Introduction
Oral squamous cell carcinoma (OSCC) is a type of
malignant tumor with a high degree of malignancy and invasion.
Following a single surgery, there are clear defects and severe
deformities in the maxillofacial of patients, and tumor relapse is
likely to occur. The effect of a single radiotherapy or
chemotherapy is not satisfactory, and there are many side effects.
The study of OSCC has focused on integrated sequential therapy,
although there has been difficulty in this research area, for
example, the cure rate is not high, the prognosis is poor and the
postoperative survival quality is not high. Thus, there is an
urgent requirement to explore and seek the best combination in
order to obtain the best treatment effect (1).
Phosphatidylinositol-3-kinase (PI3K) signaling is
involved in numerous cellular functions, including cell
proliferation, differentiation, apoptosis and glucose
transportation (2). It was revealed
that signaling of PI3K and its downstream molecule protein kinase
B/RAC-alpha serine/threonine-protein kinase (PKB or Akt) are
associated with human tumor occurrence and development (3). Integrin-linked kinase (ILK) is a
Serine/Threonine protein kinase, which can be activated in a
PI3K-dependent manner by a growth factor or integrin (4). ILK is activated through phosphorylation
of the downstream substrates of PKB/Akt and inhibition of glycogen
synthase kinase (GSK) 3, so that the extracellular signals are
passed downstream, and regulate cell growth, differentiation and
migration (5,6). In a number of tumors, ILK is
overexpressed, and overexpression or constitutive activation of ILK
is associated with tumor formation, invasion and metastasis.
Inhibition of ILK activity can lead to cell cycle arrest and
apoptosis (7,8), making it an ideal target for gene
therapy of tumors. However, for single-gene therapy there are
deficiencies and side effects (it cannot cure the disease and may
trigger new genetic mutations), and it is difficult to achieve the
best treatment effect (4).
Hyperthermia is an important means of adjuvant
therapy. Compared with surgery, radiotherapy and chemotherapy,
hyperthermia has minimally invasive or even non-invasive and
non-toxic side effects, and it can effectively kill tumor cells and
improve the quality of life of patients (9–11).
However, in the process of hyperthermia, it was revealed that when
the repetitions of hyperthermia are increased, the effect of
hyperthermia is reduced, and a thermal tolerance phenomenon has
been reported (9,10). Due to the existence of thermal
tolerance mechanisms, the clinical application of hyperthermia is
limited. Therefore, it is important to improve the efficiency of
clinical hyperthermia and to remove the thermal tolerance of tumor
tissues in order to improve sensitivity to hyperthermia.
Heat shock protein (HSP) expression is mediated by
heat shock factor 1 (HSF1) (12).
Previous studies have demonstrated that PI3K can influence HSF1
activity, thus affecting the expression of HSP (10). One such study demonstrated that
inhibition of the PI3K/Akt signaling pathway can reduce the
expression of HSP70 (13). Another
previous study has demonstrated that activation of Akt
[phosphorylated-Akt; (p)-Akt] can promote the phosphorylation of
GSK3β and lead to inactivation of GSK3β (6). Inactivation of GSK3β leads to HSF1
activation (14), which may be the
reason that PI3K inhibits the expression of HSP70 induced by
hyperthermia.
In a previous study from our group (15), an ILK-siRNA lentiviral expression
vector was successfully constructed and transfected into tongue
squamous cell carcinoma Tca8113 cells. Subsequent to silencing ILK,
Tca8113 cell growth, proliferation and migration were inhibited,
and apoptosis increased. Following hyperthermia, similar changes
were observed in Tca8113 cells. Therefore, the present study aimed
to investigate whether a combination of ILK silencing and
hyperthermia can induce a synergistic sensitizing effect and
exhibit an improved antitumor effect.
By way of gene therapy, the impact of the biological
functions of ILK gene silencing combined with hyperthermia on
Tca8113 cells was observed, and it was investigated whether
combination therapy is the synergistic sensitizing effect. The
relevant mechanisms were analyzed, and an objective basis was
provided for gene silencing therapy and hyperthermia combined
treatment of OSCC.
Materials and methods
Cell line and cell culture
Human tongue squamous cell carcinoma Tca8113 cells
were obtained from the State Key Laboratory of Oral Diseases,
Sichuan University (Chengdu, China) and cultured in Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS;
Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) and 1%
penicillin/streptomycin at 37°C in a humidified atmosphere with 5%
CO2. Cells were routinely passaged every 2–3 days by
trypsinization (Gibco; Thermo Fisher Scientific, Inc.). Cells at
the logarithmic growth phase were used in the present study.
Hyperthermia of Tca8113 cells
With reference to the literature (16), eight time points for heating the cells
were selected as follows: NT (no heating), 0 h (cells were
subjected to heat shock at 45°C for 30 min, and then allowed to
recover by incubation in a 37°C chamber for an additional 0, 2, 4,
6, 8, 10 and 24 h).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Each experiment was conducted in duplicate and
repeated 3 times. RT-qPCR was performed using the SYBR Premix ExTaq
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was
extracted from Tca8113 cells using QIAGEN RNeasy mini kit (cat. no.
74104; Qiagen GmbH, Hilden, Germany) and isolated using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The total RNA
was reverse transcribed into cDNA using a PrimeScript RT reagent
kit with gDNA Eraser (cat. no. DRR037A; Perfect Real Time; Takara
Biotechnology Co., Ltd., Dalian, China) according to the
manufacturer's protocol. The reverse transcription reaction
conditions were as follows: 95°C for 5 min, followed by 35 cycles
of 94°C for 45 sec, 55°C for 45 sec and 75°C for 15 sec. The
resulting cDNA was quantified by a RT-qPCR mRNA SYBR-Green
detection kit (Takara Biotechnology Co., Ltd.) according to the
manufacturer's protocol. Each reaction was performed in a total
volume of 20 µl (10 µl Premix, 0.8 µl forward primer, 0.8 µl
reverse primer, 2 µl cDNA 6.4 µl dH2O), using SYBR-Green
PCR reagents (Takara Biotechnology Co., Ltd.). The following
primers were used: ILK forward, 5′-TTTGCAGTGCTTCTGTGGGAA-3′ and
reverse, 5′-CTACTTGTCCTGCATCTTCTC-3′; HSF1 forward,
5′-CCAGCAACAGAAAGTCGTCAAC-3′ and reverse,
5′-GGCTATACTTGGGCATGGAATG-3′; and human-actin forward,
5′-CCACGAAACTACCTTCAACTCC-3′ and reverse,
5′-GTGATCTCCTTCTGCATCCTGT-3′. qPCR was performed using QuantStudio™
3 and 5 Real-Time PCR systems (Thermo Fisher Scientific, Inc.) The
reaction conditions were as follows: 95°C for 30 sec, followed by
40 cycles of 95°C for 5 sec and 60°C for 31 sec. The relative
expression levels of mRNA in each sample were calculated using the
2−ΔΔCq method (14). The
products were analyzed on 1% agarose gels and observed under
ultraviolet light (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Western blot analysis
Whole cell extracts were harvested in radio
immuno-precipitation assay buffer (150 mM NaCl, 50 nM Tris pH 8.0,
0.5% sodium deoxycholate, 0.1% Triton X-100, 0.1% SDS, 2 mM EDTA
and 5% glycerol) and homogenized for 5 min at 447 × g and harvested
at room temperature. Total protein concentrations were determined
using a BCA assay (Nanjing Kaiji Biotechnology Development Co.
