Introduction
Thyroid cancer was the most common malignant
endocrine tumor diagnosed in 2006 in the USA (1,2). Thyroid
cancer is also the seventh most common type of cancer in Canadians,
and there were ~5,650 cases of thyroid cancer diagnosed in 2012
(1,2).
Concurrently, equal trends in the increase in incidence rate have
been identified all over the world (3–14). The
age-standardized incidence rate of thyroid cancer has increased
from 1.1/100,000 to 6.1/100,000 for males, and from 3.3/100,000 to
22.2/100,000 for females, from 1970 to 1972 in the USA (1,15). A
previous study indicated that the thyroid cancer incidence rate in
Canada was the fastest increasing rate in the world, t trends in
the incidence rate of thyroid cancer have demonstrated a 6.8%
increase for males and 6.9% increase for females per annum between
1998 and 2007 (16–18). Most recently, the number of new cases
of thyroid cancer is estimated to be 12.9 per 100,000 men and women
annually in 2015 in the US (19,20).
At present, previous studies (17,19,21) have
suggested that gene therapy is the most promising and effective
therapeutic method for thyroid cancer. The principle of gene
therapy depends on the intracellular conversion of a relatively
non-toxic pro-drug (or drug gene) to a toxic drug (therapeutic
protein) through gene transcription and translation processes. The
gene therapy method exhibits more advantages than conventional
chemotherapy, as it limits the pro-drug-induced toxicity to the
targeted cells (17,19,21–23). The
surrounding cells and tissues are not affected by systemic
toxicity. In previous years, the cytosine deaminase (CD) and
sodium iodide symporter (NIS) genes have been employed as
therapeutic genes in certain studies. Bentires-Alj et al
(24) investigated the feasibility of
CDA suicide gene therapy in a model of peritoneal
carcinomatosis. Kogai and Brent (23)
used the NIS gene to target cancer cells as an effective
therapeutic method. Therefore, the present study used the CD
and NIS genes to treat thyroid cancer cells.
With the exception of gene therapy, 5-fluorocytosine
(5-FC) and Na131I have also been used in cancer therapy
combined with gene therapy: Kucerova et al (25) utilized CD-mesenchymal stromal
cells/5-FC as an effective gene therapeutic tool. Zimmer et
al (26) also used
Na131I to mediate radiochemical therapy. Therefore, in
the present study, 5-FC and Na131I were combined
together to act as an assistant therapy tool for thyroid
cancer.
Following the enzyme/pro-drug systems developed and
applied in clinical practice, herpes simplex virus-1 thymidine
kinase (HSV-tk) has been used in previous years. HSV-tk is an
enzyme that may convert pro-drugs to toxic products in targeted
cells (21). In the absence of the
drug, constitutive expression of the HSV-tk gene does not
exert any harmful effects on normal cell growth. A previous study
has also suggested that transgenic animals transfected with the
HSV-tk gene have not suffered toxicity effects (21). A minimal promoter region may be
located in the progression elevated gene-3 (PEG-3), which is
associated with malignant transformation and tumor progression
(26). PEG-3 may initiate the
expression of other genes in tumor cells (27,28).
Therefore, in the present study, the PEG-3 gene was used as
the promoter for CDA and SLC5A5 gene expression in
tumor cells.
The present study attempted to develop CDA
and SLC5A5 therapy through a replication-defective
adenovirus encoding human CDA and SLC5A5
(Ad-CDA-SLC5A5) genes to treat human thyroid cancer
cells.
Materials and methods
Cell lines and cell culture
The human thyroid cancer TT cell line and the
adenovirus-transformed human embryonic kidney 293 cell line (used
as an expression tool for recombinant proteins) were purchased from
the American Type Culture Collection (ATCC; Manassas, VA, USA). All
these cells were grown in Dulbecco's modified Eagle's medium (DMEM;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (Thermo Fisher Scientific, Inc.) at 37°C in
the presence of 5% CO2.
