Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common malignancy and the third leading cause of cancer-related
mortality in the world (1). Only 20%
of HCC patients are candidates for resection (2) due to multifocal disease or poor liver
function reserve as a result of the underlying cirrhosis. Liver
transplantation is an alternative curative treatment for early HCC,
but is often unfeasible due to the shortage of liver
transplantation donors. Therefore, various non-surgical therapies
have been introduced. Among these, local ablative therapies have
been developed for the management of small HCC (3). Currently, local ablative therapies
compete with resection and liver transplantation as curative
treatments for small HCC.
Percutaneous radiofrequency ablation (RFA) is one of
the most widely used local ablation therapies for HCC, with a high
complete ablation rate of >85% for solitary tumors <5 cm in
diameter or up to 3 tumors with a maximum diameter of 3 cm
(4,5).
Two previous randomized controlled trials have suggested that RFA
is as effective as resection in terms of overall survival (OS) and
disease-free survival times (6,7).
Increasing evidence demonstrates that RFA for small HCC (<3 cm)
may result in comparable survival time with partial hepatectomy
(7,8).
However, RFA is associated with a high incidence of postoperative
recurrence. It has been reported that the cumulative 5-year
recurrence rate of patients undergoing RFA is >70% (8,9).
Previous studies have demonstrated that the systemic
inflammatory response (SIR) serves an important role in the
progression of cancer (10,11), including the proliferation, invasion,
recurrence and metastasis of tumors. SIR is associated with OS and
postoperative survival times in patients of several cancer types
(12–14). As typical representatives of
inflammatory factors, the neutrophil to lymphocyte ratio (NLR) and
the platelet to lymphocyte ratio (PLR) have become a research focus
for a number of malignancies. Published data also suggest that an
elevated NLR may be associated with a worse prognosis in patients
with HCC who have undergone resection, orthotopic liver
transplantation or transcatheter arterial chemoembolization (TACE)
(15–18). Several clinical studies in advanced
HCC have demonstrated that an elevated NLR or PLR reflects an
inflammatory process elicited by cancer cells, and is associated
with unfavorable clinicopathological features (15,17,19).
To the best of our knowledge, few studies have
evaluated the association between the NLR, the PLR and the
prognosis of HCC patients following RFA treatment. Therefore, the
present study evaluated the value of a novel inflammation-based
prognostic system, the combination of the neutrophil to lymphocyte
ratio and the platelet to lymphocyte ratio (CNP), in patients
undergoing RFA for HCC.
Materials and methods
Patients and samples
A retrospective analysis was conducted of 287
patients with HCC who had undergone RFA at Guangdong General
Hospital (Guangzhou, Guangdong, China) between January 2010 and
December 2014. Written informed consent was obtained from patients
prior to treatment and the study was approved by the Ethics
Committee of Guangdong General Hospital. A diagnosis of HCC was
based on the criteria of the American Association of the Study of
Liver Disease (20). The inclusion
criteria were as follows: i) Patient age of 18–75 years; ii) a
solitary HCC tumor ≤5.0 cm in diameter or multiple HCC lesions each
≤3 in diameter; iii) an Eastern Cooperative Oncology Group
Performance Status (ECOG-PS) (21) of
0; iv) liver function Child-Pugh (22) class A or B cirrhosis; v) RFA as the
first-line anticancer treatment for HCC; vi) preoperative NLR and
PLR data obtained <1 week prior to RFA; vii) no other
malignancies that may determine the prognosis; and viii) no
extrahepatic metastases. The exclusion criteria were as follows: i)
Radiological evidence of invasion into the major portal/hepatic
vein branches; ii) the presence of extrahepatic metastases; iii)
previous chemotherapy and/or radiotherapy; iv) previous
anti-inflammatory medicines within 1 week; v) active infection at
the time of blood sampling to establish NLR and PLR; vi) severe
coagulation disorders; and vii) loss to follow-up within 3 months
post-treatment.
RFA procedure
RFA was performed on an inpatient basis using an RFA
system (RITA Medical Systems Inc., Mountain View, CA, USA). The
pathological features (size, number, shape and border) of the
tumors were identified prior to surgery and the access routes were
determined by contrast-enhanced computed tomography (CT) or
ultrasound. All procedures were performed percutaneously, under
general or local anesthetic, by two qualified interventional
radiologists with the guidance of real-time ultrasonography or
X-ray. Each ablation cycle lasted between 5 and 12 min. For tumors
≤3.0 cm, a single ablation was performed. For tumors >3.0 cm,
multiple overlapping ablations were performed. The range of
ablation was extended 0.5–1.0 cm into the surrounding non-cancerous
tissues to ensure complete coverage.
RFA was deemed successful based on the following CT
observations: i) The ablation zone was beyond the original tumor
borders; ii) the margin of the ablation zone was clear and smooth;
and iii) no arterial enhancement or abnormal wash-out was detected
within or around the tumor.
Follow-up
Dual-phase spiral CT was performed 4–6 weeks
post-treatment. Short-term response was assessed using the modified
Response Evaluation Criteria In Solid Tumors (m-RECIST) (23), based on the CT images acquired 1 month
after RFA. Residual viable tumor tissue was considered to be
present if enhancement areas were observed within the tumor at
either the arterial or the portal venous phase. In these cases,
further RFA treatment was administered.
All patients were followed up in the Oncology Clinic
of the Gangdong General Hospital. Follow-up involved physical
examination, blood tests, including liver functions tests and
α-fetoprotein (AFP) level assessment, and abdominal CT/magnetic
resonance imaging (MRI) every 3 months for the first 2 years, every
4–6 months for the subsequent 3 years and annually thereafter. If
extrahepatic recurrence was suspected (on the basis of clinical
symptoms or an unexplained elevation in AFP level), chest CT, brain
MRI and whole-body bone scintigraphy were also performed. The
patients were censored on the date of mortality or the date of last
follow-up if tumor recurrence was not diagnosed. The last follow-up
date for the present study was December 2015.