Ltd., Jiangsu, China). Total proteins were loaded per lane and
separated on an SDS-PAGE gel by electrophoresis, and transferred to
a nitrocellulose membrane (the mass of protein loaded per lane was
40 µg, the percentage of the concentrate gel was 4%, the percentage
of the isolate gel was 10%). The nitrocellulose membrane was
blocked with 5% skim milk in TBST (137 mM Cl, 20 mM Tris-HCl pH
7.6, 0.1% Tween-20), and incubated at 37°C for 1 h. Membranes were
probed with the indicated primary antibodies, against anti-ILK
(cat. no. 3862; 1:100; Cell Signaling Technology, Inc., Danvers,
MA, USA), anti-p-Akt (cat. no. 9271; 1:300; Cell Signaling
Technology, Inc.), AKT (Ser-473) (cat. no. 9272; 1:300; Cell
Signaling Technology, Inc.), p-GSK3β (Ser-9) (cat. no. BS6365;
1:100; BioWorld Technology, Inc., St. Louis Park, MN, USA), GSK3β
(cat. no. BS4084; 1:100; BioWorld Technology, Inc.), anti-p-HSF1
(Ser-303/307) (cat. no. 2108-1; 1:100; Epitomics; Abcam, Cambridge,
UK), anti-HSF-1 (cat. no. 2043-1; 1:100; Epitomics; Abcam), anti-B
cell-2-associated X protein (Bax; cat. no. BS6420;1:100; BioWorld
Technology, Inc.) and anti-GAPDH (cat. no. 5174; 1:1,000; Cell
Signaling Technology, Inc.) at 4°C overnight. The following day,
membranes were incubated with the Horseradish Peroxidase Rabbit
anti-Horseradish Polyclonal Antibody (cat. no. LS-C74277, 1:5,000;
LifeSpan Biosciences, Inc.) at room temperature for 1 h. Proteins
were detected using ECL luminous fluid (cat. no. WBKLS0050; EMD
Millipore, Billerica, MA, USA), and autographed onto an X-ray
film.
Transduction and hyperthermia
Tca8113 cells were grown at a density of
5×104/well in a 12-well plate in RPMI-1640 medium until
they reached 30–50% confluency and incubated with 5% CO2
in air. ILK-shRNA-lentivirus was introduced for 12 h when the
multiplicity of infection was 50. After 5 days, cells were heated
at 45°C for 30 min. Subsequent to heating for 8 h at 37°C in the
CO2 incubator; cells were centrifuged for 5 min at 447 ×
g at room temperature, the upper cleaning fluid was removed and
cells were harvested at room temperature.
MTT assay
Cells were seeded at a density of
1×103/well on 96-well plates and incubated at 37°C
overnight, and further incubated with 0.5 mg/ml MTT (Sigma-Aldrich;
Merck KGaA). After 4 h of incubation, the medium was replaced with
150 ml DMSO (Sigma-Aldrich; Merck KGaA) and heated for 10 min.
Absorbance was then measured at a wavelength of 490 nm using a
multiwell spectrophotometer (Bio-Rad Laboratories, Inc.). Cell
inhibition rate (%) was calculated as follows: The inhibition rate
= (control group - experiment group)/control group ×100%. The
experiment was repeated three times.
Colony formation assay
Tumor cells were plated at a density of 100/well on
6-well plates and cultured at 37°C. Following culture for 3 weeks,
colonies were stained with crystal violet (0.005%) for 20 min at
37°C and images were captured by optical microscopy (Olympus
Corporation, Tokyo, Japan). The colony formation rate was then
calculated as follows: The colony formation rate = number of
colonies/number of inoculated cells ×100.
Cell cycle analysis
Flow cytometry was used to analyze the cell cycle.
Initially, cells were cultured in serum-free RPMI-1640 medium for
24 h at 37°C to induce cell cycle synchronization. Floating and
attached cells were collected and centrifuged for 5 min at 447 × g
at room temperature prior to washing with cold PBS. Cells were then
fixed in 70% cold ethanol overnight at 4°C. RNase A (100 µl; Sigma
Aldrich; Merck KGaA) and a fluorochrome solution containing 400 µl
propidium iodide (BestBio, Shanghai, China) and 1% Triton X-100
were added and cells were incubated in the dark at room temperature
for 30 min. Cell cycle analysis was performed using a Coulter Epics
XL/XL-MCL flow cytometer (Beckman Coulter, Inc., Brea, CA, USA) and
analyzed using Quantity One software (version 3.0; Bio-Rad
Laboratories, Inc.). All experiments were performed three
times.
Ethics statement
Animal care and use were conducted in accordance
with the Animal Research Institute Committee guidelines of Sichuan
University (Chengdu, China). Mice were housed in a
temperature-controlled room at 37°C with appropriate dark-light
cycles (10 h of light and 14 h of darkness), fed with a regular
diet and maintained under the care of the Laboratory Animal Unit,
Sichuan University. The mice were sacrificed by cervical
dislocation. The present study was approved by the Committee of
Animal Research, Institute of Sichuan University (Sichuan,
China).
Xenograft tumor model and
treatments
Tca8113 cells (2×106) in 100 µl medium
were harvested for 5 min at 447 × g at room temperature and
injected subcutaneously into the hind dorsalis pedis of 60 SPF
BALB/cnu/nu female mice (Charles River Japan, Japan) at the
Laboratory Animal Unit, Sichuan University (6 weeks old; weight,
18–22 g). After 1 week, 108 TU ILK-shRNA-lentivirus and
NC-shRNA-lentivirus (NC) were injected into tumors, respectively,
suspended in 50 µl RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% FBS and 5 mg/ml polybrene
(Shanghai GeneChem Co., Ltd., Shanghai, China). Mice were heated in
a water bath at 45°C for 30 min and were administered a second
hyperthermic dose after 1 week.
Tumor size and weight measurement
Each tumor was measured weekly with a caliper every
3 days, and the following formula was used to calculate tumor
volumes = 1/2xAxB2, where A and B represent the larger
and smaller tumor diameters, respectively (15). Mice were sacrificed at 28 days after
first hyperthermia. The tumor tissue was removed, weighed and the
heart, liver, brain, spleen, lung and kidney of mice were removed,
fixed with 4% paraformaldehyde for 24 h at room temperature and
embedded.
Hematoxylin and eosin (H&E)
staining
Paraffin-embedded mouse extracted organs sections,
including the heart, liver, brain, spleen, lung or kidney
(sectioned at 4 µm) were prepared by a routine procedure (16).
