Introduction
Endometrial carcinoma (EC) is one of the most common
malignancies in the female reproductive system. It typically occurs
in perimenopausal women approximately 50 years of age. Recent
studies have shown that the incidence of EC is rising yearly
(1). According to statistical data,
the morbidity of EC ranks second among female reproductive tract
tumors, second only to cervical cancer (2). It has been found that EZH2 is a core
component of the family of Polycomb group proteins (3). Studies have shown that EZH2 has abnormal
expression in gastric (4), esophageal
(5), liver cancer (6,7) and other
malignant tumors and is closely related to tumor growth,
development and prognosis. However, there has been very little
research on the expression of EZH2 protein in the tissues of
patients with EC. Moreover, EZH2 is highly expressed in a variety
of tumors and can regulate the expression of genes to promote cell
proliferation, invasion and metastasis; therefore, EZH2 may be a
potential target for the treatment of EC (8,9). In this
study, an immunohistochemical assay was used to detect the
expression of EZH2 protein in endometrial carcinoma and adjacent
tissues obtained from a total of 104 patients. In addition, the
correlation between EZH2 expression and clinicopathological
features was analyzed to explore the effects of inhibition of EZH2
expression on the proliferation and invasion of endometrial
carcinoma RL-952 cells.
Materials and methods
Cell line
Human endometrial carcinoma cell line RL-952 cells
were purchased from the Shanghai Academy of Life Sciences Cell Bank
(Shanghai, China).
Reagents
Lipofectamine 2000 transfection reagent was
purchased from Invitrogen (Carlsbad, CA, USA); small interfering
RNA (si-RNA) was synthesized by Shanghai Gemma (Pudong New Area,
Shanghai, China); rabbit anti-human EZH2 monoclonal antibody and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody were
purchased from Abcam (Cambridge, UK); immunohistochemical SP kit,
DAB color kit and hematoxylin were purchased from Zhongjin Jinqiao
(Beijing, China); horseradish peroxidase conjugated secondary
antibody was purchased from Santa Cruz Biotechnology (Dallas, TX,
USA); Transwell Chamber was purchased from Corning Inc. (Corning,
NY, USA); and CCK-8 kit was purchased from Nanjing Kaiji Biological
Co., Ltd. (Nanjing, China).
Sample collection
A total of 104 patients admitted to Daqing Longnan
Hospital from May, 2014 to June, 2016 with the pathological
diagnosis of EC were selected as subjects. All patients underwent
total uterine, double attachment resection and pelvic
lymphadenectomy. Patients had not received chemotherapy,
radiotherapy or biological targeted therapy before surgery. The
patients ages ranged from 31 to 67 years (average age, 47.56±15.49
years). According to the degree of pathological differentiation, 38
cases were well differentiated, 34 cases were moderately
differentiated and 32 cases were poorly differentiated. According
to the revised staging for carcinoma adopted by the 2009
International Union of Obstetrics and Gynecology (FIGO), 26 cases
were in stage I, 31 cases were in stage II, 29 cases were in stage
III, and 18 cases were in stage IV. Pathological types: endometrial
adenocarcinoma was present in 59 cases, clear cell carcinoma in 23
cases and serous papillary carcinoma in 22 cases. A total of 104
corresponding adjacent tissue samples were obtained from the
patients. This study was approved by the Medical Ethics Committee
of Daqing Longnan Hospital (Heilongjiang, China), and all patients
and their families signed the informed consent.
Determination of EZH2 expression in
endometrioid adenocarcinoma and adjacent tissues by an
immunohistochemical assay
The paraffin embedded tissue blocks of patients with
EC were collected and 4 µm consecutive paraffin sections were cut
and heated at 65°C for 2 h. After dewaxing and hydration, an
immunohistochemical SP method was used in accordance with the
protocol. Sections were incubated with anti-EZH2 primary antibody
(1:500) overnight at 4°C followed by 3 washes in phosphate-buffered
saline (PBS). The sections were then incubated with
enzyme-conjugated secondary antibody at 37°C for 1 h; after
washing, the sections were incubated with diaminobenzidine (DAB) as
the chromogen and counterstained with hematoxylin. Assessment of
results was as follows: EZH2 positive cells showed brown or yellow
granules in the nuclei. Six different fields of view were randomly
selected under microscope (Olympus, Tokyo, Japan) at ×200
magnification and the result was evaluated according to the
percentage of positive cells and the severity of the pigment.
First, scoring according to the severity of the pigment was:
negative as 0 points; light yellow as 1 point; brown as 2 points;
strong brown as 3 points. Second, scoring according to the
percentage of positive cells in the total cells was: 0–30% as 1
point, 30–70% as 2 points and 70–100% as 3 points. Two scores were
multiplied for each section: 3 points or more represented positive
expression; otherwise, the scores represented negative
expression.
Transfection of small interfering RNA
by cell culture medium
The endometrial cancer RL-952 cells were removed
from the −80°C refrigerator and placed in a 37°C water bath until
they were completely thawed. The cells were then incubated in a
37°C incubator and the medium was replaced periodically.
Lipofectamine 2000 transfection reagent was used to transfect
siRNA-EZH2 three times and the control group siRNAs once into
endometrial cancer RL-952 cells, respectively, for 4 samples total.