OS and recurrence-free survival (RFS) times were
assessed. The OS time was defined as the time between termination
of RFA and the date of mortality or the last follow-up. The RFS
time was defined as the time between termination of RFA and the
first recording of disease recurrence or the date of mortality in
patients without evidence of disease recurrence. Recurrence
included local recurrence, distant intrahepatic recurrence and
extrahepatic metastasis. Local recurrence was defined as tumor
recurrence within or at the periphery of the ablated lesion on
CT/MRI after complete ablation had been confirmed on the first
post-ablation CT scan; distant intrahepatic recurrence was defined
as a separate new lesion in the liver >2 cm away from the
primary lesion and extrahepatic metastasis was defined as any tumor
lesion outside of the liver.
When recurrent tumors were diagnosed, patients
received appropriate management, including repeated RFA,
percutaneous ethanol injection therapy, TACE, resection surgery,
liver transplantation, chemotherapy, radiotherapy or supportive
treatment.
CNP evaluation
Data on preoperative blood cell counts were
extracted retrospectively from the medical records. All white blood
cell and differential counts were taken within 1–3 days prior to
RFA. The NLR was defined as the absolute neutrophil count divided
by the absolute lymphocyte count and PLR was defined as the
absolute platelet count divided by the absolute lymphocyte count.
The recommended cut-off values of the preoperative NLR and PLR were
determined using receiver operating characteristic (ROC) curve
analysis.
Statistical analysis
Statistical analyses were performed using SPSS 21.0
statistical software (IBM Corp., Armonk, NY, USA). Baseline
continuous variables were expressed as the mean ± standard
deviation or the median and were compared using one-way analysis of
variance with post-hoc Bonferroni's correction. Categorical data
were presented as frequencies and were analyzed using the Pearson
χ2 test or Fisher's exact test. Correlation analysis was
performed using Pearson's and Spearman's correlation analyses.
Survival curves were calculated using Kaplan-Meier analysis, and
the difference in survival rates between the groups were compared
using the log-rank test. The primary endpoint was OS and the
secondary endpoint was RFS. Univariate analysis was used to assess
significant differences in the clinicopathological characteristics
that influence survival following RFA. Multivariate analysis was
performed using Cox's regression analysis for significant variables
identified by univariate analysis. Risk ratios with a 95%
confidence interval were used to quantify the strength of the
association between predictors and survival. All statistical tests
were two-sided, and P<0.05 was considered to indicate a
statistically significant difference. Bonferroni's correction was
performed on the survival curve data, so P<0.017 was considered
to indicate a statistically significant difference in survival
curve analysis.
Results
CNP evaluation
The recommended cut-off values of the preoperative
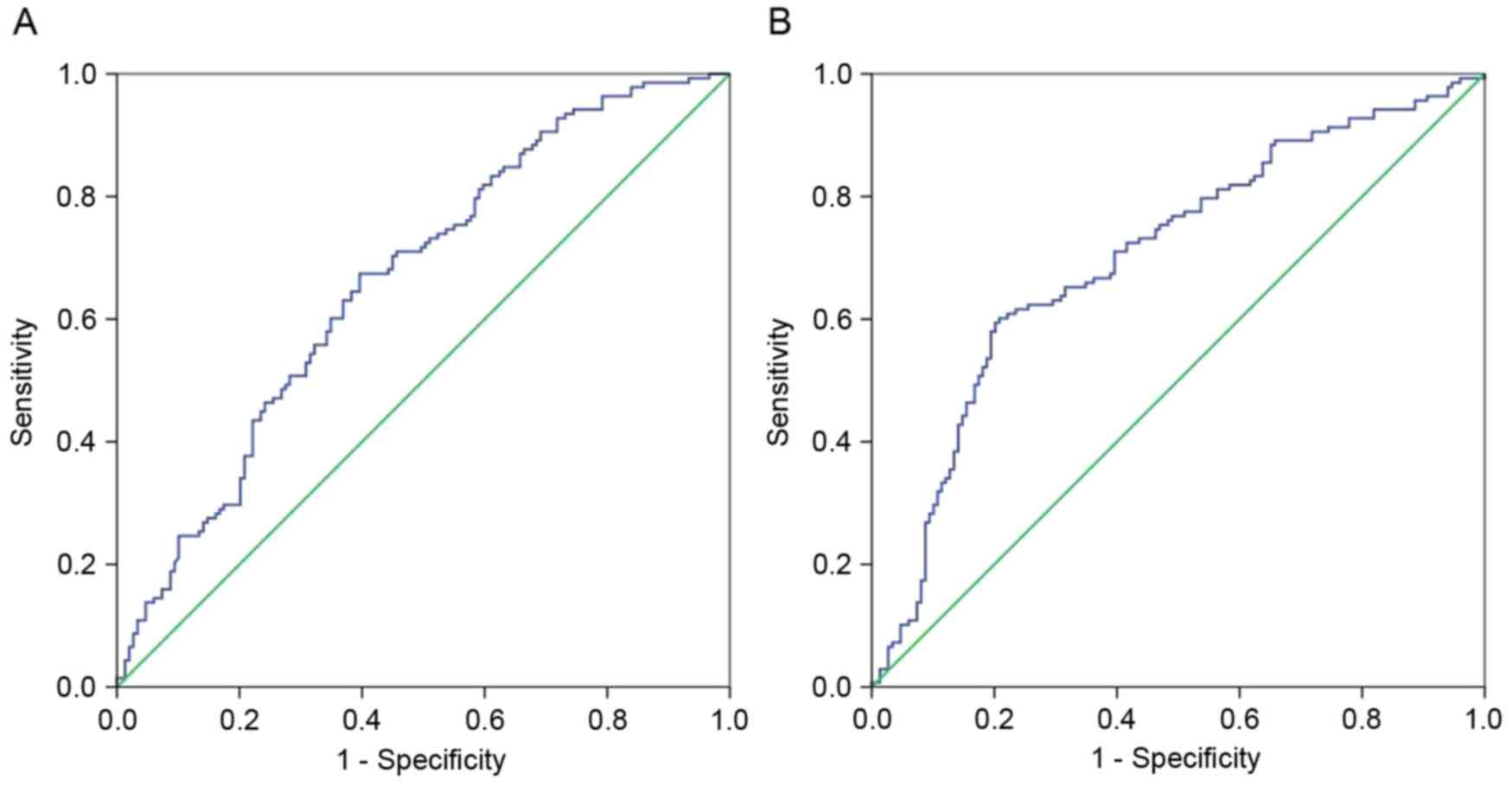
NLR and PLR were determined using ROC curve analysis (Fig. 1). The recommended cut-off value for
the NLR was based on the most prominent point on the ROC curve for
sensitivity and specificity (0.674 and 0.604, respectively). These
two parameters indicated a cut-off value of 2.58, and the area
under the ROC curve (AUC) was 0.663. Similarly, the ROC curve for
PLR recommended a cut-off value of 131.78 for sensitivity and
specificity (0.601 and 0.792, respectively), and the AUC was
0.703.