Immunohistochemistry
Immunohistochemical staining was performed using the
streptavidin-biotin-peroxidase complex method. The primary
antibodies used were: ILK (cat. no. 3862; 1:100; Cell Signaling
Technology, Inc.), p-Akt (cat. no. 9271; 1:300; Cell Signaling
Technology, Inc.), total Akt (cat. no. 9272; 1:300; Cell Signaling
Technology, Inc.), p-GSK3β (cat. no. BS6365; 1:100; BioWorld
Technology, Inc.), total GSK3β (cat. no. BS4084; 1:100; BioWorld
Technology, Inc.), Bax (cat. no. bs2538; 1:100, BioWorld
Technology, Inc.), p-HSF1 (cat. no. pS303/307; 2108-1, 1:100;
Epitomics; Abcam) and total HSF1 (2043–1; 1:100, Epitomics; Abcam).
Normal rabbit IgG (cat. no. 2729S; Cell Signaling Technology, Inc.)
was substituted for each primary antibody as a negative control.
All sections were incubated for 1 h at room temperature, and then
were incubated overnight at 4°C. After being washed with
phosphate-buffered saline (PBS), sections were incubated with 100
µl biotinylated goat anti-rabbit IgG (cat. no. PAB9401; Abnova;
Taipei City, Taiwan) for 30 min at room temperature. They were then
washed three times with PBS, treated with streptavidineperoxidase
reagent for 30 min, and rewashed with PBS three times. The
reactions were visualised with DAB kits (cat. no. ST033; Guangzhou
Whiga Technology Co., Ltd., Guangzhou, China) as chromogen, and
sections were counterstained with haematoxylin. (Sigma-Aldrich;
Merck KGaA) for 2 min at room temperature, until the nucleus to
blue.
A total of four high-power fields were analyzed at
×400 magnification with a light microscope (Olympus Corporation,
Toyko, Japan) from a single representative tissue section (4ct).
Fields of interest were selected to reflect the area of highest
intensity staining and highest percentage of cells that were
positive, and scored and the mean was recorded. The percentage of
positive cells was assessed by counting manually. Each sample field
was assigned a value between 0 and 4 (0, negative; 1, 15% of the
cells with positive staining; 2, 5–50% of the cells with positive
staining; 3, >50% of the cells with weak staining and 4, >50%
of the cells with strong staining) for ILK, p-Akt, p-GSK3β and Bax
(14). The proportion of
p-HSF1-positive cells was determined by counting 100 cells in six
random fields from each section.
Terminal
deocynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) analysis
Tissue sections of the tumors were fixed in 10%
neutral buffered formalin (Thermo Fisher Scientific, Inc.) for 24 h
at 4°C and were prepared in a routine manner, washed in 0.01 M PBS
and incubated with 50 l TUNEL reaction mixture (prepared by mixing
a terminal deoxynucleotidyl transferase enzyme solution with a
solution containing a fluorescence-producing agent in a 1:1 ratio)
at 37°C for 60 min. The negative control sections were treated with
PBS to replace terminal deoxynucleotidyl transferase using the
rubber sealing process. In total, five high-power fields at ×400
magnification were analyzed through a fluorescence microscope
(Leica Microsystems, Inc., Buffalo Grove, IL, USA) from a single
representative tissue section, selected to reflect the area of
highest intensity staining and highest percentage of cells nuclei
that were stained red, were scored.
Statistical analysis
Measurement data are expressed as the mean ±
standard deviation. All experimental and control groups were
compared using one-way analysis of variance. Multiple comparisons
between the groups were performed using Student-Neuman-Keuls test.
All statistical tests were performed using SPSS statistical
software, version 13.0 (SPSS, Inc., Chicago, IL, USA). P-values
were two-tailed. P<0.05 was considered to indicate a
statistically significant difference.
Results
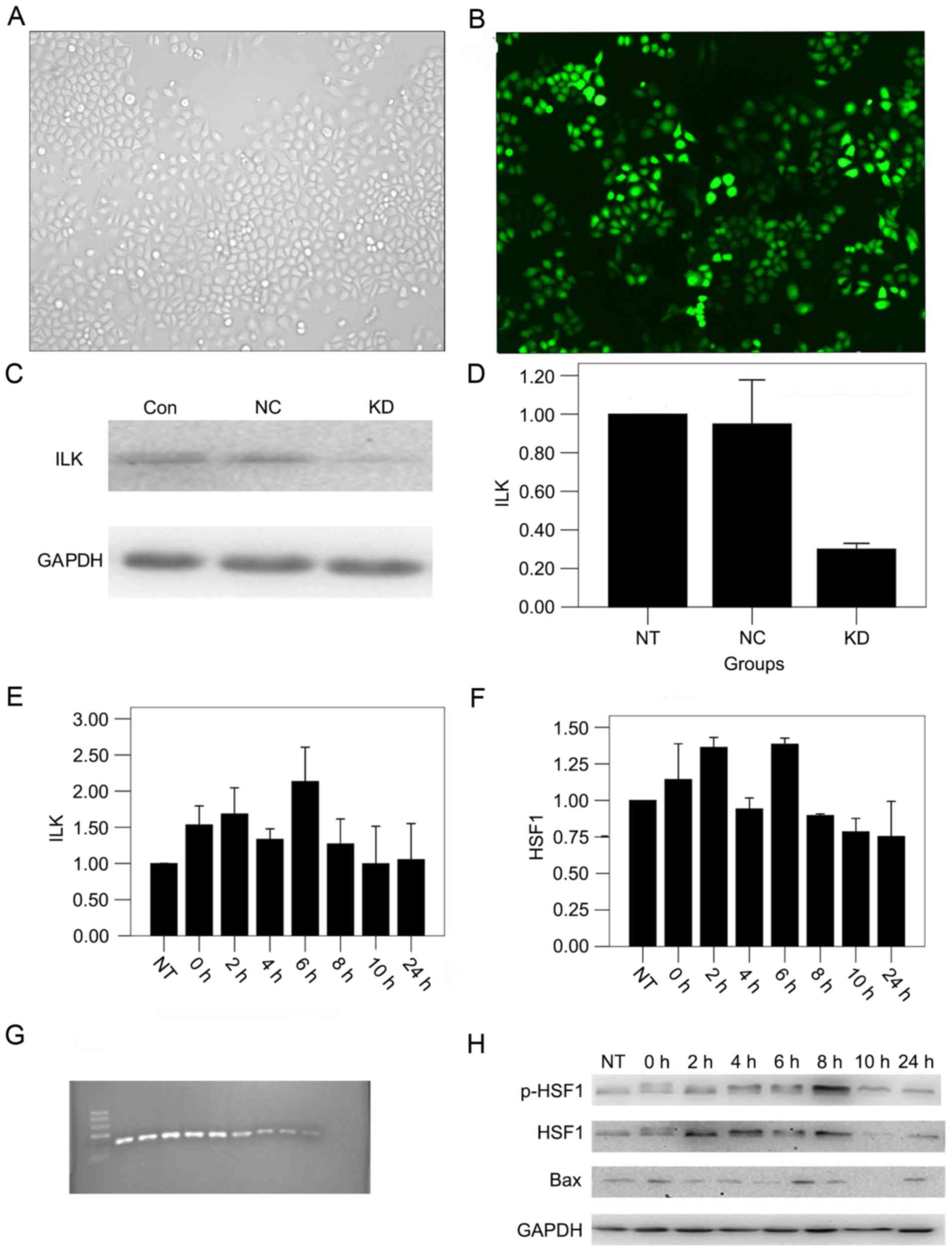
shRNA transfection effectively
decreases the expression of ILK in OSCC cells
To observe the function of ILK in OSCC, ILK
expression was knocked down in the OSCC cell line. Specific ILK
downregulation was confirmed by reduced expression of ILK mRNA and
protein in cells transfected with shRNAs. A large number of cells
illuminated bright green, which represented the high transfection
efficiency. Green fluorescence was not detected in non-transfected
cells. The transduction efficiency was 90% (Fig. 1A and B) and the silencing efficiency
was 75%, calculated by the mean of qPCR and western blot analysis
(Fig. 1C and D).
