Introduction
MicroRNAs (miRNAs/miRs) typically contain 20–24
nucleotides and do not code for proteins, but instead modulate the
expression of specific target genes. miRNAs bind to 3′-untranslated
regions of target mRNA molecules to repress translation or enhance
degradation (1). As each miRNA has
multiple targets, a single microRNA may produce diverse effects by
functioning as a tumor initiator or suppressor (2). miRNAs may regulate various critical
signaling networks to modulate carcinogenesis (3–6). The
differential expression of miR-26b has been demonstrated in several
types of tumor, including lung (7),
cervical (8), tongue (9) and breast cancer (10), suggesting that it has specific roles
in tumor pathology. Previous studies conducted with clinical
samples of breast tumor tissue have indicated that miR-26b may
inhibit tumor formation, and that its overexpression induces
apoptosis and inhibits proliferation (10–12).
DNA damage-regulated autophagy modulator 1 (DRAM1)
is a lysosomal protein upregulated by p53 that induces the
macro-autophagy activity associated with cell death; DRAM is
critical for the induction of programmed cell death, contributing
to the tumor-suppressive effects of p53 (13–15).
Despite the previous studies on miR-26b, the
functions of miR-26b and its target genes remain to be fully
characterized, including in human breast cancer. In the present
study, the function of miR-26b in breast cancer was determined
using the human breast cancer cell line MCF7. The results of the
present study demonstrated low-expression of miR-26b in breast
cancer tumor tissue. In addition, it was identified that miR-26b
downregulated DRAM1 by targeting the 3′-UTR directly. Inhibiting
miR-26b upregulated DRAM1 and increased irradiation-induced
autophagy. Therefore, the results of the present studymay providea
novel insight for targeting therapy of cancer.
Materials and methods
Tissue samples
Three paired samples of breast cancer tissue and
adjacent noncancerous tissue were provided by the Breast and
Thyroid Surgery Department of the China-Japan Union Hospital
(Changchun, China) between June 2013 and December 2013, with a mean
age of 41 years (range, 37–49 years). All samples of breast cancer
tissue had been pathologically confirmed as invasive ductal
carcinoma. None of the patients had been treated with chemotherapy
or radiation therapy prior to surgery. The study was approved by
the China-Japan Union Hospital of Jilin University and informed,
written consent was provided by all patients.
Cell culture and transfection
Human MCF7 breast cancer cells were purchased from
the Institute of Basic Medical Sciences, Chinese Academy of Medical
Sciences (Beijing, China) and cultured at 37°C in Dulbecco's
Modified Eagle's Medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) containing fetal bovine serum (10%;
HyClone; GE Healthcare, Logan, UT, USA), penicillin (100 units/ml),
and streptomycin (100 units/ml), in a humidity-controlled incubator
with an atmosphere containing 5% CO2. Following culture,
the cells were transfected with 100 nmol of an miR-26b mimic or
inhibitor, or a negative control miRNA (NC; GenePharma, Shanghai,
China) using Lipofectamine™ 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol; they
were then exposed to ionizing radiation (IR) as subsequently
described. The NC was designed not to target any human gene. The
sequence for the miR-26b mimic was sense,
5′-UCAAGUAAUUCAGGAUAGGU-3′, antisense, 5′-CUAUCCUGAAUUACUUGAAUU-3′;
the sequence for the miR-26b inhibitor was sense,
5′-ACCUAUCCUGAAUUACUUGAA-3′, antisense,
5′-CAGUACUUUUGUGUAGUACAA-3′; the sequence for the NC was sense,
5′-UUCUCCGAACGUGUCACGUTT-3′, antisense,
5′-ACGUGACACGUUCGGAGAATT-3′. Specific siRNAs for human DRAM
(catalog no. sc-96209) and control siRNA (catalog no. sc-44230)
were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA).
Irradiation
Cells were given for IR 24 h after transfection and
harvested by centrifugation at 13,201 × g for 3 min at room
temperature for analysis. IR was delivered at a rate of 0.38 Gy/min
(180 kV; 18 mA) using an XSZ-Z20/20 X-ray generator (Kang Jia
Instrument Equipment Co., Ltd., Dandong, China).