PEG-3 gene clone and adenoviral vector
construction
PEG-3 was amplified using rat genomic DNA
(cat. no. 69238; EMD Millipore, Billerica, MA, USA) as the template
with forward primer 5′-TATAGTCAGCTCTAGAAGCCATCTCACCAGCCCAG-3′ and
reverse primer 5′-CCGGGGATCCTCTAGAGTGTCTGGCCTAGAAAGGG-3′ (SBS
Genetech Co., Ltd., Beijing, China). pSB539-4 (22) was ligated into the pAV-murine
cytomegalovirus-green fluorescent protein (GFP)-3FLAG vector
(VB161208-1123ehs; Cyagen Biosciences, Santa Clara, CA, USA) for
the generation of the recombinant Ad-PEG-3-CDA-SLC5A5
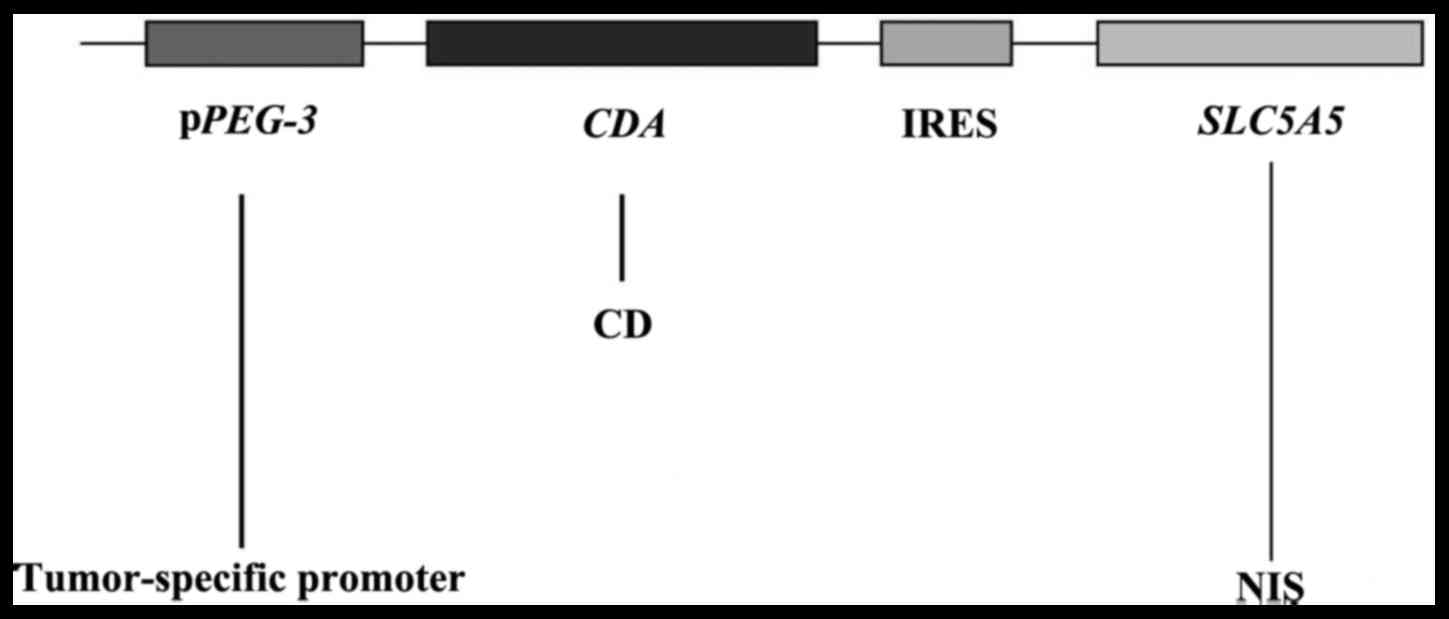
digested by XbaI. A diagrammatic sketch for the
double-cistron vector under the regulation of the tumor-specific
promoter PEG-3 gene is presented in Fig. 1.
Adenovirus infection
On the day prior to viral infection, TT cells
(3.6×105 cells/well) were plated in each well of 6-well
plates. When the cells reached 70–90% confluence, the culture
medium was aspirated and the cell monolayer was washed with
pre-warmed sterile PBS.