Detection of the optimal time for gene
silencing
In order to obtain the optimal time point of thermal
tolerance, the expression of thermal-associated factors was
measured. If silencing ILK sensitized hyperthermia at a specific
time point, then silencing ILK should sensitize hyperthermia more
in other low thermal tolerance conditions. By qPCR analysis, it was
revealed that the expression of ILK mRNA was highest 6 h after
hyperthermia (Fig. 1E), and HSF1
expression peaked 2 and 6 h after hyperthermia (Fig. 1F). Western blot analysis revealed that
the expression of p-HSF1 protein peaked 8 h after hyperthermia, and
the expression of Bax protein was highest 6 h after hyperthermia
(P<0.01; Fig. 1G and H). Finally,
8 h post-hyperthermia was found to be the optimal time for gene
silencing.
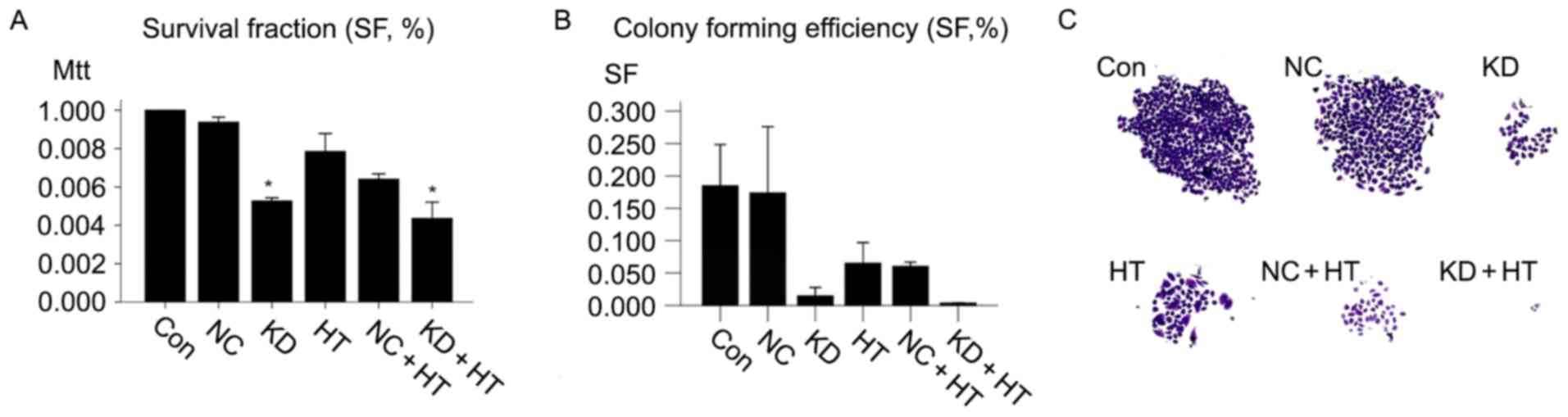
ILK silencing combined with
hyperthermia inhibits cell proliferation, migration and cell cycle
in vitro
The contribution of ILK silencing combined with
hyperthermia to OSCC cell proliferation was explored. MTT assays
were employed in the present study. The results demonstrated that
the (KD + HT group; ILK silencing combined with hyperthermia group)
grew significantly more slowly compared with other groups
(P<0.01). No significant difference was detected in cells
treated with NC-shRNA-lentivirus compared with untreated cells
(Fig. 2A).
The present study sought to determine the
contribution of ILK silencing combined with hyperthermia to OSCC
cell migration. Colony forming assays were employed in the present
study; the colony formation rate of untreated cells was
0.185±0.007, while that of the NC group was 0.174±0.011. The colony
formation rate of the KD + HT group (0.004±0.000) was significantly
lower compared with the KD group (0.015±0.001), and the colony
formation rates of two groups were significantly lower compared
with the other groups (P<0.01; Fig. 2B
and C).
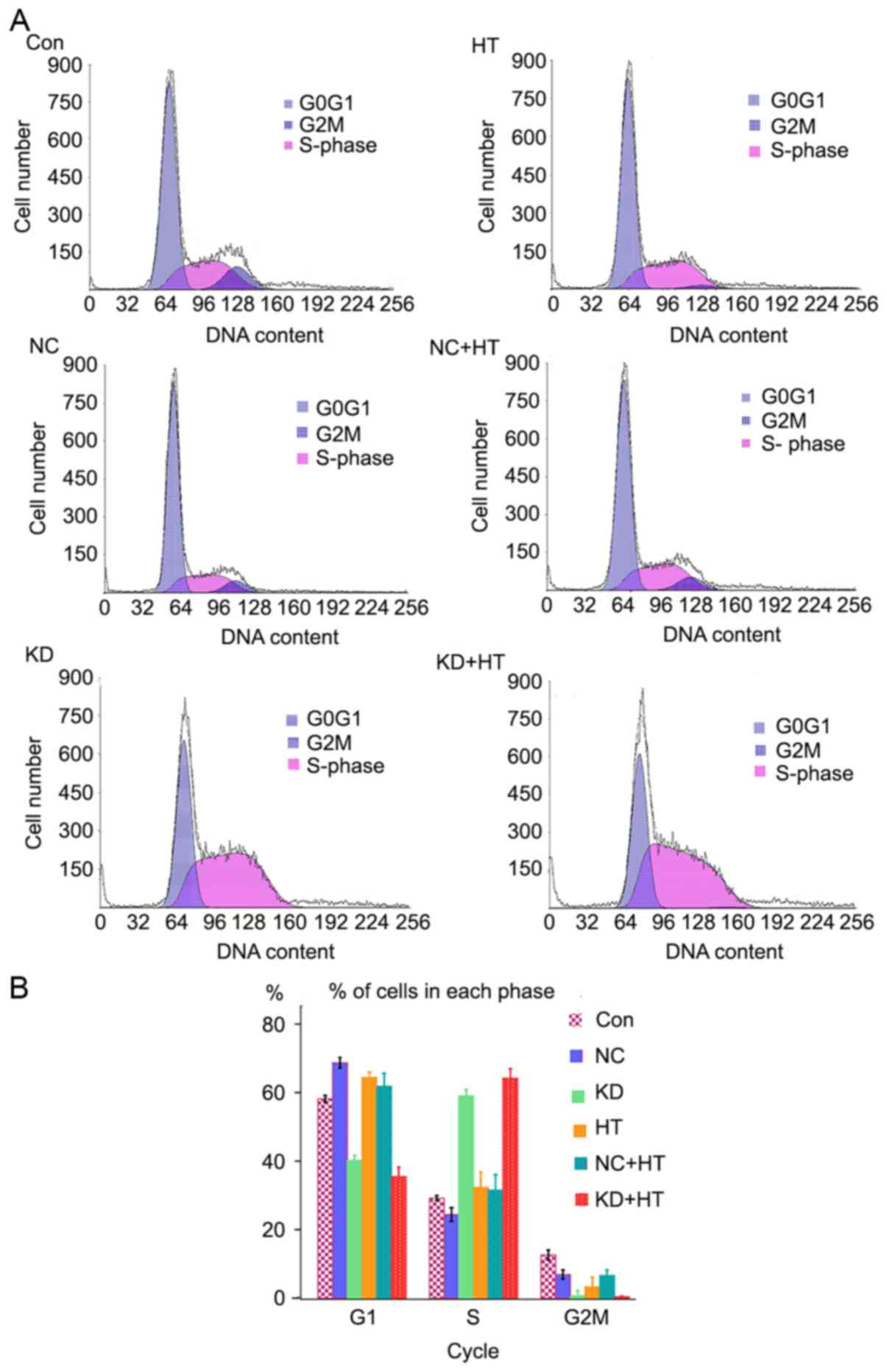
Flow cytometry was used to assess changes in the
cell cycle. Cell retention in the S phase of the KD and KD + HT
groups was significantly increased compared with the other groups
(P<0.01), and that of the KD + HT group was increased compared
with the KD group (Fig. 3). This
indicated that the cells were blocked in G2 phase, and could not
undergo mitosis, resulting in apoptosis.