Detection of miR-26b by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
At 48 h after transfection, total cellular RNA was
extracted using the TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). The RNA samples were then frozen at −80°C until
use in further analyses. The expression of miRNA was detected
usinga Hairpin-it™ miRNAs RT-PCR Quantization kit, containing
miR-26b primers and U6 primers (E22002; GenePharma) and an MX3000p
thermocycler (Stratagene; Agilent Technologies, Inc., Santa Clara,
CA, USA). Reverse transcription was performed with a 20 ng sample
of RNA, which was incubated for 60 min at 42°C followed by 5 min at
95°C. Following synthesis, the cDNA template was diluted 80-fold
with nuclease-free H2O. Then qPCR was performed as
follows: Denaturation at 95°C for 10 min, followed by 40
amplification cycles of 95°C for 10 sec and 60°C for 1 min
(ramp-rate, 1.6°C/sec). All samples were normalized to the U6
expression level using the 2−ΔΔCq method (16).
Plasmid construction
DRAM1 was identified as a potential target of
miR-26b with TargetScan (http://www.targetscan.org/), which was used to
identify autophagy-associated genes. The wild-type (WT)
3′-untranslated region (3′UTR) of DRAM1, including the putative
miR-26b-binding site, was extracted by performing PCR with genomic
DNA obtained from the extracted blood of a healthy human donor. DNA
was extracted using Rapid DNA extraction and detection kit (Tiangen
Biotech Co., Ltd., Beijing, China). The amplified product was then
cloned into the HindIII and XhoI sites of the pMIR
vector (Promega Corporation, Madison, WI, USA). PCR was also
performed to generate a DRAM1 3′UTR with a mutated miR-26b binding
site. The primer sequences were WT forwards,
5′-GTCAAGCTTCTGCAGCACATCCAGGACTTGAATTTCATTACGAGTTCCT-3′, WT
reverse, 5′-CCACTCGAGACCAGGCGATACAGACTATT-3′; mutant forwards,
5′-GTCAAGCTTCTGCAGCACATCCAGGACAACAATTTCATTACGAGTTCCT-3′, mutant
reverse, 5′-CCACTCGAGACCAGGCGATACAGACTATT-3′. PCR was performed as
follows: Denaturation at 95°C for 5 min, 40 amplification cycles of
95°C for 10 sec, 55°C for 15 sec and 72°C for 90 sec, followed by
72°C for 5 min (ramp-rate, 1.6°C/sec). PrimeSTAR® Max
DNA Polymerase (R045Q; Takara Biotechnology Co., Ltd., Dalian,
China) was used for PCR amplification. DNA sequencing was used to
verify the identities of the PCR products incorporated into the
plasmids.
Luciferase reporter assays
MCF7 cells were cultured in 96-well plates
(5×104 cells/well). Lipofectamine® 2000 was
used to transfect the cells with 200 ng of pMIR-DRAM1 3′UTR and 5
ng of the pRL-SV40 vector containing the Renilla luciferase
gene used to normalize transfection efficiency (Promega
Corporation), in addition to 100 nmol of the miR-26b mimic or NC.
At 48 h after transfection, the Dual-Luciferase Reporter assay
(Promega Corporation) was used to examine firefly and
Renilla luciferase activities. Each transfection was
repeated 5 times in three independent experiments.
Western blot analysis
MCF7 cells were irradiated for 24 h (a time selected
based on a time-course curve) and harvested; 1 ml lysis buffer (50
mM NaCl, 10 mM HEPES, 1 mM EDTA, 1 mM DTT, 1% NP-40, 0.1% SDS and 1
mM PMSF) and 10 µl protease inhibitor cocktail (P8340;
Sigma-Aldrich; Merck KGaA) was added to the cell pellets.
Subsequently, protein was quantified using BCA Protein Assay kit
(P0012S; Beyotime Institute of Biotechnology, Haimen, China).
SDS-PAGE was used to separate 60 µg of total cellular protein
extract; the separated bands were transferred onto a nitrocellulose
membrane for western blotting. Membranes were blocked with 5%
skimmed milk in Tris-Buffered Saline Tween-20 for 1 h at room
temperature. Subsequently, horseradish peroxidase-conjugated
secondary antibodies [HRP-Goat anti-Rabbit IgG (cat no.
bs-0295G-HRP) and HRP-Goat anti-Mouse IgG (cat no. bs-0296G-HRP;
both 1:2,000); Boster Biological Technology, Pleasanton, CA, USA]
was added for 1 h at room temperature. Primasry antibodies were
used against DRAM1 (1:300; ab72171; Abcam; Cambridge, UK), light
chain 3 (LC3) I and II (1:300; 4108S; Cell Signaling Technology;
Danvers, MA, USA), and GAPDH (1:1,000; sc-32233; Santa Cruz
Biotechnology, Inc.). Immunoreactive bands were detected by
chemiluminescence (sc-2048; Santa Cruz Biotechnology, Inc.). The
staining intensities of individual proteins were measured using
ImageJ software (version 1.41; National Institutes of Health,
Bethesda, MD, USA), and expressed relativeto the intensity of the
GAPDH band.