The recombinant generation of
Ad-PEG-3-CDA-SLC5A5 was additionally amplified in 293
low-passage cells. Viral particles were purified using cesium
chloride density gradient ultracentrifugation (54,645 × g for 20 h
at 4°C). 293 cells in serum-free DMEM were transfected with Ad-GFP
to identify the optimal conditions using Lipofectamine®
2000 (cat. no. 18324–111; Invitrogen; Thermo Fisher Scientific,
Inc.). The uptake of Ad-PEG-3 vector was detected by
fluorescence microscopy (magnification, ×100) following
transfection. Additionally, the transfected TT cells were
co-cultured with PHH, Hep3B, HuH7 and CCLP1 cell lines (purchased
from ATCC), with the aforementioned culture conditions, to study
interactions between cell populations in respect to targeting gene
expression (29).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
One-Step SYBR® PrimeScript™ RT-PCR kit II
was purchased from Clontech Laboratories, Inc., (Mountainview, CA,
US). Total RNA was isolated from cultured cells using an RNAiso
Plus kit (1 ml/5×106 cells; Takara Bio, Inc.). The
concentration and purity of RNA were detected by an ultraviolet
spectrometer. cDNA was generated according to the One-Step
SYBR® PrimeScript™ RT-PCR kit II protocol. CDA
fragments were amplified with forward primer,
5′-GGAAAACGGGAAAGTTGCATCA-3′ and reverse primer,
5′-GCCTTCTCCCGCTTAGAGAC-3′. Primers for the qPCR of the mouse
SLC5A5 gene were: Forward, 5′-AGCAGGCTTAGCTGTATCCC-3′ and
reverse, 5′-AGCCCCGTAGTAGAGATAGGAG-3′, to yield 235-bp products.
Primers for the reference gene, rat β-actin, were as follows:
Forward 5′-ATCTGGCACCACACCTTC-3′ and reverse
5′-AGCCAGGTCCAGACGCA-3′. DNA amplification was conducted in a
PerkinElmer thermocycler 2400 (PerkinElmer, Inc., Waltham, MA, USA)
using an initial denaturation step at 95°C for 8 min, followed by
30 cycles of amplification with denaturation at 95°C for 30 sec,
annealing at 58°C for 30 sec, and extension at 72°C for 30 sec,
ending with a final extension at 72°C for 7 min. The
2−ΔΔCq method was used to quantify the expression levels
(30).
Western blot analysis
Transfected TT cells were lysed using
radioimmunoprecipitation assay lysis buffer (Abcam, Cambridge, MA,
USA). After centrifugation at 12,000 × g for 20 min at 4°C, protein
concentrations were determined using a Bicinchoninic Acid Protein
Assay kit (Beyotime Institute of Biotechnology, Haimen, China).
Total protein (5 µg/ml/lane) was denatured in protein Laemmli
loading buffer (Abcam), separated by 10% SDS-PAGE, and then
transferred to a polyvinylidene difluoride membranes (EMD
Millipore). Tris-buffered saline-Tween 20 (TBST) solution
supplemented with 10% non-fat dry milk (Abcam) was used to block
the membrane for 2 h at room temperature. The blots were then
incubated with primary CD antibody [AID antibody (2D3); cat. no.
sc-101417; 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA] and NIS (NIS-G-5) antibody (cat. no. sc-514487; 1:1,000; Santa
Cruz Biotechnology, Inc.) overnight at 4°C. The blots were washed
three times, for 10 min each, in TBST followed by incubation for 1
h at room temperature with goat horseradish peroxidase-conjugated
anti-mouse secondary antibodies (cat. no. 31430; 1:10,000; Thermo
Fisher Scientific, Inc.). Blots from three independent trials were
developed using enhanced chemiluminescent reagents (Beyotime
Institute of Biotechnology). β-actin (anti-β-actin; cat. no.
ab8229; 1:1,000; Abcam) was used as a control. Band intensities
were quantified by scanning densitometry using the Quantity One
software v. 4.6 (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
MTT assay
MTT assay was performed to evaluate the cell
viability in culture. The cells were seeded onto a 96-well plate at
a concentration of 1.0×105 cells/ml and a volume of 90
µl/well. Different concentrations of adenovirus
(2×105-1×106 PFU/ml) were applied to culture
wells in triplicate. Dimethyl sulfoxide was used as a negative
control. Following incubation at 37°C with 5% CO2 for 48
h, a mixture of 0.1 ml phenazine methosulfate and MTT (5 mg/ml) was
added to each well with a volume of 50 µl. The plates were
additionally incubated at 37°C for 2 h to allow MTT formazan
production. The absorbance was determined with an ELISA reader
(Thermo Fisher Scientific, Inc.) at a test wavelength of 450 nm and
a reference wavelength of 690 nm.