ILK silencing combined with
hyperthermia promotes apoptosis
The expression of Bax could regulate cell apoptosis.
Western blot analysis was employed to measure the change of the
associated protein in the present study. It was found that the
expression of p-Akt and p-GSK3β were reduced, resulting in
downregulation of p-HSF1 expression, and eventually leading to
upregulation of the apoptosis-associated protein Bax. The variation
tendency of protein expression in the KD + HT group was comparable
to that in the KD group, however the effect was more significant
(P<0.01; Fig. 4A and B).
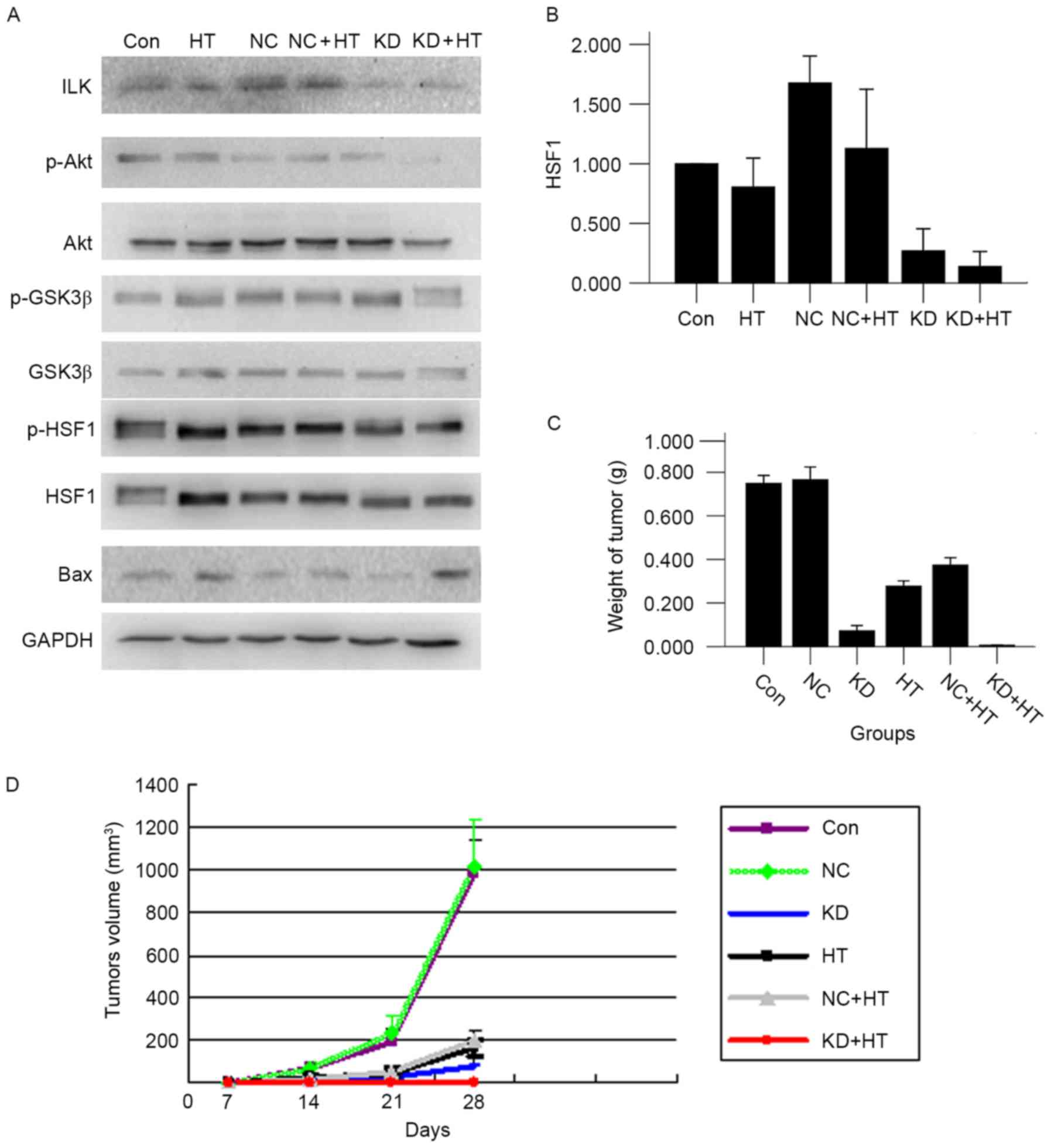 | Figure 4.(A) By western blot analysis, ILK
silencing combined with hyperthermia could inhibit the proteins
level of p-Akt, p-GSK3β and p-HSF1, and promote the expression of
Bax protein (P<0.01). (B) The p-HSF1 expression was lowest in
the KD + HT group. (C) ILK silencing combined with hyperthermia
reduced tumor weight (P<0.01). (D) ILK silencing combined with
hyperthermia reduced tumor volume (P<0.01). ILK, integrin-linked
kinase; p-, phosphorylated; GSK3β, glycogen synthase kinase 3β;
HSF1, heat shock factor 1; Bax, B cell-2-associated X protein; KD,
ILK silencing; HT, hyperthermia; NC, negative control; Con,
control. |
In vivo analysis: ILK silencing
combined with hyperthermia inhibits tumor growth
Following inoculation for 7 days, the xenograft
tumor formed. Mice were sacrificed at 28 days after the first
hyperthermia. The tumor volume of the KD + HT group was the
smallest, followed by that of the KD group; subsequent to
treatment, it was revealed that the tumor volume and weight of the
KD + HT group were the smallest in all groups (P<0.01 vs. all
other groups; Fig. 4C and D). The
tumor surface of the two groups were yellow-green, the membrane was
intact, and adhesion and infiltration were not observed compared
with all other groups (Fig. 5A).