The CNP was calculated as follows: Patients with an
elevated NLR (>2.58) and an elevated PLR (>131.78) were
allocated a score of 2, and patients exhibiting one or neither of
these factors were allocated a score of 1 or 0, respectively.
Patient characteristics
A total of 287 patients were enrolled in the present
study [male:female, 215 (74.9%):72 (25.1%)]. The mean age was
56.1±14.2 years, with an age range of 27–75 years. Table I presents the clinical background
characteristics of the patients in the 3 groups divided according
to their CNP. The present study demonstrated that CNP was
associated with liver cirrhosis (P=0.015) and Child-Pugh class
(P=0.024). No significant differences were observed between the CNP
and the hepatitis B surface antigen (HBsAg), the number of tumors,
the tumor diameter or the AFP level (P>0.05). In addition, there
was a positive correlation between the PLR and the NLR (r=0.534;
P<0.001; Fig. 2).
 | Table I.Associations between general clinical
variables and CNP in hepatocellular carcinoma patients undergoing
radiofrequency ablation. |
Table I.
Associations between general clinical
variables and CNP in hepatocellular carcinoma patients undergoing
radiofrequency ablation.
| Variables | CNP0 (n=118) | CNP1 (n=81) | CNP2 (n=88) | P-value |
|---|
| Sex |
|
|
| 0.258 |
|
Male | 89 | 65 | 61 |
|
|
Female | 29 | 16 | 27 |
|
| Age, years |
|
|
| 0.352 |
|
≤55 | 51 | 43 | 39 |
|
|
>55 | 67 | 38 | 49 |
|
| HBsAg |
|
|
| 0.606 |
|
Positive | 72 | 53 | 51 |
|
|
Negative | 46 | 28 | 37 |
|
| Liver
cirrhosis |
|
|
| 0.015 |
|
Presence | 79 | 63 | 74 |
|
|
Absence | 39 | 18 | 14 |
|
| Number of
tumors |
|
|
| 0.798 |
| 1 | 87 | 58 | 67 |
|
| ≥2 | 31 | 23 | 21 |
|
| Tumor diameter,
cm |
|
|
| 0.824 |
| ≤3 | 76 | 55 | 56 |
|
|
3–5 | 42 | 26 | 32 |
|
| Tumor location near
intrahepatic vessels |
|
|
| 0.761 |
|
Yes | 41 | 30 | 35 |
|
| No | 77 | 51 | 53 |
|
| Child-Pugh
class |
|
|
| 0.024 |
| A
grade | 92 | 61 | 54 |
|
| B
grade | 26 | 20 | 34 |
|
| AFP, ng/ml |
|
|
| 0.690 |
|
<400 | 43 | 34 | 36 |
|
|
≥400 | 75 | 47 | 52 |
|
| Prothrombin time
prolongation, sec |
|
|
| 0.163 |
| ≤3 | 71 | 48 | 42 |
|
|
>3 | 47 | 33 | 46 |
|
Association between CNP and various
clinicopathological characteristics of HCC
The associations between CNP and different
clinicopathological features of HCC were analyzed (Table II). Significant differences were
identified among the groups with regard to the absolute neutrophil
count (CNP 0 vs. CNP 1, P<0.001; CNP 1 vs. CNP 2, P=0.139), the
absolute lymphocyte count (CNP 0 vs. CNP 1, P<0.001; CNP 1 vs.
CNP 2, P<0.001), the total platelet count (CNP 0 vs. CNP 1,
P=0.120; CNP 1 vs. CNP 2, P<0.001), the total bilirubin level
(mol/l) (CNP 0 vs. CNP 1, P=0.043; CNP 1 vs. CNP 2, P=0.012), the
NLR (CNP 0 vs. CNP 1, P<0.001; CNP 1 vs. CNP 2, P<0.001) and
the PLR (CNP 0 vs. CNP 1, P<0.001; CNP 1 vs. CNP 2,
P<0.001).
 | Table II.Correlations between CNP and
clinicolaboratory variables. |
Table II.
Correlations between CNP and
clinicolaboratory variables.
| Variable | CNP0 (n=118) | CNP1 (n=81) | CNP2 (n=88) | P-value (0 vs.