The protocol was performed according to the user manual. Three
primers, EZH2-Homo-1741 (si-EZH2-1), EZH2-Homo-803 (si-EZH2-2) and
EZH2-Homo-2167 (si-EZH2-3), were designed to ensure the efficiency
of the transfection. The sequences are shown in Table I.
 | Table I.siRNA-EZH2 primer sequences. |
Table I.
siRNA-EZH2 primer sequences.
| siRNA | Sense strand sequence
(5′-3′) | Antisense strand
sequence (3′-5′) |
|---|
| si-EZH2-1 |
GCUCCUCUAACCAUGUUUATT |
UAAACAUGGUUAGAGGAGCTT |
| si-EZH2-2 |
GGAUCACCGAGAUGAUAAATT |
UUUAUCAUCUCGGUGAUCCTT |
| si-EZH2-3 |
GAGGGAAAGUGUAUGAUAATT |
UUAUCAUACACUUUCCCUCTT |
Determination of EZH2 protein
expression in endometrial carcinoma RL-952 cells by western blot
analysis
After transfection, the total protein in the cells
was extracted according to the RAPI lysis and extraction buffer
manual. Protein concentration was quantified by Coomassie brilliant
blue method. A total of 60 µg of protein was separated by 10%
SDS-PAGE and the separated protein was transferred to PVDF membrane
after 1.5 h of electrophoresis. The membrane was incubated in 5%
skim milk powder at room temperature for 1 h, and then rabbit
anti-human EZH2 primary antibody (1:1,500) was added and the
membrane was incubated overnight at 4°C. After washing the membrane
with PBS, the secondary antibody IgG (1:2,000) was added and the
membrane was incubated again at 37°C for 2 h. ECL was added on the
membrane and blots were developed in the dark. Images were recorded
with a gel imaging system (Bio-Rad Laboratories, Irvine, CA, USA)
and the gray-scale values were calculated. GADPH was used as the
internal reference and the ratio of EZH2 to GAPDH protein was
interpreted as the relative expression level of EZH2.
Determination of cell proliferative
ability by MTT after interference of EZH2 gene
The cells were collected after transfection (control
group, cells transfected with control siRNAs; negative control
group, normal untransfected cells; si-EZH2 group, cells transfected
with si-EZH2-3) and cultured in an incubator. The number of cells
was 5,000 in each well, with 5-wells for each group. After
incubation at 37°C and 5% CO2 for 24 h, 5 µl of 5 mg/ml
MTT was added to each well. After 4 h, 200 µl of dimethyl sulfoxide
(DMSO) was added to each well with shaking for 10 min. The
absorbance value of each well at 490 nm was detected by a
microplate reader (Bio-Rad 680). The absorbance values at days 1,
2, 3 and 4 were detected by MTT assay and the cell growth curve was
plotted.
Detection of cell invasion ability by
a Transwell assay after interference of EZH2 gene
The cells were collected after transfection (control
group, cells transfected with control siRNAs; negative control
group, normal untransfected cells; si-EZH2 group, cells transfected
with si-EZH2-3). Matrigel was diluted to a 1:1 ratio with precooled
serum-free culture medium and 20 µl of diluted Matrigel was evenly
added to the above culture wells made of 8 µm polycarbonate
membrane at 37°C until fully solidified. A total of 200 µl cells
diluted with serum-free medium (1×105 cells/ml) were
then added at the lower culture wells and the Transwell plate was
incubated at 37°C and 5% CO2 for 24 h. The Transwell
plate was removed and fixed with methanol for 30 min; 0.1% crystal
violet was added for 10 min for staining. After carefully wiping
the bottom of the wells with a wet cotton swab to remove Matrigel
and non-invasive cells, the wells were observed under an inverted
microscope (Leica Microsystems, Wetzlar, Germany). Five fields of
view were randomly selected and the cells were counted, with the
average number of cells interpreted as the cells passing through
the basement membrane.
Statistical analysis
Data were analyzed using SPSS 21.0 software (IBM,
New York, NY, USA). Measurement data between the two groups were
compared by t-test and among multiple groups it was analyzed by the
analysis of variance. The enumeration of data among groups was
compared by the χ2 test. Differences with a P<0.05
were considered statistically significant.