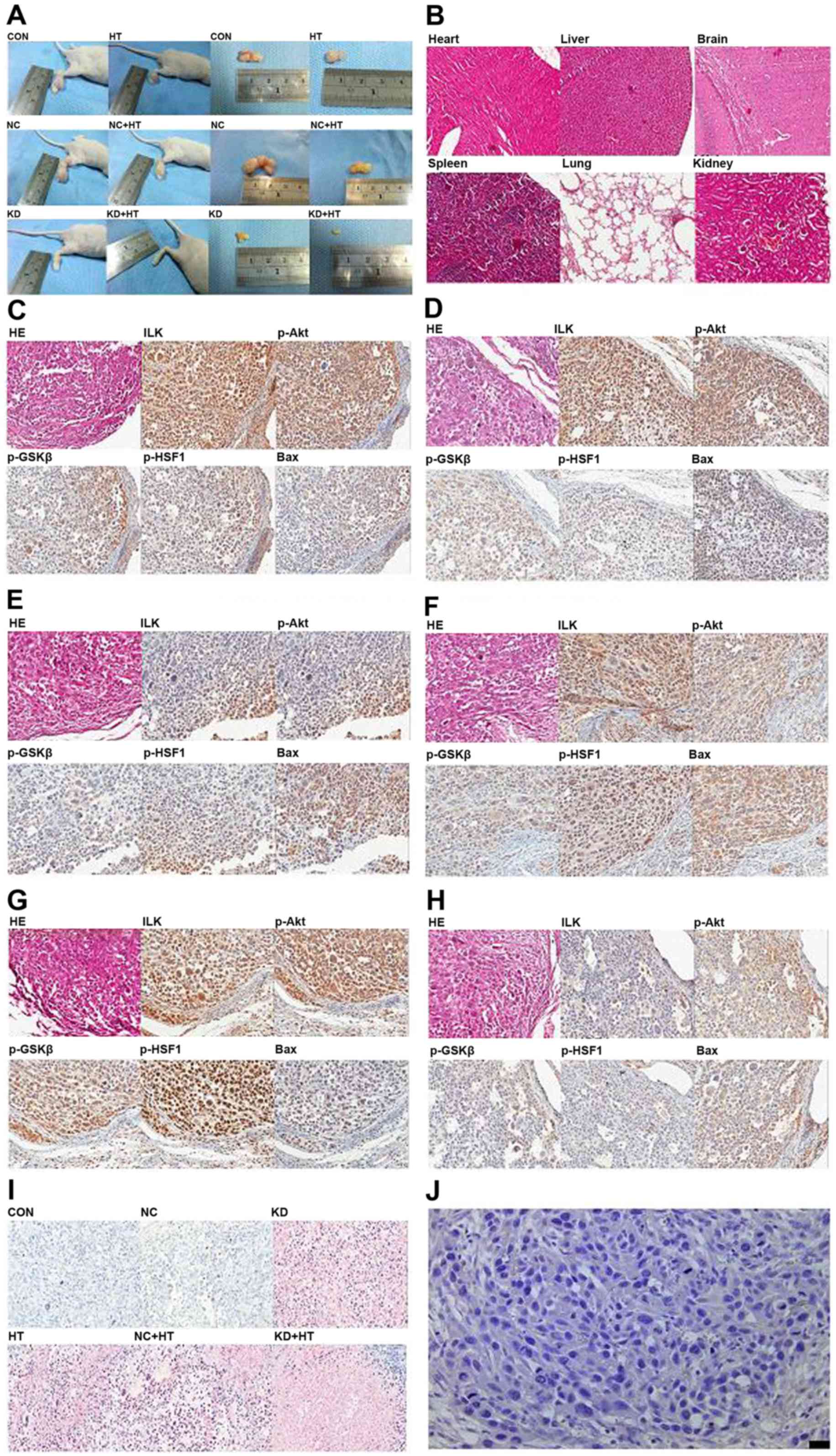 | Figure 5.In vivo experiments of ILK
silencing combined with hyperthermia (×400). (A) A total of 7 days
after inoculation, the xenograft tumor formed. (B) There was no
significant organic damage in heart, liver, brain, spleen, lung and
kidney of nude mice following ILK silencing combined with
hyperthermiaby H&E staining (×100). (C) Results of H&E
staining and immunohistochemical analysis in the control group.
Polygonal cells were in dense flocks, nuclei were large and deeply
stained, the expression of ILK, p-Akt, p-GSK3β and p-HSF1 was high,
and the expression of Bax was low. (D) The figures of the NC group
were same as the control group. (E) Results of H&E staining and
IHC analysis in the KD group. (F) Results of H&E staining and
IHC analysis in the HT group. (G) Results of the NC + HT group were
same as the HT group. (H) Results of the KD + HT group were similar
to the HT group. (I) TUNEL analysis was used to detect cell
apoptosis. Cell apoptosis was significantly increased in the KD
group and KD + HT group. Fewer apoptotic cells were found in the HT
group and the NC + HT group, and the smallest number of apoptotic
cells were found in the control and NC groups. (J) IgG negative
control. ILK, integrin-linked kinase; HT, hyperthermia; p-,
phosphorylated; GSK3β, glycogen synthase kinase 3β; HSF1, heat
shock factor 1; Bax, B cell-2-associated X protein; H&E,
hematoxylin and eosin; NC, negative control; Con, control; HT,
hyperthermia; KD, ILK silencing. |
In vivo analysis
Microscope observation of xenograft tumor
No significant organic damage was observed in the
heart, liver, brain, spleen, lung or kidney by H&E staining
(Fig. 5B). Subsequent to H&E
staining, polygonal cells were in dense flocks and nuclei were
large and deeply stained in the control and the NC group; tumor
cells were distributed sparsely, some cells shrunk and became round
and nuclei were pyknotic and darkly stained in the HT group and the
NC + HT group; tumor cells were small and distribution was sparse,
most cells shrunk and became round and nuclei were pyknotic and
darkly stained in the KD group and the KD + HT group (Fig. 5C-H).
Immunohistochemical analysis detected positive ILK,
p-Akt and p-GSK3β expression, predominantly in the cytoplasm and
occasionally in the nucleus, while positive expression of p-HSF1
was detected predominantly in the nucleus and occasionally in the
cytoplasm. Positive Bax expression was detected mainly in the
cytoplasm and occasionally in the membrane. The results of
quantitative analysis are presented in Table I. Positive p-HSF1 expression in the KD
+ HT group was the least detectable (P<0.01; Fig. 5C-H).
 | Table I.Quantitative analysis of ILK, p-Akt,
p-GSK3β, p-HSF1 and Bax expression detected by immunohistochemical
analysis. |
Table I.