Monodansylcadaverine (MDC) staining
assay
MCF7 cells (4×105 cells/well) were seeded
in 60-mm cell culture dishes and let sit overnight at room
temperature. Each group of cells received the specified dose of IR.
After 24 h, they were washed twice in cold PBS solution. The cells
were incubated in DMEM containing 50 mM MDC for 1 h at 37°C to
label the autophagic vacuoles; they were then washed with PBS and
fixed by a 15 min immersion in 4% paraformaldehyde solution at room
temperature. The cells were analyzed for fluorescence with a
FACSort Flow Cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Data were analyzed using Cell Quest software (version 5.1; BD
Biosciences) and FlowJo software (version 7.6.5; Tree Star Inc.,
Ashland, OR, USA).
Statistical analysis
The Student's t test and the χ2 test were
used to analyze experimental data, and data are presented as the
mean ± standard deviation using SPSS software (version 12.0; SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-26b expression is reduced in
breast cancer tissue and irradiated MCF7 cells
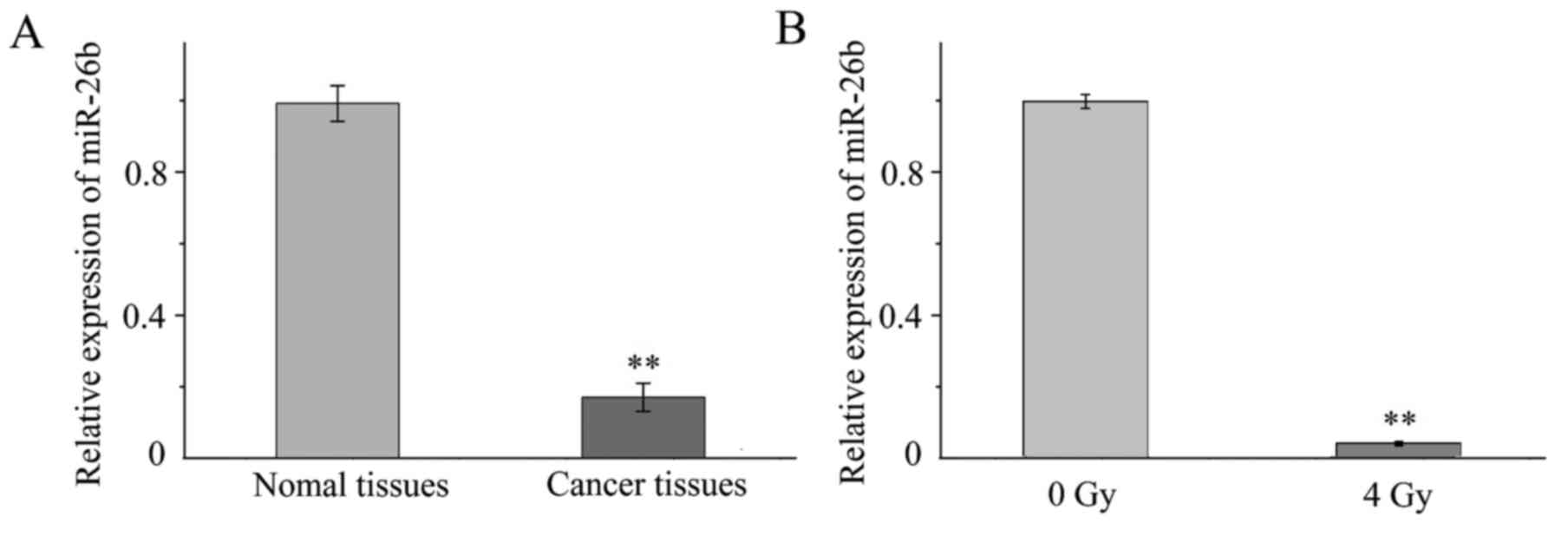
RT-qPCR was performed to investigate the expression
of miR-26b in tissue samples and MCF7 cells. It was demonstrated
that the expression of miR-26b was significantly lower in the three
samples of breast cancer tissue when compared with expression in
the noncancerous adjacent tissue (P<0.001; Fig. 1A). In addition, miR-26b expression was
significantly downregulated in MCF7 cells exposed to 4-Gy IR
compared with MCF7 cells that were not irradiated (P<0.01;
Fig. 1B).