Results
Expression of EZH2 protein in the
tissues of patients with endometrial carcinoma
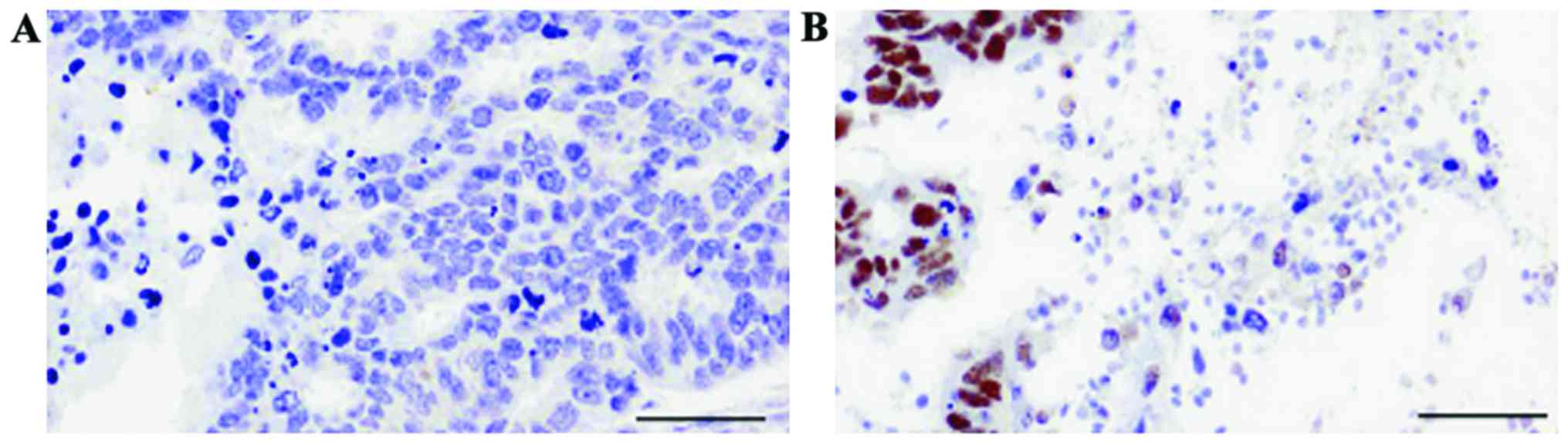
Under microscope, EZH2 protein was observed in the
nuclei showing brown or yellow particles. In this study,
immunohistochemical results showed that, among 104 cases of EC
specimens, 71 cases showed positive expression of EZH2, with an
expression rate of 68.27%. In contrast, among 104 cases of adjacent
tissue, positive expression of EZH2 was evident in 25 cases, with a
24.03% expression rate. The expression of EZH2 in endometrial
carcinoma tissue was significantly higher than that in adjacent
tissue (P<0.05) (Fig. 1 and
Table II).
 | Table II.Expression of EZH2 in endometrial
cancer and adjacent tissues. |
Table II.
Expression of EZH2 in endometrial
cancer and adjacent tissues.
|
|
| EZH2 expresssion |
|---|
|
|
|
|
|---|
| Group | No. of cases | − | + | Positive rate
(%) |
|---|
| Endometrial cancer
tissue | 104 | 33 | 71 | 68.27 |
| Adjacent tissue | 104 | 79 | 25 | 24.03 |
| χ2 |
|
| 17.861 |
|
| P-value |
|
| 0.017 |
|
Relationship between the EZH2 positive
expression and the clinical characteristics of patients
The expression of EZH2 in EC was not correlated with
the menopausal status or age of patients (P>0.05), but was
correlated with the histological grade, depth of tumor invasion,
lymph node metastasis and TNM stage (P<0.05) (Table III).
 | Table III.Relationship between the positive
expression of EZH2 and the clinical characteristics of
patients. |
Table III.
Relationship between the positive