Quantitative analysis of ILK, p-Akt,
p-GSK3β, p-HSF1 and Bax expression detected by immunohistochemical
analysis.
| Groups | ILK | p-Akt | p-GSK3β | Bax | p-HSF1 |
|---|
| CON | 4 | 4 | 4 | 2 |
78.6730±3.86401 |
| NC | 4 | 4 | 4 | 2 |
78.7240±2.77414 |
| HT | 3 | 3 | 3 | 2 |
59.1380±1.96891 |
| NC+HT | 3 | 3 | 3 | 2 |
59.1980±3.01508 |
| KD | 2 | 2 | 2 | 3 |
29.4220±3.13326 |
| KD+HT | 2 | 2 | 2 | 3 |
22.0400±2.11737 |
In TUNEL analysis, cell apoptosis was significantly
increased in the KD group and the KD + HT group. The highest number
of apoptotic cells was observed in the KD + HT group, fewer
apoptotic cells were found in the HT group and the NC + HT group,
and the fewest number of apoptotic cells was observed in the
control group and NC group (P<0.01; Fig. 5I).
Discussion
At present (17–23), it is
recognized internationally that cancer progression is a
multi-factorial, multi-step, multi-stage process, which involves a
variety of changes in gene expression. Subsequent to being
subjected to thermal stimulation, the cells produce a heat shock
response, and their role in the resistance to heat and other stress
response is enhanced (24). Cells
express HSPs to fight heat stress, and the expression of HSPs is
regulated by HSF1 (12). Therefore,
the efficacy of hyperthermia can be assessed through the activation
of HSF1, that is, the expression level of phosphorylated HSF1. In a
study by He et al (17), HeLa
cells were heated at 45°C for 30 min, and the cells were then
allowed to recover at 37°C for 0, 2, 4, 6, 8, 10 and 24 h, and it
was revealed that the DNA binding activity of HSF1 was highest
following 8 h. In another study by Bijur and Jope (25), neuroblastoma SH-SY5Y cells were heated
at 45°C for 30 min, allowed to recover at 37°C for 0, 2, 4, 6, 8,
10 and 24 h, and the DNA binding activity of HSF1 was found to be
highest after 4 h. In the present study, Tca8113 cells were heated
at 45°C for 30 min, and the cells were allowed to recover at 37°C
for 0, 2, 4, 6, 8, 10 and 24 h. The mRNA of HSF1 was highest after
6 h and the protein of HSF1 was highest after 8 h, which is
consistent with the experimental results of He et al
(17). The rewarming time was
different between the highest levels of HSF1 mRNA and the highest
levels of HSF1 protein, which may be the reason that RNA was
expressed substantially earlier than the protein. The heat
sensitivity was worst and the thermal tolerance was highest after 8
h, so subsequent experiments were performed at this time point.
After silencing ILK, if the sensitivity of the thermotherapy
increased at the given time point, the sensitivity of the
thermotherapy should increase more significantly in other time
points (because the other time points represented thermal tolerance
conditions).
Changes of Tca8113 cell proliferation, migration and
cell cycle were detected. It was revealed that hyperthermia
combined with ILK gene silencing could significantly inhibit cell
proliferation and migration and Tca8113 cells were arrested in the
S phase. Tumor cells could not undergo mitosis, and apoptosis
eventually occurred. This is in contrast from previous studies
(26,27), which reported that inhibition of ILK
leads to tumor cells stagnating in the G1 phase.
The phosphorylation sites of Akt Ser473 can be
completely suppressed when ILK is inhibited in certain tumor cells
(28,29). The present results were in agreement
with this observation, but in OSCC cells, it was demonstrated that
silencing ILK can significantly reduce the expression of p-HSF1.
Previous studies have shown (30–32) that
inhibition of the PI3K/Akt pathway can significantly improve the
sensitivity of hyperthermia. It was also revealed that the
expression of p-Akt, p-GSK3β and p-HSF1 were significantly reduced
following ILK silencing. Therefore, ILK silencing significantly
improved the sensitivity of hyperthermia. In addition, the
apoptosis-associated protein Bax was found to be significantly
increased in the KD + HT group, but was not significantly increased
in the KD group and other groups, which indicated that ILK
silencing combined with hyperthermia significantly increased
apoptosis of Tca8113 cells. In contrast, it was also observed that
p-Akt and p-GSK3β expression in the KD + HT group was reduced
compared with those of the KD group, indicating that hyperthermia
increased the ILK silencing effect as feedback, producing an
increased inhibitive effect on the PI3K/Akt signaling pathway.
Finally, in vivo experiments revealed that
the transplanted tumor volume and weight in the KD + HT group were
significantly reduced compared with other groups, and organ damage
was not detected, which indicated the efficacy of the treatment.
Similar results were obtained with in vitro experiments by
the PI3K/Akt pathway and hyperthermia-associated protein
immunohistochemistry and TUNEL analysis. In in vivo
experiments, ILK silencing led to the sensitivity of hyperthermia
increase in nude mice and hyperthermia could feedback to enhance
the effect of ILK silencing; the combination therapy had a
synergistic antitumor effect.
To conclude, the present study investigated the
potential to withstand cancer-associated activities of ILK
silencing combined with hyperthermia against OSCC in vitro
and in vivo. The results demonstrated that ILK silencing led
to Tca8113 cells and their transplanted tumors exhibiting increased
hyperthermia sensitivity; and hyperthermia could feedback to
enhance the effect of ILK silencing, consequently inhibiting the
PI3K/Aktsignaling pathway. Therefore, the combination therapy had a
synergistic antitumor effect.
Acknowledgements
The present study was supported by the project in
Sichuan Province Scientific and Technological Support (grant no.
2011SZ0156), Hefei Science and Technology Research Program (grant
no. 2015HK163) and Hefei's Independent Innovation Policy ‘Borrow
and Replace’ Project (grant no. 2017).
References
|
1
|
Perri F, Muto P, Aversa C, Daponte A,
Della Vittoria G, Pepe S and Caponigro F: Integrated therapeutic
approaches in head and neck cancer: The importance of
multidisciplinary team management. Anticancer Agents Med Chem.
13:834–843. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Antoniv TT and Ivashkiv LB:
Interleukin-10-induced gene expression and suppressive function are
selectively modulated by the PI3K-Akt-GSK3 pathway. Immunology.