1) | P-value (1 vs.
2) | P-value |
|---|
| ALT, U/l |
38.7±30.5 |
45.3±33.9 |
41.2±36.4 | – | – | 0.627 |
| AST, U/l |
41.6±33.8 |
47.2±35.7 |
49.8±41.6 | – | – | 0.483 |
| Absolute neutrophil
count, n |
3.72±1.43 |
4.87±2.48 |
5.29±1.70 | <0.001 | 0.139 | <0.001 |
| Absolute lymphocyte
count, n |
2.05±0.61 |
1.59±0.73 |
1.22±0.40 | <0.001 | <0.001 | <0.001 |
|
γ-glutamyltransferase, U/l |
70.7±45.7 |
65.8±49.3 |
75.1±40.8 | – | – | 0.207 |
| Total platelets,
n |
169.90±62.35 |
186.42±80.55 |
243.13±80.00 | 0.120 | <0.001 | <0.001 |
| Albumin, g/dl |
3.78±0.66 |
4.12±0.81 |
3.91±0.73 | – | – | 0.774 |
| Total bilirubin,
mol/l |
16.6±9.1 |
18.9±8.3 |
22.1±11.4 | 0.043 | 0.012 | 0.028 |
| NLR |
1.92±0.83 |
3.11±1.00 |
4.64±2.14 | <0.001 | <0.001 | <0.001 |
| PLR |
84.63±23.09 |
124.39±52.55 |
206.22±63.30 | <0.001 | <0.001 | <0.001 |
Pattern of recurrence in patients with
HCC following RFA
The present follow-up study demonstrated that
179/287 (62.4%) patients developed recurrence. Among these
patients, 151 (52.6%) developed intrahepatic recurrence (37 with
local recurrence and 114 with distant intrahepatic recurrence). A
total of 46 patients (16.0%) developed extrahepatic metastasis,
including 18 cases of concurrent intrahepatic and extrahepatic
recurrences.
To further evaluate the association between CNP and
tumor recurrence in these patients, the pattern of recurrence was
analyzed and the results are presented in Table III. The present study demonstrated
that, although no significant differences were identified in the
local recurrence rate among the three groups, the patients with an
elevated CNP had significantly higher distant intrahepatic
recurrence (52.3, 34.6 and 33.9% for CNP groups 2, 1 and 0,
respectively; CNP 0 vs. CNP 1, P=0.922; CNP 1 vs. CNP 2, P=0.020)
and extrahepatic recurrence rates (25.0, 18.5 and 7.6% for CNP
groups 2, 1 and 0, respectively; CNP 0 vs. CNP 1, P=0.020; CNP 1
vs. CNP 2, P=0.309) compared with their low-CNP counterparts. In
addition, 3, 6, and 9 patients had concurrent intrahepatic and
extrahepatic recurrences in the CNP 0, CNP 1 and CNP 2 groups,
respectively (data not shown).
 | Table III.Correlation between pattern of
recurrence and CNP in patients with hepatocellular carcinoma. |
Table III.
Correlation between pattern of
recurrence and CNP in patients with hepatocellular carcinoma.
| Recurrence | CNP 0, n (%) | CNP 1, n (%) | CNP 2, n (%) | P-value (0 vs.
1) | P-value (1 vs.
2) | P-value |
|---|
| Type of
recurrence |
| Local
recurrence | 18 (15.3) | 13 (16.0) | 6 (6.8) | – | – | 0.123 |
| Distant
intrahepatic recurrence | 40 (33.9) | 28 (34.6) | 46 (52.3) | 0.922 | 0.020 | 0.015 |
|
Extrahepatic recurrence | 9 (7.6) | 15 (18.5) | 22 (25.0) | 0.020 | 0.309 | 0.003 |
| Total
patients | 64 (54.2) | 50 (61.7) | 65 (73.9) | – | – | 0.016 |
| Treatment for
recurrence |
| Repeat
RFA | 22 | 13 | 25 | – | – | 0.105 |
|
Others | 42 | 37 | 40 | – | – | 0.241 |
OS and RFS
Kaplan-Meier analysis and log-rank tests
demonstrated a significant difference in the median OS time among
the three groups (23.2, 50.0 and 62.8 months for CNP groups 2, 1
and 0, respectively; CNP 0 vs. CNP 1, P<0.001; CNP 1 vs. CNP 2,
P<0.001) (Fig. 3A). In addition,
there were also significant differences in the RFS time among the
three groups (18.8, 32.9 and 40.2 months for CNP groups 2, 1 and 0,
respectively; CNP 0 vs. CNP 1, P=0.012; CNP 1 vs. CNP 2, P=0.004)
(Fig. 3B). Thus, the CNP was able to
clearly classify patients into three independent groups.
Prognostic factors associated with the
OS time of HCC patients undergoing RFA
Univariate and multivariate analyses were next
performed to assess the association between clinical
characteristics and the OS, the results of which are represented in
Table IV. Univariate analysis
demonstrated that the OS was significantly associated with high
HBsAg (P=0.041), presence of liver cirrhosis (P=0.024), large tumor
diameter (P=0.011), high Child-Pugh class (P=0.017), an elevated
NLR (P=0.018), an elevated PLR (P=0.012) and an elevated CNP
(P<0.001).
 | Table IV.Univariate and multivariate analysis
of different factors associated with overall survival in
hepatocellular carcinoma patients treated with radiofrequency
ablation. |
Table IV.