expression of EZH2 and the clinical characteristics of
patients.
|
|
| EZH2 positive
expression |
|---|
|
|
|
|
|---|
| Pathological
features | Total no. of
cases | No. of cases (%) |
χ2-value | P-value |
|---|
| Menopause |
|
|
|
|
|
Yes | 64 | 46 (71.88) | 0.142 | >0.05 |
| No | 40 | 26 (65.00) |
|
|
| Age (years) |
|
|
|
|
|
≥50 | 80 | 56 (70.00) | 0.473 | >0.05 |
|
<50 | 24 | 16 (66.67) |
|
|
| Histology
grade |
|
|
|
|
| I | 42 | 30 (71.43) | 13.287a | <0.05 |
| II | 30 | 22 (73.33) |
|
|
|
III | 32 | 19 (59.38) |
|
|
| Depth of
infiltration |
|
|
|
|
|
Shallow | 38 | 24 (63.16) | 5.692a | <0.05 |
|
Deep | 66 | 47 (71.21) |
|
|
| Lymph node
metastasis |
|
|
|
|
| No | 58 | 38 (65.52) | 8.472a | <0.05 |
|
Yes | 46 | 33 (71.74) |
|
|
| TNM stage |
|
|
|
|
| I,
II | 42 | 28 (66.67) | 6.489a | <0.05 |
| III,
IV | 62 | 45 (72.58) |
|
|
Determination of protein expression
before and after interference of the EZH2 gene by western blot
analysis
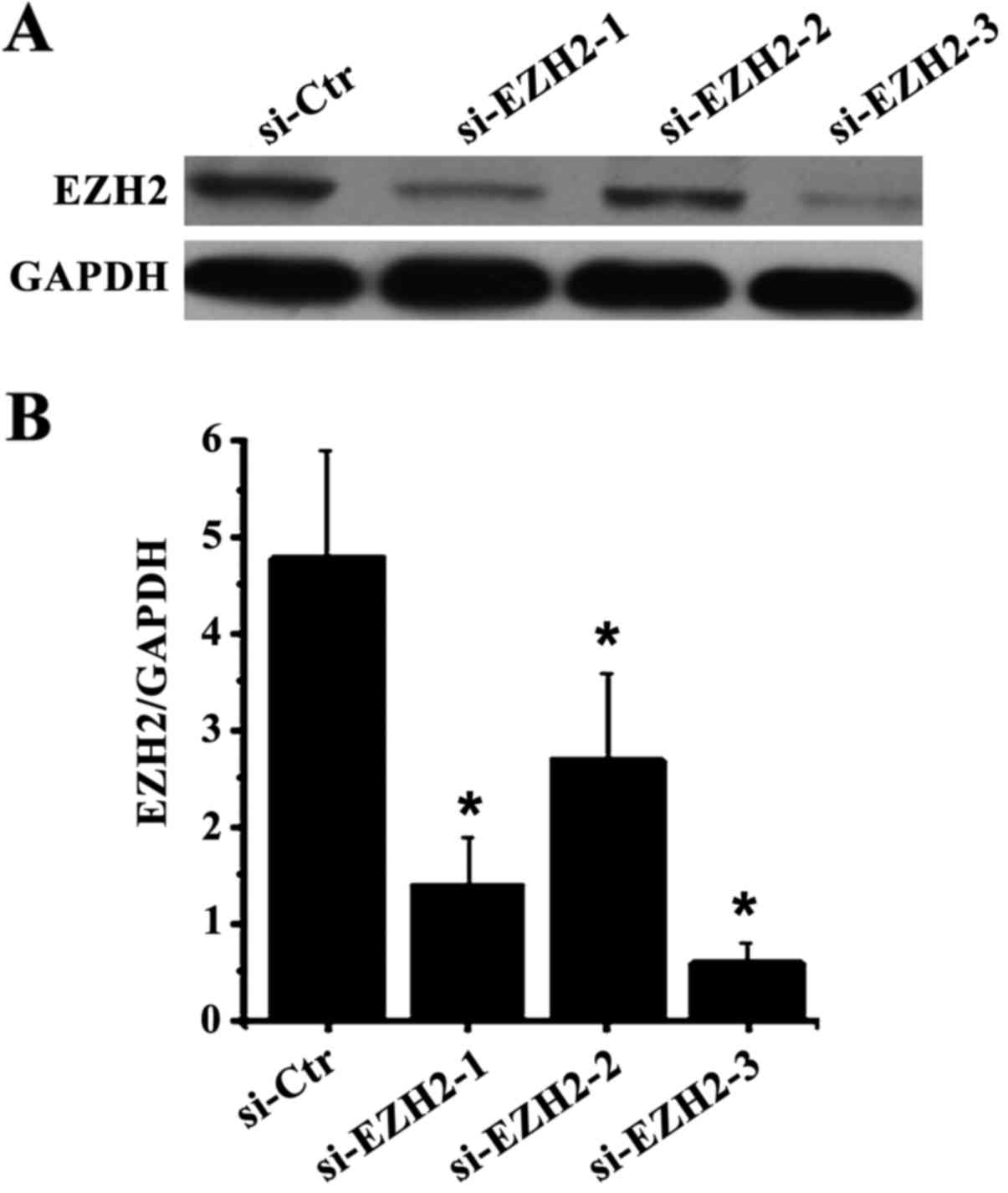
As shown in Fig. 2,
compared with the control group, the expression of EZH2 in cells
was significantly inhibited after the three siRNA-EZH2 samples were
transfected into endometrial carcinoma RL-952 cells, in which the
inhibition of the third siRNA was the most markedly pronounced.
Effect of the EZH2 gene interference
on cell proliferative ability
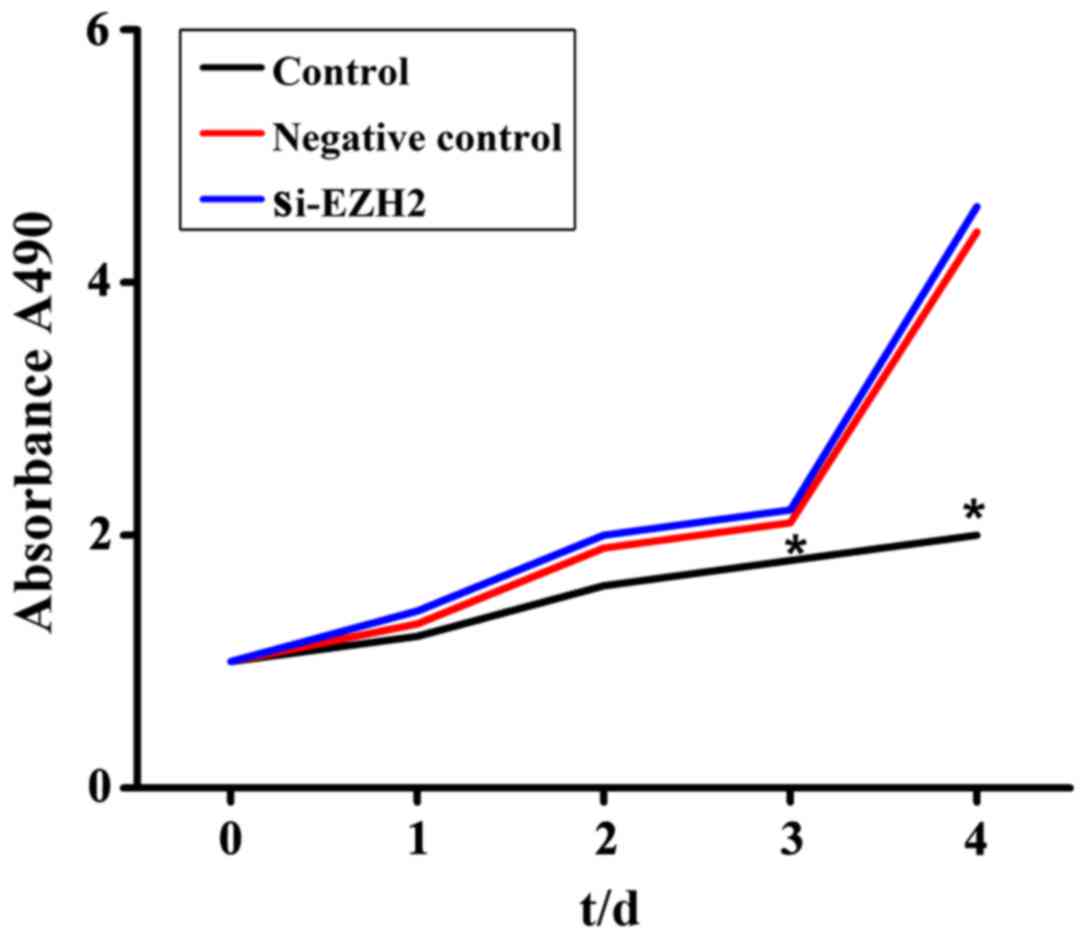
Compared with the control group, the proliferative
ability of the cells was significantly decreased after being
treated with siRNA-EZH2-3, and the difference was statistically
significant (P<0.05) (Fig. 3).