Statistical analysis
Statistical analyses were performed using SPSS
v.16.0 software (SPSS, Inc., Chicago, IL, USA). Values were
reported as the mean ± standard deviation. Kruskal-Wallis tests
followed by Mann-Whitney U tests were used to determine the
statistical significance of the data. P<0.05 was considered to
indicate a statistically significant difference.
Results
PEG-3 gene cloning and determination
of multiplicity of infection (MOI) in 293 cells
pSB539 is highly homologous to the
PEG-3 promoter (1,835 bp), which targets cancer cell lines
(26,27). To verify the cloning of the
PEG-3 gene and the transfection efficiency of
Ad-PEG-3 vector in 293 cells, the PEG-3 gene was
amplified by PCR, and the uptake of Ad-PEG-3 vector was
detected by fluorescence microscopy following transfection. The PCR
results indicated that PEG-3 mRNA was successfully cloned
into the Ad-vector, which was also transfected into the 293 cells
(Fig. 2A). The results of microscopy
observation demonstrated highly efficient transfection when the
virus was diluted to a MOI of 105 (~1×106
cells/ml with virus at a MOI of 5; Fig.
2B).
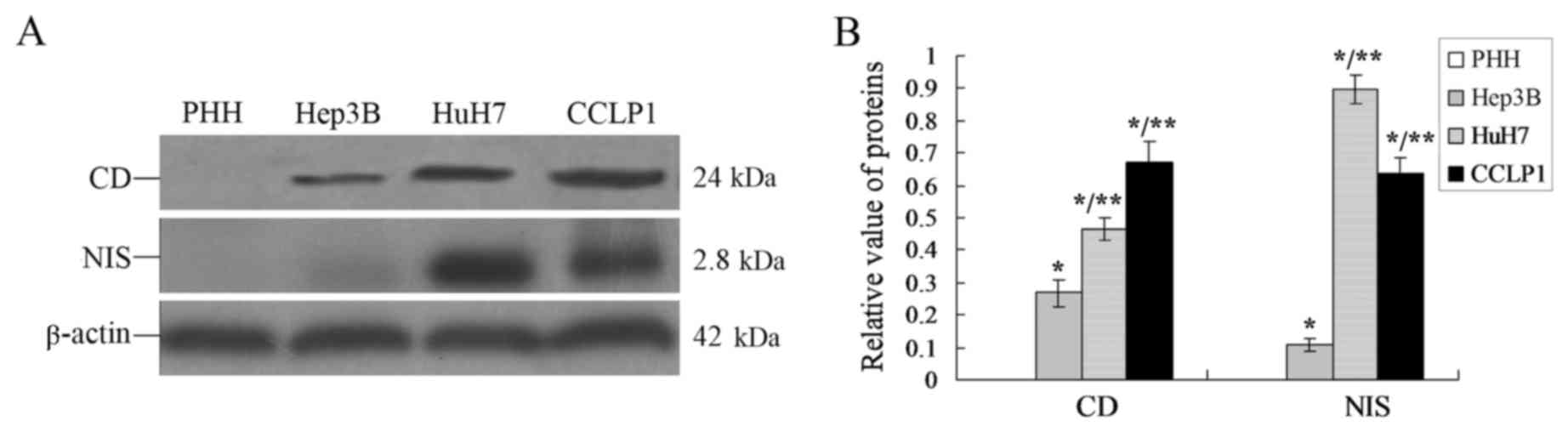
CD and NIS proteins express highly in
TT cells
From the results of Fig.
2, it was identified that the PEG-3 gene had been
successfully expressed in TT cells, which may trigger the positive
expression of downstream genes such as CDA and
SLC5A5. Western blot analyses were performed and the results
demonstrated that there were differences in CD and NIS protein
expression levels in TT cells when they were co-cultured with
different cell lines (PHH, Hep3B, HuH7 or CCLP1; Fig. 3).