Univariate and multivariate analysis
of different factors associated with overall survival in
hepatocellular carcinoma patients treated with radiofrequency
ablation.
|
|
| Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factors | Univariate
P-value | Risk ratio | 95% CI | P-value |
|---|
| Sex
(male/female) | 0.785 | – | – | – |
| Age (≤55/>55
years) | 0.466 | – | – | – |
| Number of tumors
(1/≥2) | 0.487 | – | – | – |
| HBsAg
(positive/negative) | 0.041 | – | – | – |
| Liver cirrhosis
(presence/absence) | 0.024 | – | – | – |
| Tumor diameter
(≤3/3-5 cm) | 0.011 | 1.543 | 1.032–2.588 | 0.005 |
| Location near
intrahepatic vessels (yes/no) | 0.127 | – | – | – |
| Total bilirubin
(≤17.1/>17.1 µmol/l) | 0.543 | – | – | – |
| Child-Pugh class
(A/B) | 0.017 | 1.693 | 1.078–2.796 | 0.019 |
| NLR
(≤2.58/>2.58) | 0.018 | – | – | – |
| PLR
(≤131.78/131.78) | 0.012 | 1.732 | 1.093–2.956 | 0.024 |
| CNP (0/1/2) | <0.001 | 2.183 | 1.251–3.564 | <0.001 |
Multivariate analysis was performed with the Cox
proportional hazards model using the clinical characteristics
revealed to be significantly associated with the OS (P<0.05) by
univariate analysis (Table IV). The
results indicate that the high Child-Pugh class (P=0.019), large
tumor diameter (P=0.005), an elevated CNP (P<0.001) and PLR
(P<0.001) were independent prognostic factors of OS. In
addition, the results of the present study revealed that CNP (RR,
2.183; P<0.001) is superior to PLR (RR, 1.732; P=0.024) as a
predictive factor in patients with HCC (Table IV).
Prognostic factors associated with the
RFS of patients with HCC undergoing RFA
Univariate and multivariate analyses were performed
to assess the association between clinical characteristics and the
RFS (Table V). Univariate analysis
revealed that the RFS was significantly associated with high number
of tumors (P=0.024), high HBsAg (P=0.046), presence of liver
cirrhosis (P=0.031), location near intrahepatic vessels (P=0.021),
an elevated NLR (P=0.033), an elevated PLR (P=0.028) and an
elevated CNP (P<0.001).
 | Table V.Univariate and multivariate analysis
of prognostic factors associated with recurrence-free survival in
hepatocellular carcinoma patients treated with radiofrequency
ablation. |
Table V.
Univariate and multivariate analysis
of prognostic factors associated with recurrence-free survival in
hepatocellular carcinoma patients treated with radiofrequency
ablation.
|
|
| Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factors | Univariate
P-value | Risk ratio | 95% CI | P-value |
|---|
| Sex
(male/female) | 0.965 | – | – | – |
| Age (≤55/>55
years) | 0.189 | – | – | – |
| Number of tumors
(1/≥2) | 0.024 | 1.821 | 1.194–3.068 | 0.008 |
| HBsAg
(positive/negative) | 0.046 | – | – | – |
| Liver cirrhosis
(presence/absence) | 0.031 | – | – | – |
| Tumor diameter
(≤3/3-5 cm) | 0.078 | – | – | – |
| Location near
intrahepatic vessels (yes/no) | 0.021 | 1.642 | 1.084–2.765 | 0.017 |
| Total bilirubin
(≤17.1/>17.1 µmol/l) | 0.061 | – | – | – |
| Child-Pugh class
(A/)B | 0.685 | – | – | – |
| NLR
(≤2.58/>2.58) | 0.033 | – | – | – |
| PLR
(≤131.78/131.78) | 0.028 | – | – | – |
| CNP (0/1/2) | <0.001 | 1.965 | 1.252–3.207 | <0.001 |
Multivariate analysis was performed with the Cox
proportional hazards model using the characteristics revealed to
have statistical significance (P<0.05) by univariate analysis
(Table V). The results indicated that
high number of tumors, location near intrahepatic vessels and an
elevated CNP were statistically significant independent prognostic
factors of RFS (P=0.008, P=0.017 and P<0.001, respectively).
Discussion
There is a strong association between inflammation
and cancer. SIR is associated with overall or postoperative
survival in patients of several cancer types (12–14,24). The
change in tumor-associated inflammatory cells reflects the degree
of inflammatory response to the tumor, with a higher inflammatory
response often indicating a poor prognosis (25). Due to the convenient and economically
viable nature of blood sampling, neutrophils, platelets and
lymphocytes are common inflammatory markers that form the composite
indices NLR and PLR, and reflect the host inflammatory status.
There is increasing evidence that the NLR or the PLR may be used as
clinical indicators of the host inflammatory response and immune
status, and an elevated NLR or PLR has been revealed to be a strong
predictor of poor survival in certain types of malignancies
(17,26–30).
Previous studies have investigated the prognostic value of
combining the blood routine indices in patients with esophageal
squamous cell carcinoma or colorectal cancer (31,32), These
studies established a novel inflammation-based system, named CNP
(the combination of NLR and PLR) or COP-NLR (the combination of
platelet count and NLR), and found that CNP and COP-NLR were useful
predictors of postoperative survival in cancer patients. The
present study analyzed the potential prognostic value of CNP (the
combination of NLR and PLR) in HCC patients who had received RFA.
To the best of our knowledge, this is the first study to examine
the prognostic value of CNP for predicting the prognosis of
patients with HCC following treatment with RFA. The present study
demonstrated that CNP is associated with tumor progression and
thus, can be regarded as an independent prognostic biomarker of
poor prognosis in patients who have undergone treatment with RFA
for HCC.
A complex tumor microenvironment is one of the most
important factors in a cancer prognosis. Several studies have
confirmed that the interactions between the tumor itself and the
SIR will lead to tumorigenesis (33).