Effect of the EZH2 gene interference
on cell invasion ability
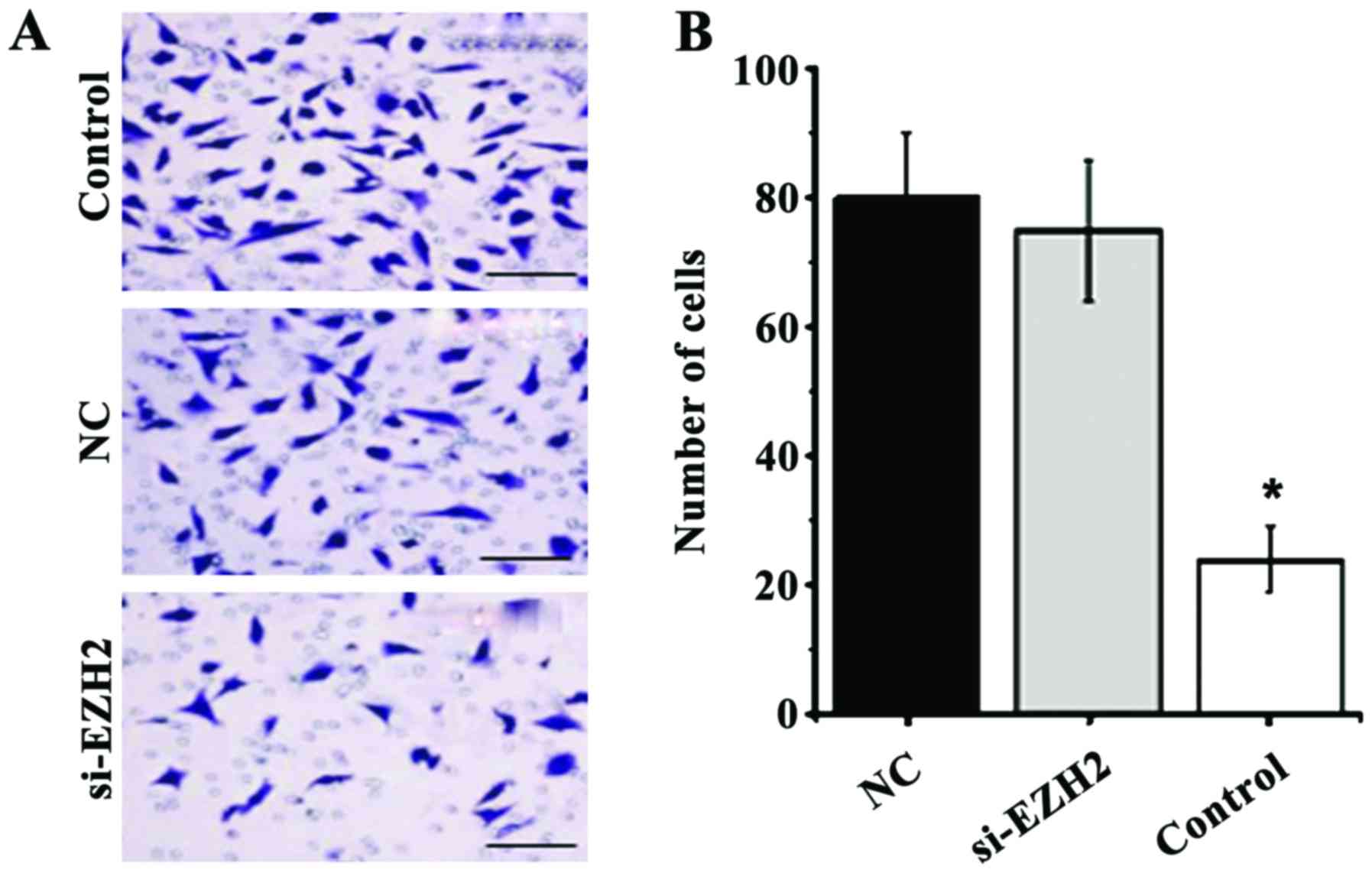
The Transwell results showed that the number of
cells passing through the basement membrane was significantly
reduced after interference of the EZH2 gene with siRNA-EZH2-3
(Fig. 4A). The average number of
cells in the si-EZH2 group was significantly higher than that in
the control group and the negative control group (P<0.05)
(Fig. 4B).