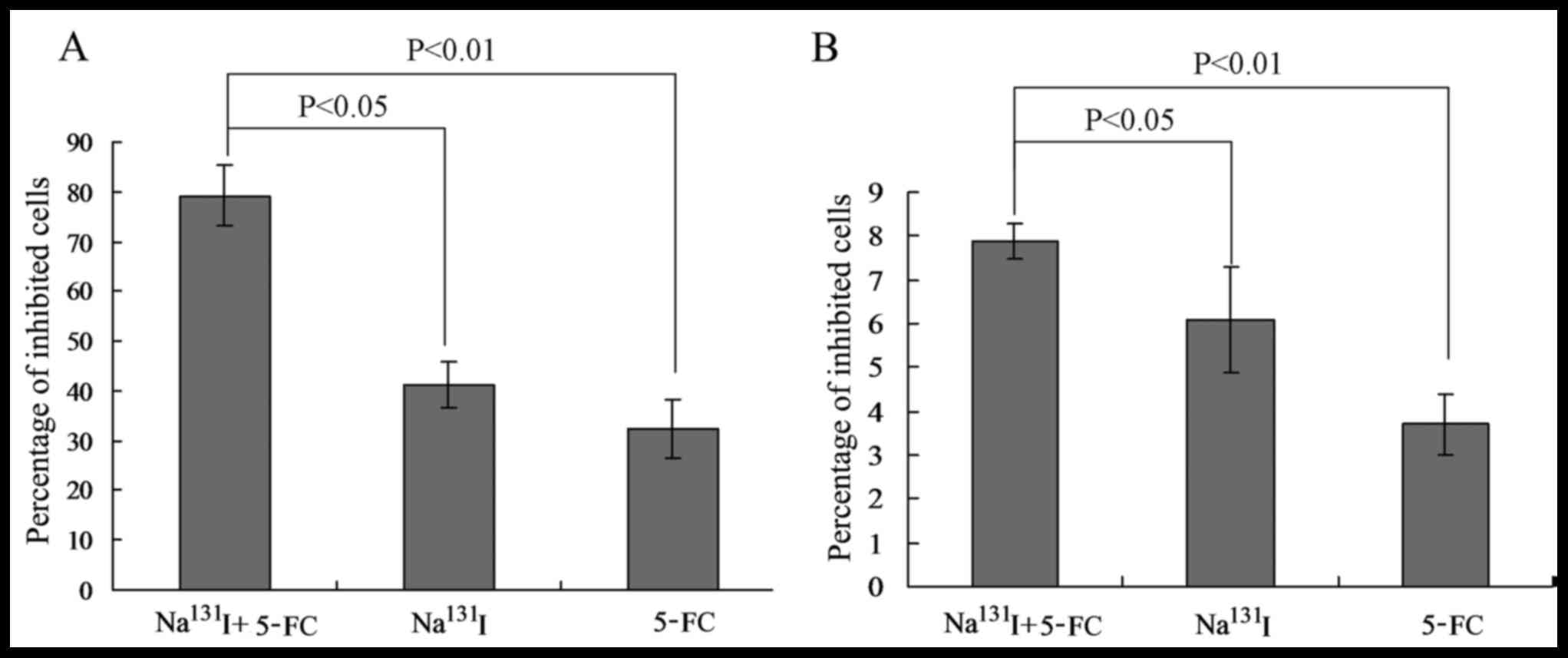
Na131I combined with 5-FC
decreases living human thyroid cancer cell viability
The effect of Ad-PEG-3 vector transfection on
human thyroid living cells was determined by MTT assay. The number
of living cells was calculated as 1- the optical density reading at
600 nm. The MTT assay results indicated that either
Na131I or 5-FC could inhibit TT living cells
significantly at 24, 48, 72 or 96 h when treated with different
combinations (Table I and Fig. 4). Particularly, the Na131I
combined with 5-FC group exhibited a significantly decreased number
of living cells compared with that of the Na131I and
5-FC single treatment groups (P<0.05 and P<0.01,
respectively; Fig. 4A). Concurrently,
the living cell numbers for untransfected TT cells, used as the
control in the present study, were also significantly decreased
when treated with Na131I and 5-FC in combination
compared with that of the Na131I and 5-FC single
treatment groups (P<0.05 and P<0.01, respectively; Fig. 4B).
 | Table I.Examination of the percentage of
living cells in transfected and untransfected TT cells treated with
Na131I and 5-FC. |
Table I.