The main features of a tumor-associated inflammatory response are
the infiltration of leukocytes, the production of cytokines, the
remodeling of tissue and angiogenesis (34). Due to the high incidence of hepatitis
B in China, the majority of HCC patients are also infected with
hepatitis B, the persistent inflammation of which affects the
development of HCC (35).
A change of NLR and PLR could be interpreted as a
relative increase in the number of neutrophils and the platelet
count, or a relative decrease in the number of lymphocytes. There
are a number of generally accepted explanations for this imbalance.
Firstly, neutrophils can promote tumor growth and invasion by
releasing vascular endothelial growth factor (VEGF), an important
factor in promoting tumor angiogenesis (36), and there is a significant negative
correlation between tumor angiogenic ability and the prognosis of
the patient (37). Additionally,
inflammatory cells and tumor cells can release a series of
inflammatory mediators, including cell growth factor (CXCL8),
matrix metalloproteinase 8 and the anti-apoptotic factor, nuclear
factor-κB, to promote the growth, invasion and metastasis of the
tumor (38), and to induce the
involvement of more inflammatory cells. The excessive release of
inflammatory mediators leads to oxidative damage, DNA mutation and
an altered tumor microenvironment, which further promote cell
transformation, and tumor cell growth and reproduction (10).
Secondly, clinical and experimental studies have
revealed that malignancy is often associated with thrombocytosis.
Platelets can secrete several types of growth factor, including
platelet-derived growth factor, platelet factor 4, transforming
growth factor-β, thrombospondin-1 and VEGF, which stimulate the
proliferation and differentiation of tumor cells and the
degradation of the extracellular matrix. Furthermore, platelets can
form an adhesion bridge, enabling tumor cells to spread to other
locations, including the capillaries, thereby promoting the growth,
invasion and metastasis of the tumor (39). Additionally, certain pro-inflammatory
cytokines [including interleukin (IL)-1 and IL-6] may promote the
proliferation of megakaryocytes and stimulate the differentiation
of megakaryocytes to platelets in the bone marrow, leading to
further thrombocytosis (40). As
their numbers increase, platelets will release more growth factors
to stimulate the growth and proliferation of the tumor, thereby
aggravating the disease, reducing the survival and the efficacy of
treatment in cancer patients. For this reason, elevated levels of
neutrophils and platelets may lead to a worse prognosis in cancer
patients. The present study not only confirmed that the OS time of
patients with an elevated CNP is significantly shorter than that of
their low-CNP counterparts, but also observed that patients with an
elevated CNP are more likely to develop distant intrahepatic
recurrence and extrahepatic recurrence.
Thirdly, lymphocytes are one of the most important
components of antitumor immunity. A reduced number of lymphocytes
is suggestive of abnormal immune mechanisms and a decline in
antitumor immunity, and also creates an environment that enables
tumor invasion and metastasis (41).
In addition to promoting tumor growth and diffusion, a low number
of lymphocytes results in an increased CNP, which is consistent
with the results of the present study confirming that CNP is
associated with survival times and that a higher CNP was associated
with a worse prognosis.
Under normal conditions, the NLR and the PLR
maintain a relative dynamic balance. The increase in the NLR and
PLR does not indicate the imbalance of any single indicator among
neutrophils, platelets or lymphocytes. NLR and PLR can
comprehensively reflect the tumor inflammation and immune status in
the body, and once this dynamic balance is broken (for example by
the relative increase of neutrophils and platelets, or a relative
reduction in lymphocytes), the balance between the tumor
inflammatory response and antitumor inflammation response will be
destroyed, normal immune function will be impaired, and the
patient's ability to fight the tumor will decline. This indicates
that the host is in a state of antitumor immunosuppression and that
the inflammatory response will develop towards the promotion of
tumor progression, leading to poor patient prognosis.
In the present study, univariate analysis
demonstrated that NLR, PLR and CNP were predictive of OS and RFS
times in HCC patients who had undergone RFA. The Kaplan-Meier
analysis and log-rank tests also demonstrated that CNP was able to
clearly classify patients into three independent groups, results
which assisted in illustrating that patients with a higher NLR and
PLR had a worse prognosis. In addition to CNP, PLR was also
revealed to be an independent prognostic indicator of OS by
multivariate analysis. In addition, CNP is an independent
prognostic indicator of RFS, but no prognostic value was
demonstrated by PLR in the recurrence of HCC.
Furthermore, the present study emphasized the
association between CNP and the pattern of recurrence in HCC
patients following RFA, and CNP was revealed to be associated with
distant intrahepatic recurrence and extrahepatic recurrence. Since
the majority of the Chinese patients with liver cancer have
hepatitis B infections, persistent inflammation has always been
associated with the progression of the disease in HCC patients.
Inflammatory mediators produced by tumor-associated inflammatory
responses, as well as abnormalities in the immune mechanism and a
reduction of antitumor immunity caused by the reduction in the
number of lymphocytes, provide favorable conditions for tumor
invasion and metastasis. This suggests that a higher inflammatory
response always indicates a poor prognosis and that a patient with
an elevated CNP is more likely to develop distant intrahepatic
recurrence and extrahepatic recurrence.
Multivariate analysis also identified other factors,
including the Child-Pugh class and the tumor diameter, as
independent predictive factors for OS, and the number of tumors as
an independent predictor of tumor recurrence, which was consistent
with the results of previous studies (42–44). In
particular, location near the intrahepatic vessels was revealed to
be an imperative risk factor for HCC recurrence following RFA
therapy. It has been demonstrated that blood flow promotes heat
loss, which may account for the reduced effectiveness of RFA
(45).
The present study is a retrospective analysis of a
small patient population with a certain degree of heterogeneity.
Therefore, future studies require larger sample sizes to further
validate the prognostic capability of CNP in HCC patients who have
undergone RFA treatment.