miR-26b may regulate IR-induced
autophagy in MCF7 cells
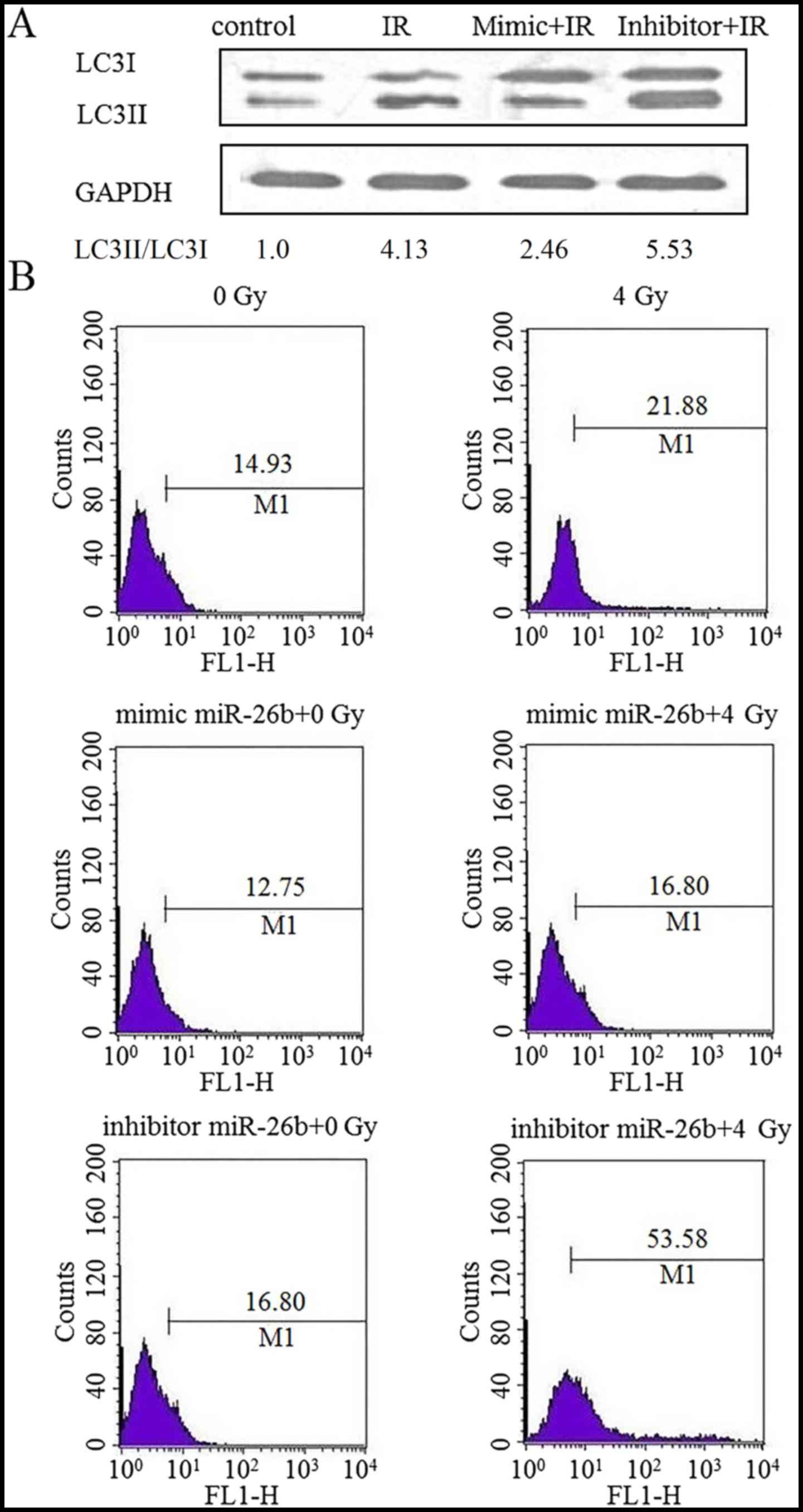
The effect of miR-26b on autophagy in MCF7 cells was
examined by performing a western blot analysis for LC3II protein
expression. Cells exposed to IR exhibited an increased level of
LC3II protein expression, which was suppressed following
transfection with an miR-26b mimic. Furthermore, LC3II protein was
overexpressed in cells treated with the miR-26b inhibitor
(P<0.01; Fig. 2A). MDC staining
demonstrated that exposure to IR increased the rate of autophagy by
21.88% in the control cells, by 16.80% in the miR-26b mimic-treated
cells and by 53.58% in cells treated with the miR-26b inhibitor,
suggesting that miR-26b can inhibit IR-induced autophagy in MCF7
cells (P<0.001; Fig. 2B).