Examination of the percentage of
living cells in transfected and untransfected TT cells treated with
Na131I and 5-FC.
|
| Percentage of
living cell in transfected TT cells | Percentage of
living cell in untransfected TT cells |
|---|
|
|
|
|
|---|
| Treatment | 24 h | 48 h | 72 h | 96 h | 24 h | 48 h | 72 h | 96 h |
|---|
|
Na131I+5-FC |
|
|
|
|
|
|
|
|
| (KBq/ml +
µg/ml) |
|
|
|
|
|
|
|
|
|
3,700+5.0 | 7.7±0.4 | 23.2±3.5 | 23.2±3.5 | 79.1±6.1 | 2.5±1.8 | 3.5±1.5 | 6.4±4.3 | 7.9±4.9 |
|
370+0.5 | 6.2±1.8 | 14.5±2.7 | 35.1±4.8 | 47.2±7.1 | 1.7±0.8 | 3.2±1.6 | 5.1±3.5 | 5.1±4.1 |
|
37+0.05 | 3.4±1.2 | 7.9±3.1 | 18.7±3.3 | 35.4±6.2 | 1.7±1.6 | 3.2±1.9 | 4.1±3.5 | 5.2±2.8 |
|
3.7+0.005 | 1.1±0.4 | 3.8±2.8 | 11.8±4.5 | 20.1±3.8 | 1.8±0.7 | 2.8±1.2 | 2.9±1.8 | 3.3±1.7 |
| Na131I
(KBq/ml) |
|
|
|
|
|
|
|
|
|
3,700 | 5.2±0.8 | 11.8±2.2 | 30.1±5.6 | 41.2±4.7 | 1.7±0.6 | 1.7±0.8 | 5.5±2.9 | 6.1±3.5 |
|
370 | 2.7±1.0 | 3.3±1.1 | 8.8±2.7 | 19.7±3.8 | 0.8±0.3 | 1.0±0.5 | 5.0±1.3 | 4.1±2.8 |
| 37 | 1.6±0.8 | 1.4±0.5 | 4.2±2.4 |
8.7±3.1 | 0.7±0.5 | 3.0±2.1 | 4.5±1.8 | 4.1±1.4 |
|
3.7 | 0.6±0.5 | 1.5±0.8 | 2.5±1.3 |
4.3±0.2 | 1.1±0.4 | 1.8±0.9 | 2.3±0.8 | 2.3±0.8 |
| 5-FC (µg/ml) |
|
|
|
|
|
|
|
|
|
5.0 | 3.5±0.5 | 8.6±1.2 | 25.2±4.0 | 32.3±5.8 | 2.1±0.9 | 2.0±1.1 | 3.6±2.0 | 3.7±3.1 |
|
0.5 | 1.5±0.6 | 2.5±1.5 |
7.2±2.3 | 11.2±2.9 | 3.0±2.1 | 2.5±1.1 | 2.9±1.2 | 3.9±1.6 |
|
0.05 | 1.6±0.9 | 2.6±1.2 |
2.3±1.8 |
3.9±1.8 | 2.3±0.9 | 2.5±0.4 | 2.9±1.8 | 3.8±2.4 |
|
0.005 | 1.4±0.6 | 2.7±2.2 |
3.5±2.0 |
3.6±1.8 | 1.2±0.5 | 3.8±2.2 | 2.8±1.4 | 2.9±1.6 |
Discussion
At present, the most significant problem for cancer
gene therapy is the delivery of the therapeutic gene to the
targeted tumor cells or tissues (17,21).
Indeed, almost all clinical trials currently being performed depend
on direct intra-tumor injection of the vector (27). In order to overcome this problem,
scientists have created certain vectors such as engineered
adenoviral vectors and cationic liposomes (14,15).
However, some vectors are not able to be expressed in various types
of human cancer (31). In the present
study, the pAV-murine cytomegalovirus-GFP-3FLAG vector was used to
transport the therapeutic genes. A previous study indicated that
NIS expression is primarily controlled by the thyroid-selective
transcription factors paired box gene 8 (Pax-8) and NK2 homeobox 1
(Nkx2.1) in thyroid cancer (31).
Pax-8 and Nkx2.1 target the NIS upstream enhancer through the
cardiac homeobox transcription factor Nkx2 (16,32).
Previous advances propose additional improvements to
CDA suicide gene therapy (32). The uracil phosphoribosyl transferase
(UPRT) gene from Escherichia coli encodes uracil
phosphoribosyltransferase, which converts uracil and
5-phosphoribosyl-1-R-diphosphate to uridine monophosphate (UMP).