Discussion
Recent studies have found that the EZH2 gene and
protein are highly expressed in a variety of malignant tumors,
while non-expression or a low level of EZH2 expression is evident
in normal or adjacent tissue (10,11). It
has been reported that EZH2 was highly expressed in breast
(12), ovarian (13), non-small cell lung (14), colorectal cancer (15) and other tumor tissues, and the
positive expression rates, as determined by immunohistochemical
assays, were 71.7, 49.7, 62.3 and 91.9%, respectively. Several
researchers world-wide have also reported on the relationship
between EZH2 expression and clinicopathological features. Chang
et al (16) collected a total
of 128 samples from patients with the pathological diagnosis of
breast cancer and analyzed the expression of EZH2 in the tissues by
immunohistochemical staining. The results showed that the
expression of EZH2 in the tissues of breast cancer at stages III
and II was 60.9%, which was significantly higher than 18.7%
expression observed in stage I (P<0.05). Cai et al
(17) analyzed 212 biopsies from
patients with hepatocellular carcinoma and the results showed that
diagnostic sensitivity and specificity of EZH2 expression for
hepatocellular carcinoma were 95.8 and 97.8%, respectively,
suggesting that EZH2 could have an important clinical role in the
diagnosis of hepatocellular carcinoma. In addition, they compared
the relationship between EZH2 expression and the clinical stage and
survival in patients with hepatocellular carcinoma. The results
showed that the expression of EZH2 was only correlated with the
clinical stage and survival time of patients, but not with age,
gender, tumor size, personal history of liver cirrhosis, degree of
differentiation or other factors. Rao et al (18) analyzed 179 cases of ovarian cancer
samples and the results suggested that highly expressed EZH2 had a
significant correlation with the clinical stage and survival rate,
but not with the patient's age or gender. A total of 1443 cases of
breast cancer tissues were immunohistochemically stained by a
concurrent study, and the results showed that high expression of
EZH2 was closely related to tumor size and pathological type.
Long-term follow-up observation showed that high expression of EZH2
had important clinical value in the prognosis of distant tumor
metastasis (19). All these studies
indicated that EZH2 was highly expressed in different malignancies,
and no significant correlation was observed between its expression
and the age or gender of patients. However, EZH2 expression has a
certain correlation with the clinical stage and survival time;
therefore, it is possible for EZH2 to be a tumor-diagnosed
biological marker. The results of this study demonstrated that the
expression of EZH2 in endometrial carcinoma tissue (68.27%) was
significantly higher than that in adjacent tissue (24.03%)
(P<0.05). The expression of EZH2 in endometrial carcinoma tissue
was not correlated with the menopausal status or age of the
patients (P>0.05): however, it was correlated with histological
grade, depth of tumor invasion, lymph node metastasis and TNM stage
(P<0.05). These correlations are consistent with findings of
other research studies (20–22).
EZH2 is highly expressed in EC and has a certain
correlation with the clinical stage and survival time of patients,
but the exact mechanistic activity of EZH2 in EC is still unclear.
Therefore, this study aimed to further explore the possible
mechanism of EZH2 in the occurrence and development of endometrial
cancer through the interference of the EZH2 gene in endometrial
cancer RL-952 cells. We used siRNA to inhibit the expression of the
EZH2 gene in endometrial carcinoma RL-952 cells and observed the
changes in cellular and biological characteristics. Many studies
have shown that EZH2 can promote the proliferation and invasion of
endometrial cancer RL-952 cells (23–25), which
is similar to its effect on other malignant tumors. Therefore,
inhibition of EZH2 can significantly decrease the proliferation of
RL-952 cells. The invasion and metastasis of cells is one of the
most detrimental features of malignant tumors. According to the
tumor cell invasion three-step hypothesis proposed by Liotta et
al (26–28), at the molecular level, the process of
invasion is divided into three steps of adhesion, degradation and
migration: first, tumor cells need contact with the basement
membrane. After forming a localized stable tumor, proteases
secreted by tumor cells degrade the extracellular basement
membrane. Then, tumor cells initiate vascular or lymphatic
metastasis under the influence of chemokines. In this study,
Matrigel was used to detect the invasive ability of endometrial
carcinoma RL-952 cells, and the results showed that the invasive
ability of the cells was significantly decreased in vitro
after downregulating the EZH2 gene. Wang et al (29) specifically inhibited the EZH2 gene in
bladder cancer T24 cells and demonstrated that the invasive ability
of T24 cells was significantly attenuated. In addition, further
studies have reported that the invasive ability of breast cancer
cells is significantly reduced upon decreasing the expression of
EZH2 gene in the breast cancer cell lines, CAL51 and MDA-MB-231
(30–32). Several studies have shown that, in
non-small cell lung (33), cervical
(34), gastric cancer (35) and other malignant tumors, the tumor
cell invasion ability is significantly inhibited after
downregulating of EZH2 gene expression, which corresponds to the
results of this study.
In conclusion, EZH2 is closely related to the
development of EC and can function as a biomarker for its diagnosis
and progression. It can enhance the proliferative ability of
endometrial cancer RL-952 cells and promote cell invasion in
metastasis. Conversely, its downregulation can inhibit the invasive
ability of EC cells, preventing its proliferation.
