lung cancers arising from lung
adenocarcinomas after therapy with tyrosine kinase inhibitors.
Oncotarget. 7:34240–34249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahn S, Hwang SH, Han J, Choi YL, Lee SH,
Ahn JS, Park K, Ahn MJ and Park WY: Transformation to small cell
lung cancer of pulmonary adenocarcinoma: Clinicopathologic analysis
of six cases. J Pathol Transl Med. 50:258–263. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sen J and Belli A: S100B in
neuropathologic states: The CRP of the brain? J Neurosci Res.
85:1373–1380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ikeda Y and Umemura K: Analysis of
reference values of serum S100B concentrations of Japanese adults.
Rinsho Byori. 53:395–399. 2005.(In Japanese). PubMed/NCBI
|
|
11
|
Hidaka H, Endo T, Kawamoto S, Yamada E,
Umekawa H, Tanabe K and Hara K: Purification and characterization
of adipose tissue S-100b protein. J Biol Chem. 258:2705–2709.
1983.PubMed/NCBI
|
|
12
|
Cocchia D, Michetti F and Donato R:
Immunochemical and immuno-cytochemical localization of S-100
antigen in normal human skin. Nature. 294:85–87. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kindblom LG, Lodding P, Rosengren L,
Baudier J and Haglid K: S-100 protein in melanocytic tumors. An
immunohistochemical investigation of benign and malignant
melanocytic tumors and metastases of malignant melanoma and a
characterization of the antigen in comparison to human brain. Acta
Pathol Microbiol Immunol Scand A. 92:219–230. 1984.PubMed/NCBI
|
|
14
|
Takahashi K, Isobe T, Ohtsuki Y, Sonobe H,
Yamaguchi H and Akagi T: S-100 protein positive human T-lymphocyte.
Am J Clin Pathol. 83:69–72. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Selinfreund RH, Barger SW, Pledger WJ and
Van Eldik LJ: Neurotrophic protein S100 beta stimulates glial cell
proliferation. Proc Natl Acad Sci USA. 88:pp. 3554–3558. 1991,
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kawata K, Liu CY, Merkel SF, Ramirez SH,
Tierney RT and Langford D: Blood biomarkers for brain injury: What
are we measuring? Neurosci Biobehav Rev. 68:460–473. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Duarte-Rojo A, Ruiz-Margáin A,
Macias-Rodriguez RU, Cubero FJ, Estradas-Trujillo J, Muñoz-Fuentes
RM and Torre A: Clinical scenarios for the use of S100β as a marker
of hepatic encephalopathy. World J Gastroenterol. 22:4397–4402.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee R, Yeung AW, Hong SE, Brose MS and
Michels DL: Principles of medical oncology. Asian Pac J Surg Oncol.
1:39–46. 2015.
|
|
19
|
Smithers BM, Li A, Kelly SL, Wilson MK,
Chaturvedi A and Samoukovic K: Staging of non-small cell lung
cancer and small cell lung cancer. Asian Pac J Surg Oncol.
1:125–140. 2015.
|
|
20
|
Kowgier M, Qiu LA and Tse H: Surgery for
small cell lung cancer. Asian Pac J Surg Oncol. 1:171–180.
2015.
|