This protein is a potential target in cancer therapy, but not
present in mammalian genomes when combining with UPRT
(33,34).
The limitation of the present study was that only
one thyroid cancer cell line, the TT cell line, was employed, which
may be not sufficient to support the function of a gene as part of
a gene therapy cancer study. Therefore, in following studies, the
same in vitro experiments of the present study should be
attempted with different thyroid cancer cell lines.
To conclude, transfection with an Ad-PEG-3
plasmid into human thyroid cancer cells may inhibit tumor growth
in vitro. This may be a useful tool for gene therapy in
human thyroid cancer and other types of cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81072185).
References
|
1
|
Wartofsky L: Increasing world incidence of
thyroid cancer: Increased detection or higher radiation exposure?
Hormones (Athens). 9:103–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang C, Lu S, Jiang J, Jia X, Dong X and
Bu P: Has-microRNA-101 suppresses and invasion by targeting Rac1 in
thyroid cancer cells. Oncol Lett. 8:1815–1821. 2014.PubMed/NCBI
|
|
3
|
Xing M: BRAF mutation in thyroid cancer.
Endocr Relat Cancer. 12:245–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Romagnoli S, Moretti S, Voce P and Puxeddu
E: Targeted molecular therapies in thyroid carcinoma. Arq Bras
Endocrinol Metab. 53:1361–1073. 2009. View Article : Google Scholar
|
|
5
|
Lin SF, Huang YY, Lin JD, Chou TC, Hsueh C
and Wong RJ: Utility of a PI3K/mTOR Inhibitor (NVP-BEZ235) for
thyroid cancer therapy. PLoS One. 7:e467262012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gild ML, Bullock M, Robinson BG and
Clifton-Bligh R: Multikinase inhibitors: A new option for the
treatment of thyroid cancer. Nat Rev Endocrinol. 7:617–624. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khan MS, Pandith AA, Hussain M, Iqbal M,
Khan NP, Wani KA, Masoodi SR and Mudassar S: Lack of mutational
events of RAS genes in sporadic thyroid cancer but high risk
associated with HRAS T81C single nucleotide polymorphism
(case-control study). Tumor Biol. 34:521–529. 2013. View Article : Google Scholar
|
|
8
|
Yazawa K, Fisher WE and Brunicardi FC:
Current Progress in Suicide Gene Therapy for Cancer. World J Surg.
26:783–789. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Springer CJ and Niculescu-Duvaz I:
Prodrug-activating systems in suicide gene therapy. J Clin Invest.
105:1161–1167. 2010. View
Article : Google Scholar
|
|
10
|
Wang Z, Wang B, Guo H, Shi G and Hong X:
Clinicopathological significance and potential drug target of
T-cadherin in NSCLC. Drug Des Devel Ther. 9:207–316.
2014.PubMed/NCBI
|
|
11
|
Chung JK: Sodium iodide symporter: Its
role in nuclear medicine. J Nucl Med. 43:1188–1200. 2002.PubMed/NCBI
|
|
12
|
Guerrieri F, Piconese S, Lacoste C,
Schinzari V, Testoni B, Valogne Y, Gerbal-Chaloin S, Samuel D,
Bréchot C, Faivre J and Levrero M: The sodium/iodide symporter NIS
is a transcriptional target of the p53-family members in liver
cancer cells. Cell Death Dis. 4:e8072013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McCormick F: Cancer gene therapy: Fringe
or cutting edge? Nat Rev Cancer. 1:130–141. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Breyer B, Jiang W, Cheng HW, Zhou L, Paul
R, Feng T and He TC: Adenoviral vector-mediated gene transfer for
human gene therapy. Curr Gene Ther. 1:49–162. 2001. View Article : Google Scholar
|
|
15
|
Aschebrook-Kilfoy B, Grogan RH, Ward MH,
Kaplan E and Devesa SS: Follicular thyroid cancer indicence
patterns in the Unites States, 1980–2009. Thyroid. 23:1015–1021.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lazar V, Bidart JM, Caillou B, Mahé C,
Lacroix L, Filetti S and Schlumberger M: Expression of the Na+/I-
symporter gene in human thyroid tumors: A comparison study with
other thyroid-specific genes. J Clin Endocrinol Metal.