132:567–577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aksamitiene E, Kiyatkin A and Kholodenko
BN: Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt
pathways: A fine balance. Biochem Soc Trans. 40:139–146. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Troussard AA, Mawji NM, Ong C, Mui A,
St-Arnaud R and Dedhar S: Conditional knock-out of integrin-linked
kinase demonstrates an essential role in protein kinase B/Akt
activation. J Biol Chem. 278:22374–22378. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marotta A, Parhar K, Owen D, Dedhar S and
Salh B: Characterisation of integrin-linked kinase signalling in
sporadic human colon cancer. Br J Cancer. 88:1755–1762. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tasian SK, Teachey DT and Rheingold SR:
Targeting the PI3K/mTOR pathway in pediatric hematologic
malignancies. Front Oncol. 4:1082014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng K, Wang G, Li C, Shan X and Liu H:
Knockdown of ILK inhibits glioma development via upregulation of
E-cadherin and downregulation of cyclin D1. Oncol Rep. 34:272–278.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao X, Xu Z, Wang Z, Wu Z, Gong Y, Zhou L
and Xiang Y: RNA silencing of integrin-linked kinase increases the
sensitivity of the A549 lung cancer cell line to cisplatin and
promotes its apoptosis. Mol Med Rep. 12:960–966. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dicheva BM and Koning GA: Targeted
thermosensitive liposomes: An attractive novel approach for
increased drug delivery to solid tumors. Expert Opin Drug Deliv.
11:83–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamamoto D, Inui T, Tsubota Y, Sueoka N,
Yamamoto C, Kuwana K and Yamamoto M: The utility of hyperthermia
for local recurrence of breast cancer. World J Surg Oncol.
10:2012012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang XH, He YW, Tang YL, Wu JL, Gao XP,
Xiao GZ and Mao ZY: Thermochemotherapy of lower lip squamous cell
carcinoma without metastases: An experience of 31 cases. J
Craniomaxillofac Surg. 38:260–265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tao Y, Guo Y, Liu W, Zhang J, Li X, Shen
L, Ru Y, Xue Y, Zheng J, Liu X, et al: AKT inhibitor suppresses
hyperthermia-induced Ndrg2 phosphorylation in gastric cancer cells.
Braz J Med Biol Res. 46:394–404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schaeffer DF, Assi K, Chan K, Buczkowski
AK, Chung SW, Scudamore CH, Weiss A, Salh B and Owen DA: Tumor
expression of integrin-linked kinase (ILK) correlates with the
expression of the E-cadherin repressor snail: An
immunohistochemical study in ductal pancreatic adenocarcinoma.
Virchows Arch. 456:261–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao Z, Liu F, Yin P, Wan C, He S, Liu X,
Zhao H, Liu T, Xu J and Guo S: Inhibition of heat-induced apoptosis
in rat small intestine and IEC-6 cells through the AKT signaling
pathway. BMC Vet Res. 9:2412013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao D, Tang XF, Yang K, Liu JY and Ma XR:
Over-expression of integrin-linked kinase correlates with aberrant
expression of Snail, E-cadherin and N-cadherin in oral squamous
cell carcinoma: implications in tumor progression and metastasis.
Clin Exp Metastasis. 29:957–969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakagawa T, Ohnishi K, Kosaki Y, Saito Y,
Horlad H, Fujiwara Y, Takeya M and Komohara Y: Optimum
immunohistochemical procedures for analysis of macrophages in human
and mouse formalin fixed paraffin-embedded tissue samples. J Clin
Exp Hematop. 57:31–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He B, Meng YH and Mivechi NF: Glycogen
synthase kinase 3beta and extracellular signal-regulated kinase
inactivate heat shock transcription factor1 by facilitating the
disappearance of transcriptionally active granules after heat
shock. Mol Cell Biol. 18:6624–6633. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen X and Zhang J, Han C, Dai H, Kong X,
Xu L, Xia Q, Zhang M and Zhang J: A sexual dimorphism influences
bicyclol-induced hepatic heat shock factor 1 activation and
hepatoprotection. Mol Pharmacol. 88:38–47. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rahman MA, Amin AR, Wang D, Koenig L,
Nannapaneni S, Chen Z, Wang Z, Sica G, Deng X, Chen ZG and Shin DM:
RRM2 regulates Bcl-2 in head and neck and lung cancers: A potential
target for cancer therapy. Clin Cancer Res. 19:3416–3428. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de la Torre NG, Buley I, Wass JA and
Turner HE: Angiogensis and lymphangiogenesis in thyroid
proliferative lesions: Relationship to type and tumour behaviour.
Endocr Relat Cancer. 13:931–944. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marchesin V, Castro-Castro A, Lodillinsky
C, Castagnino A, Cyrta J, Bonsang-Kitzis H, Fuhrmann L, Irondelle
M, Infante E, Montagnac G, et al: ARF6-JIP3/4 regulate endosomal
tubules for MT1-MMP exocytosis in cancer invasion. J Cell Biol.
211:339–358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qian DC, Byun J, Han Y, Greene CS, Field
JK, Hung RJ, Brhane Y, Mclaughlin JR, Fehringer G, Landi MT, et al:
Identification of shared and unique susceptibility pathways among
cancers of the lung, breast, and prostate from genome-wide
association studies and tissue-specific protein interactions. Hum
Mol Genet. 24:7406–7420. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zeise E and Rensing L: Hyperthermia
pre-treatment protects rats IPC-81 leukaemia cells against heat-
and hydrogen peroxide-induced apoptosis. Int J Hyperthermia.
18:344–360. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bijur GN and Jope RS: Opposing actions of
phosphatidylinositol 3-kinase and glycogen synthase kinase-3beta in
the regulation of HSF-1 activity. J Neurochem. 75:2401–2408. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu XY, Liu N, Liu W, Song SW and Guo KJ:
Silencing of the integrin-linked kinase gene suppresses the
proliferation, migration and invasion of pancreatic cancer cells
(Panc-1). Genet Mol Biol. 35:538–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Q, Li C, Zhang YY, Chen W, Lv JL, Sun J
and You QS: Silencing of integrin-linked kinase suppresses in
vivo tumorigenesis of human ovarian carcinoma cells. Mol Med
Rep. 7:1050–1054. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Persad S, Attwell S, Gray V, Delcommenne
M, Troussard A, Sanghera J and Dedhar S: Inhibition of
integrin-linked kinase (ILK) suppresses activation of protein
kinase B/Akt and induces cell cycle arrest and apoptosis of
PTEN-mutant prostate cancer cells. Proc Natl Acad Sci USA. 97:pp.
3207–3212. 2000, View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Makino K, Day CP, Wang SC, Li YM and Hung
MC: Upregulation of IKKalpha/IKKbeta by integrin-linked kinase is
required for HER2/neu-induced NF-kappaB antiapoptotic pathway.
Oncogene. 23:3883–3887. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jevtov I, Zacharogianni M, van Oorschot
MM, van Zadelhoff G, Aguilera-Gomez A, Vuillez I, Braakman I, Hafen
E, Stocker H and Rabouille C: TORC2 mediates the heat stress
response in Drosophila by promoting the formation of stress
granules. J Cell Sci. 128:2497–2508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ohnishi K, Yasumoto J, Takahashi A and
Ohnishi T: LY294002, an inhibitor of PI-3K, enhances heat
sensitivity independently of p53 status in human lung cancer cells.
Int J Oncol. 29:249–253. 2006.PubMed/NCBI
|
|
32
|
Ma N, Szmitko P, Brade A, Chu I, Lo A,
Woodgett J, Klamut H and Liu FF: Kinase-dead PKB gene therapy
combined with hyperthermia for human breast cancer. Cancer Gene
Ther. 11:52–60. 2004. View Article : Google Scholar : PubMed/NCBI
|