In conclusion, as a simple, readily available
indicator, CNP has the potential to serve as a novel non-invasive
circulating marker for monitoring HCC progression. Additionally,
CNP can also be considered an effective biomarker for tracking
tumor recurrence and predicting the prognosis of HCC patients
following RFA therapy. Therefore, CNP not only appears capable of
classifying HCC patients undergoing RFA into three independent
groups, but also has potential as a novel independent unfavorable
predictor of post-operative survival in such patients.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant no. 81571785) and by the
Guangzhou Science and Technology Department, Industry Technology
Research and Development Projects (grant no. 201400000001-3).
References
|
1
|
Omata M, Lesmana LA, Tateishi R, Chen PJ,
Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT, et al: Asian
Pacific Association for the Study of the Liver consensus
recommendations on hepatocellular carcinoma. Hepatol Int.
4:439–474. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Borie F, Bouvier AM, Herrero A, Faivre J,
Launoy G, Delafosse P, Velten M, Buemi A, Peng J, Grosclaude P and
Trétarre B: Treatment and prognosis of hepatocellular carcinoma: A
population based study in France. J Surg Oncol. 98:505–509. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Poon RT, Fan ST, Tsang FH and Wong J:
Locoregional therapies for hepatocellular carcinoma: A critical
review from the surgeon's perspective. Ann Surg. 235:466–486. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Curley SA, Izzo F, Ellis LM, Vauthey J
Nicolas and Vallone P: Radiofrequency ablation of hepatocellular
cancer in 110 patients with cirrhosis. Ann Surg. 232:381–391. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Poon RT, Ng KK, Lam CM, Ai V, Yuen J and
Fan ST: Radiofrequency ablation for subcapsular hepatocellular
carcinoma. Ann Surg Oncol. 11:281–289. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lü MD, Kuang M, Liang LJ, Xie XY, Peng BG,
Liu GJ, Li DM, Lai JM and Li SQ: Surgical resection versus
percutaneous thermal ablation for early-stage hepatocellular
carcinoma: A randomized clinical trial. Zhonghua Yi Xue Za Zhi.
86:801–805. 2006.(In Chinese). PubMed/NCBI
|
|
7
|
Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH,
Zhang YQ, Lin XJ and Lau WY: A prospective randomized trial
comparing percutaneous local ablative therapy and partial
hepatectomy for small hepatocellular carcinoma. Ann Surg.
243:321–328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Livraghi T, Meloni F, Di Stasi M, Rolle E,
Solbiati L, Tinelli C and Rossi S: Sustained complete response and
complications rates after radiofrequency ablation of very early
hepatocellular carcinoma in cirrhosis: Is resection still the
treatment of choice? Hepatology. 47:82–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kao WY, Chiou YY, Hung HH, Chou YH, Su CW,
Wu JC, Huo TI, Huang YH, Lin HC and Lee SD: Risk factors for
long-term prognosis in hepatocellular carcinoma after
radiofrequency ablation therapy: The clinical implication of
aspartate aminotransferase-platelet ratio index. Eur J
Gastroenterol Hepatol. 23:528–536. 2011.PubMed/NCBI
|
|
10
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Forrest LM, McMillan DC, McArdle CS,
Angerson WJ and Dunlop DJ: Comparison of an inflammation-based
prognostic score (GPS) with performance status (ECOG) in patients
receiving platinum-based chemotherapy for inoperable non-small-cell
lung cancer. Br J Cancer. 90:1704–1706. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramsey S, Lamb GW, Aitchison M, Graham J
and McMillan DC: Evaluation of an inflammation-based prognostic
score in patients with metastatic renal cancer. Cancer.
109:205–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishizuka M, Nagata H, Takagi K, Horie T
and Kubota K: Inflammation-based prognostic score is a novel
predictor of postoperative outcome in patients with colorectal
cancer. Ann Surg. 246:1047–1051. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang ZL, Luo J, Chen MS, Li JQ and Shi M:
Blood neutrophil-to-lymphocyte ratio predicts survival in patients
with unresectable hepatocellular carcinoma undergoing transarterial
chemoembolization. J Vasc Interv Radiol. 22:702–709. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Halazun KJ, Hardy MA, Rana AA, DC IV
Woodland, Luyten EJ, Mahadev S, Witkowski P, Siegel AB, Brown RS Jr
and Emond JC: Negative impact of neutrophil-lymphocyte ratio on
outcome after liver transplantation for hepatocellular carcinoma.
Ann Surg. 250:141–151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gomez D, Farid S, Malik HZ, Young AL,
Toogood GJ, Lodge JP and Prasad KR: Preoperative
neutrophil-to-lymphocyte ratio as a prognostic predictor after
curative resection for hepatocellular carcinoma. World J Surg.
32:1757–1762. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bertuzzo VR, Cescon M, Ravaioli M, Grazi
GL, Ercolani G, Del Gaudio M, Cucchetti A, D'Errico-Grigioni A,
Golfieri R and Pinna AD: Analysis of factors affecting recurrence
of hepatocellular carcinoma after liver transplantation with a
special focus on inflammation markers. Transplantation.
91:1279–1285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kinoshita A, Onoda H, Imai N, Iwaku A,
Oishi M, Fushiya N, Koike K, Nishino H and Tajiri H: Comparison of
the prognostic value of inflammation-based prognostic scores in
patients with hepatocellular carcinoma. Br J Cancer. 107:988–993.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pugh RN, Murray-Lyon IM, Dawson JL,
Pietroni MC and Williams R: Transection of the oesophagus for
bleeding oesophageal varices. Br J Surg. 60:646–649. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McMillan DC, Canna K and McArdle CS:
Systemic inflammatory response predicts survival following curative
resection of colorectal cancer. Br J Surg. 90:215–219. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jeong JH, Lim SM, Yun JY, Rhee GW, Lim JY,
Cho JY and Kim YR: Comparison of two inflammation-based prognostic
scores in patients with unresectable advanced gastric cancer.