84:3228–3234. 1999. View Article : Google Scholar
|
|
17
|
Zhu Y, Cheng M, Yang Z, Zeng CY, Chen J,
Xie Y, Luo SW, Zhang KH, Zhou SF and Lu NH: Mesenchymal stem
cell-based NK4 gene therapy in nude mice bearing gastric cancer
xenografts. Drug Des Devel Ther. 8:2449–2462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie L, Semenciw R and Mery L: Cancer
incidence in Canada: Trends and projections (1983–2032). Health
Promot Chronic Dis Prev Can. 35 Suppl 1:S2–S186. 2015.(In English,
French). View Article : Google Scholar
|
|
19
|
Maxwell JE, Sherman SK, O'Dorision TM and
Howe JR: Medical management of metastatic medullary thyroid cancer.
Cancer. 120:3287–3301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
National Cancer Institute, . SEER stat
fact sheets: Thyroid cancer. http://seer.cancer.gov/statfacts/html/thyro.htmlJanuary
12–2015
|
|
21
|
Yip L: Molecular markers for thyroid
cancer diagnosis, prognosis, and targeted therapy. J Surg Oncol.
111:43–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chai LP, Wang ZF, Liang WY, Chen L, Chen
D, Wang AX and Zhang ZQ: In vitro and in vivo effect of 5-FC gene
therapy with TNF and CD suicide gene on human laryngeal carcinoma
cell line Hep-2. PLoS One. 8:e611362013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kogai T and Brent GA: The sodium iodide
symporter (NIS): Regulation and approaches to targeting for cancer
therapeutics. Phamacol Ther. 135:355–370. 2012. View Article : Google Scholar
|
|
24
|
Bentires-Alj M, Hellin AC, Lechanteur C,
Princen F, Lopez M, Fillet G, Gielen J, Merville MP and Bours V:
Cytosine deaminase suicide gene therapy for peritoneal
carcinomatosis. Cancer Gene Ther. 7:20–26. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kucerova L, Skolekova S, Demkova L,
Bohovic R and Matuskova M: Long-term efficiency of mesenchymal
stromal cell-mediated CD-MSC/5-FC therapy in human melanoma
xenograft model. Gene Ther. 21:874–887. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zimmer AM, Kazikiewicz JK, Rosen ST and
Spies SM: Chromatographic evaluation of the radiochemical purity of
Na131I: Effect on monoclonal antibody labeling. Int J Rad Appl
Instrum B. 14:533–534. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Su ZZ, Shi Y and Fisher PB: Subtraction
hybridization identifies a transformation progression-associated
gene PEG-3 with sequence homology to a growth arrest and DNA
damage-inducible gene. Proc Natl Acad Sci USA. 94:pp. 9125–9130.
1997, View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Su ZZ, Goldstein NI, Jiang H, Wang MN,
Duigou GJ, Young CS and Fisher PB: PEG-3, a nontransforming cancer
progression gene, is a positive regulator of cancer aggressiveness
and angiogenesis. Proc Natl Acad Sci USA. 96:pp. 15115–15120. 1999,
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Goers L, Freemont P and Polizzi KM:
Co-culture systems and technologies: Taking synthetic biology to
the next level. J R Soc Interface. 11:201400652014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pacholska A, Wirth T, Samaranayake H,
Pikarainen J, Ahmad F and Ylä-Herttuala S: Increased invasion of
malignant gliomas after 15-LO-1 and HSV-tk/ganciclovir combination
gene therapy. Cancer Gene Ther. 19:870–874. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsiao HT, Xing L, Deng X, Sun X, Ling CC
and Li GC: Hypoxia-targeted triple suicide gene therapy
radiosensitizes human colorectal cancer cells. Oncol Rep.
32:723–729. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li C, Penet MF, Wildes F, Takagi T, Chen
Z, Winnard PT, Artemov D and Bhujwalla ZM: Nanoplex delivery of
siRNA and prodrug enzyme for multimodality image-guided molecular
pathway targeted cancer therapy. ACS Nano. 4:6707–6716. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Watanabe M, Nasu Y and Kumon H:
Adenovirus-mediated REIC/Dkk-3 gene therapy: Development of an
autologous cancer vaccination therapy (Review). Oncol Lett.
7:595–601. 2014.PubMed/NCBI
|