References
|
1
|
Stålberg K, Kjølhede P, Bjurberg M,
Borgfeldt C, Dahm-Kähler P, Falconer H, Holmberg E, Staf C,
Tholander B, Åvall-Lundqvist E, et al: Risk factors for lymph node
metastases in women with endometrial cancer: A population-based,
nation-wide register study-On behalf of the Swedish Gynecological
Cancer Group. Int J Cancer. 140:2693–2700. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hwang JY, Kim DH, Bae HS, Kim ML, Jung YW,
Yun BS, Seong SJ, Shin E and Kim MK: Combined oral
medroxyprogesterone/levonorgestrel-intrauterine system treatment
for women with grade 2 stage IA endometrial cancer. Int J Gynecol
Cancer. 27:738–742. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu J, Tang Q, Yang L, Chen Y, Zheng F and
Hann SS: Interplay of DNA methyltransferase 1 and EZH2 through
inactivation of Stat3 contributes to β-elemene-inhibited growth of
nasopharyngeal carcinoma cells. Sci Rep. 7:5092017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang M, Hou J, Wang Y, Xie M, Wei C, Nie
F, Wang Z and Sun M: Long noncoding RNA LINC00673 is activated by
SP1 and exerts oncogenic properties by interacting with LSD1 and
EZH2 in gastric cancer. Mol Ther. 25:1014–1026. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu F, Gu L, Cao Y, Fan X, Zhang F and
Sang M: Aberrant overexpression of EZH2 and H3K27me3 serves as poor
prognostic biomarker for esophageal squamous cell carcinoma
patients. Biomarkers. 21:80–90. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhai R, Tang F, Gong J, Zhang J, Lei B, Li
B, Wei Y, Liang X, Tang B and He S: The relationship between the
expression of USP22, BMI1, and EZH2 in hepatocellular carcinoma and
their impacts on prognosis. Onco Targets Ther. 9:6987–6998. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang H, Yu C, Chen M, Li Z, Tian S, Jiang
J and Sun C: miR-522 contributes to cell proliferation of
hepatocellular carcinoma by targeting DKK1 and SFRP2. Tumour Biol.
37:11321–11329. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang X, Cao W, Zhang J, Yan M, Xu Q, Wu X,
Wan L, Zhang Z, Zhang C, Qin X, et al: A covalently bound inhibitor
triggers EZH2 degradation through CHIP-mediated ubiquitination.
EMBO J. 36:1243–1260. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin X, Yang C, Fan P, Xiao J, Zhang W,
Zhan S, Liu T, Wang D and Wu H: CDK5/FBW7-dependent ubiquitination
and degradation of EZH2 inhibits pancreatic cancer cell migration
and invasion. J Biol Chem. 292:6269–6280. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abdelrahman AE, Arafa SA and Ahmed RA:
Prognostic value of Twist-1, E-cadherin and EZH2 in prostate
cancer: An immunohistochemical study. Turk Patoloji Derg. Feb
4–2017.(Epub ahead of print). https://doi.org/10.5146/tjpath.2016.01392
|
|
11
|
Gardner EE, Lok BH, Schneeberger VE,
Desmeules P, Miles LA, Arnold PK, Ni A, Khodos I, de Stanchina E,
Nguyen T, et al: Chemosensitive relapse in small cell lung cancer
proceeds through an EZH2-SLFN11 axis. Cancer Cell. 31:286–299.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gong C, Yao S, Gomes AR, Man EP, Lee HJ,
Gong G, Chang S, Kim SB, Fujino K, Kim SW, et al: KOHBRA study
group: BRCA1 positively regulates FOXO3 expression by restricting
FOXO3 gene methylation and epigenetic silencing through targeting
EZH2 in breast cancer. Oncogenesis. 5:e2142016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gharpure KM, Chu KS, Bowerman CJ, Miyake
T, Pradeep S, Mangala SL, Han HD, Rupaimoole R, Armaiz-Pena GN,
Rahhal TB, et al: Metronomic docetaxel in PRINT nanoparticles and
EZH2 silencing have synergistic antitumor effect in ovarian cancer.
Mol Cancer Ther. 13:1750–1757. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Toyokawa G, Takada K, Okamoto T, Kozuma Y,
Matsubara T, Haratake N, Akamine T, Takamori S, Katsura M, Shoji F,
et al: Elevated metabolic activity on 18F-FDG PET/CT is
associated with the expression of EZH2 in non-small cell lung
cancer. Anticancer Res. 37:1393–1401. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamamoto I, Nosho K, Kanno S, Igarashi H,
Kurihara H, Ishigami K, Ishiguro K, Mitsuhashi K, Maruyama R, Koide
H, et al: EZH2 expression is a prognostic biomarker in patients
with colorectal cancer treated with anti-EGFR therapeutics.