Oncology. 83:292–299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sarraf KM, Belcher E, Raevsky E, Nicholson
AG, Goldstraw P and Lim E: Neutrophil/lymphocyte ratio and its
association with survival after complete resection in non-small
cell lung cancer. J Thorac Cardiovasc Surg. 137:425–428. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sharaiha RZ, Halazun KJ, Mirza F, Port JL,
Lee PC, Neugut AI, Altorki NK and Abrams JA: Elevated preoperative
neutrophil: Lymphocyte ratio as a predictor of postoperative
disease recurrence in esophageal cancer. Ann Surg Oncol.
18:3362–3369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang D, Yang JX, Cao DY, Wan XR, Feng FZ,
Huang HF, Shen K and Xiang Y: Preoperative neutrophil-lymphocyte
and platelet-lymphocyte ratios as independent predictors of
cervical stromal involvement in surgically treated endometrioid
adenocarcinoma. Onco Targets Ther. 6:211–216. 2013.PubMed/NCBI
|
|
29
|
Jiang N, Deng JY, Liu Y, Ke B, Liu HG and
Liang H: The role of preoperative neutrophil-lymphocyte and
platelet-lymphocyte ratio in patients after radical resection for
gastric cancer. Biomarkers. 19:444–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kwon HC, Kim SH, Oh SY, Lee S and Lee JH,
Choi HJ, Park KJ, Roh MS, Kim SG, Kim HJ and Lee JH: Clinical
significance of preoperative neutrophil-lymphocyte versus
platelet-lymphocyte ratio in patients with operable colorectal
cancer. Biomarkers. 17:216–222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feng JF, Huang Y and Liu JS: Combination
of neutrophil lymphocyte ratio and platelet lymphocyte ratio is a
useful predictor of postoperative survival in patients with
esophageal squamous cell carcinoma. Onco Targets Ther. 6:1605–1612.
2013.PubMed/NCBI
|
|
32
|
Ishizuka M, Nagata H, Takagi K, Iwasaki Y
and Kubota K: Combination of platelet count and neutrophil to
lymphocyte ratio is a useful predictor of postoperative survival in
patients with colorectal cancer. Br J Cancer. 109:401–407. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
DeNardo DG, Johansson M and Coussens LM:
Immune cells as mediators of solid tumor metastasis. Cancer
Metastasis Rev. 27:11–18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Colotta F, Allavena P, Sica A, Garlanda C
and Mantovani A: Cancer-related inflammation, the seventh hallmark
of cancer: Links to genetic instability. Carcinogenesis.
30:1073–1081. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Alison MR, Nicholson LJ and Lin WR:
Chronic inflammation and hepatocellular carcinoma. Recent Results
Cancer Res. 185:135–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kusumanto YH, Dam WA, Hospers GA, Meijer C
and Mulder NH: Platelets and granulocytes, in particular the
neutrophils, form important compartments for circulating vascular
endothelial growth factor. Angiogenesis. 6:283–287. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu
Z, Yin XY and Zheng L: Peritumoral neutrophils link inflammatory
response to disease progression by fostering angiogenesis in
hepatocellular carcinoma. J Hepatol. 54:948–955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Paramanathan A, Saxena A and Morris DL: A
systematic review and meta-analysis on the impact of pre-operative
neutrophil lymphocyte ratio on long term outcomes after curative
intent resection of solid tumours. Surg Oncol. 23:31–39. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Egan K, Crowley D, Smyth P, O'Toole S,
Spillane C, Martin C, Gallagher M, Canney A, Norris L, Conlon N, et
al: Platelet adhesion and degranulation induce pro-survival and
pro-angiogenic signalling in ovarian cancer cells. PLoS One.
6:e261252011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Stone RL, Nick AM, McNeish IA, Balkwill F,
Han HD, Bottsford-Miller J, Rupairmoole R, Armaiz-Pena GN, Pecot
CV, Coward J, et al: Paraneoplastic thrombocytosis in ovarian
cancer. N Engl J Med. 366:610–618. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kitayama J, Yasuda K, Kawai K, Sunami E
and Nagawa H: Circulating lymphocyte number has a positive
association with tumor response in neoadjuvant chemoradiotherapy
for advanced rectal cancer. Radiat Oncol. 5:472010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lam VW, Ng KK, Chok KS, Cheung TT, Yuen J,
Tung H, Tso WK, Fan ST and Poon RT: Risk factors and prognostic
factors of local recurrence after radiofrequency ablation of
hepatocellular carcinoma. J Am Coll Surg. 207:20–29. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang B, Zou J, Xia J, Ren Z, Gan Y, Wang
Y, Zhang B, Ge N, Wang D, Chen Y, et al: Risk factors for
recurrence of small hepatocellular carcinoma after long-term
follow-up of percutaneous radiofrequency ablation. Eur J Radiol.
79:196–200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim YS, Rhim H, Cho OK, Koh BH and Kim Y:
Intrahepatic recurrence after percutaneous radiofrequency ablation
of hepatocellular carcinoma: Analysis of the pattern and risk
factors. Eur J Radiol. 59:432–441. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lu DS, Raman SS, Limanond P, Aziz D,
Economou J, Busuttil R and Sayre J: Influence of large peritumoral
vessels on outcome of radiofrequency ablation of liver tumors. J
Vasc Interv Radiol. 14:1267–1274. 2003. View Article : Google Scholar : PubMed/NCBI
|