Oncotarget. 8:17810–17818. 2017.PubMed/NCBI
|
|
16
|
Chang CJ, Yang JY, Xia W, Chen CT, Xie X,
Chao CH, Woodward WA, Hsu JM, Hortobagyi GN and Hung MC: EZH2
promotes expansion of breast tumor initiating cells through
activation of RAF1-β-catenin signaling. Cancer Cell. 19:86–100.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai MY, Tong ZT, Zheng F, Liao YJ, Wang Y,
Rao HL, Chen YC, Wu QL, Liu YH, Guan XY, et al: EZH2 protein: A
promising immunomarker for the detection of hepatocellular
carcinomas in liver needle biopsies. Gut. 60:967–976. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rao ZY, Cai MY, Yang GF, He LR, Mai SJ,
Hua WF, Liao YJ, Deng HX, Chen YC, Guan XY, et al: EZH2 supports
ovarian carcinoma cell invasion and/or metastasis via regulation of
TGF-beta1 and is a predictor of outcome in ovarian carcinoma
patients. Carcinogenesis. 31:1576–1583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Neusquen LP, Filassi JR, Fristachi CE,
Carvalho KC, Dória MT, Soares Júnior JM and Piato JR: EZH2 protein
expression and tumor response to neoadjuvant chemotherapy in
locally advanced breast cancer. Rev Bras Ginecol Obstet.
38:280–286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oki S, Sone K, Oda K, Hamamoto R, Ikemura
M, Maeda D, Takeuchi M, Tanikawa M, Mori-Uchino M, Nagasaka K, et
al: Oncogenic histone methyltransferase EZH2: A novel prognostic
marker with therapeutic potential in endometrial cancer.
Oncotarget. 8:40402–40411. 2017.PubMed/NCBI
|
|
21
|
Guo ZL, Chen K, Wang XQ and Hu W:
Expression and relationship of Ezh2, Runx3 and caspase-3 in
endometrial adenocarcinoma. Zhonghua Bing Li Xue Za Zhi.
40:387–391. 2011.(In Chinese). PubMed/NCBI
|
|
22
|
Bachmann IM, Halvorsen OJ, Collett K,
Stefansson IM, Straume O, Haukaas SA, Salvesen HB, Otte AP and
Akslen LA: EZH2 expression is associated with high proliferation
rate and aggressive tumor subgroups in cutaneous melanoma and
cancers of the endometrium, prostate, and breast. J Clin Oncol.
24:268–273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ihira K, Dong P, Xiong Y, Watari H, Konno
Y, Hanley SJ, Noguchi M, Hirata N, Suizu F, Yamada T, et al: EZH2
inhibition suppresses endometrial cancer progression via
miR-361/Twist axis. Oncotarget. 8:13509–13520. 2017.PubMed/NCBI
|
|
24
|
Eskander RN, Ji T, Huynh B, Wardeh R,
Randall LM and Hoang B: Inhibition of enhancer of zeste homolog 2
(EZH2) expression is associated with decreased tumor cell
proliferation, migration, and invasion in endometrial cancer cell
lines. Int J Gynecol Cancer. 23:997–1005. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou J, Roh JW, Bandyopadhyay S, Chen Z,
Munkarah AR, Hussein Y, Alosh B, Jazaerly T, Hayek K, Semaan A, et
al: Overexpression of enhancer of zeste homolog 2 (EZH2) and focal
adhesion kinase (FAK) in high grade endometrial carcinoma. Gynecol
Oncol. 128:344–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liotta LA: Adhere, degrade, and move: The
three-step model of invasion. Cancer Res. 76:3115–3117. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liotta LA and Clair T: Cancer. Checkpoint
for invasion. Nature. 405:287–288. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liotta LA and Kohn E: Cancer invasion and
metastases. JAMA. 263:1123–1126. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang HF, Yang H, Hu LB, Lei YH, Qin Y, Li
J, Bi CW, Wang JS and Huo Q: Effect of siRNA targeting EZH2 on cell
viability and apoptosis of bladder cancer T24 cells. Genet Mol Res.
13:9939–9950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gonzalez ME, DuPrie ML, Krueger H,
Merajver SD, Ventura AC, Toy KA and Kleer CG: Histone
methyltransferase EZH2 induces Akt-dependent genomic instability
and BRCA1 inhibition in breast cancer. Cancer Res. 71:2360–2370.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moore HM, Gonzalez ME, Toy KA,
Cimino-Mathews A, Argani P and Kleer CG: EZH2 inhibition decreases
p38 signaling and suppresses breast cancer motility and metastasis.
Breast Cancer Res Treat. 138:741–752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gonzalez ME, Li X, Toy K, DuPrie M,
Ventura AC, Banerjee M, Ljungman M, Merajver SD and Kleer CG:
Downregulation of EZH2 decreases growth of estrogen
receptor-negative invasive breast carcinoma and requires BRCA1.
Oncogene. 28:843–853. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xia H, Yu CH, Zhang Y, Yu J, Li J, Zhang
W, Zhang B, Li Y and Guo N: EZH2 silencing with RNAi enhances
irradiation-induced inhibition of human lung cancer growth in
vitro and in vivo. Oncol Lett. 4:135–140.
2012.PubMed/NCBI
|
|
34
|
Liu Y, Liu T, Bao X, He M, Li L and Yang
X: Increased EZH2 expression is associated with proliferation and
progression of cervical cancer and indicates a poor prognosis. Int
J Gynecol Pathol. 33:218–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mattioli E, Vogiatzi P, Sun A, Abbadessa
G, Angeloni G, DUgo D, Trani D, Gaughan JP, Vecchio FM, Cevenini G,
et al: Immunohistochemical analysis of pRb2/p130, VEGF, EZH2, p53,
p16INK4A, p27KIP1, p21WAF1, Ki-67
expression patterns in gastric cancer. J Cell Physiol. 210:183–191.
2007. View Article : Google Scholar : PubMed/NCBI
|