DRAM1 expression in MCF7 cells is
suppressed by the overexpression of miR-26b
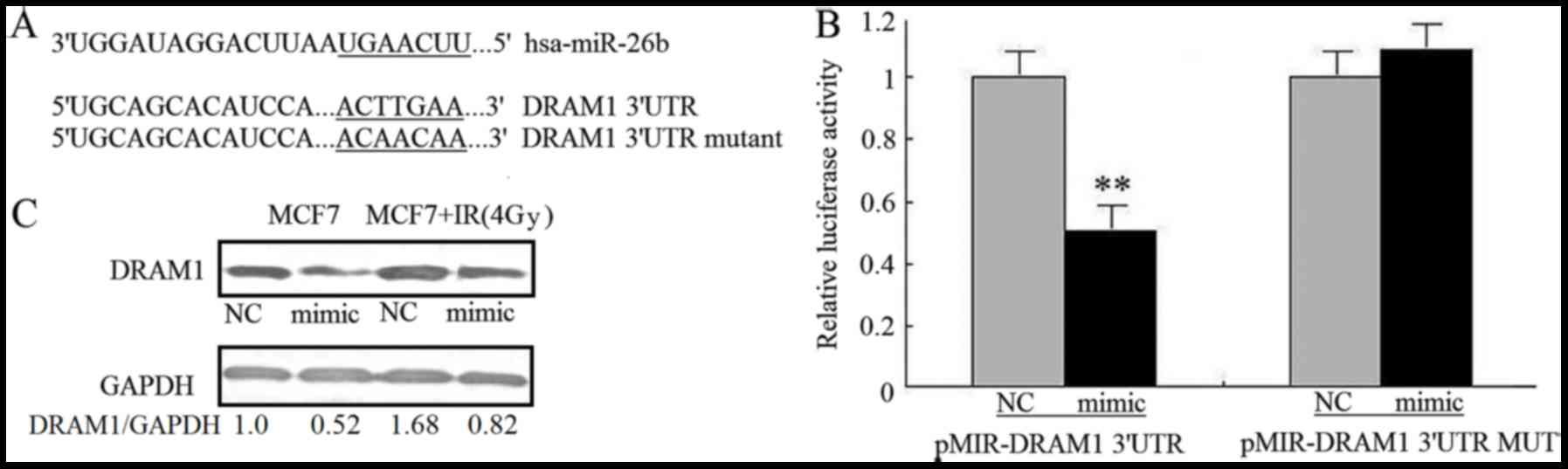
The mechanism by which miR-26b inhibits autophagy
was investigated by using TargetScanto search for
autophagy-associated potential target genes. The DRAM1 3′UTR
contained a nucleotide sequence that matched the seed sequence of
miR-26b (Fig. 3A). To obtain
experimental evidence that miR-26b targets DRAM1, the 3′-UTR of
DRAM1, including the potential miR-26b-binding sequence, was cloned
into a firefly luciferase reporter vector; an miR-26b mimic was
used to examine how miR-26b affected luciferase activity.
Noluciferase activity inhibition was observed in MCF7 cells
transfected with mutant 3′UTR vectors, where as the miR-26b mimic
significantly inhibited the luciferase activity of cells
transfected with WT DRAM1 3′UTR (P<0.01). This result
demonstrated the specificity of miR-26b for targeting the DRAM1
3′UTR (Fig. 3B). It was also
investigated how miR-26b affected the levels of endogenous DRAM1
protein in MCF7 cells. Transfection with the miR-26b mimic was
associated with a reduction in the amount of DRAM1 protein in MCF7
cells compared with the NC. MCF7 cells exposed to IR exhibited
upregulated DRAM1 protein expression; miR-26b overexpression
attenuated the stimulatory effect of IR on DRAM1 expression
(P<0.01; Fig. 3C). This result
suggests that miR-26b may inhibit IR-induced autophagy in MCF7
cells by directly targeting DRAM1.
Loss of DRAM1 inhibited IR-induced
autophagy
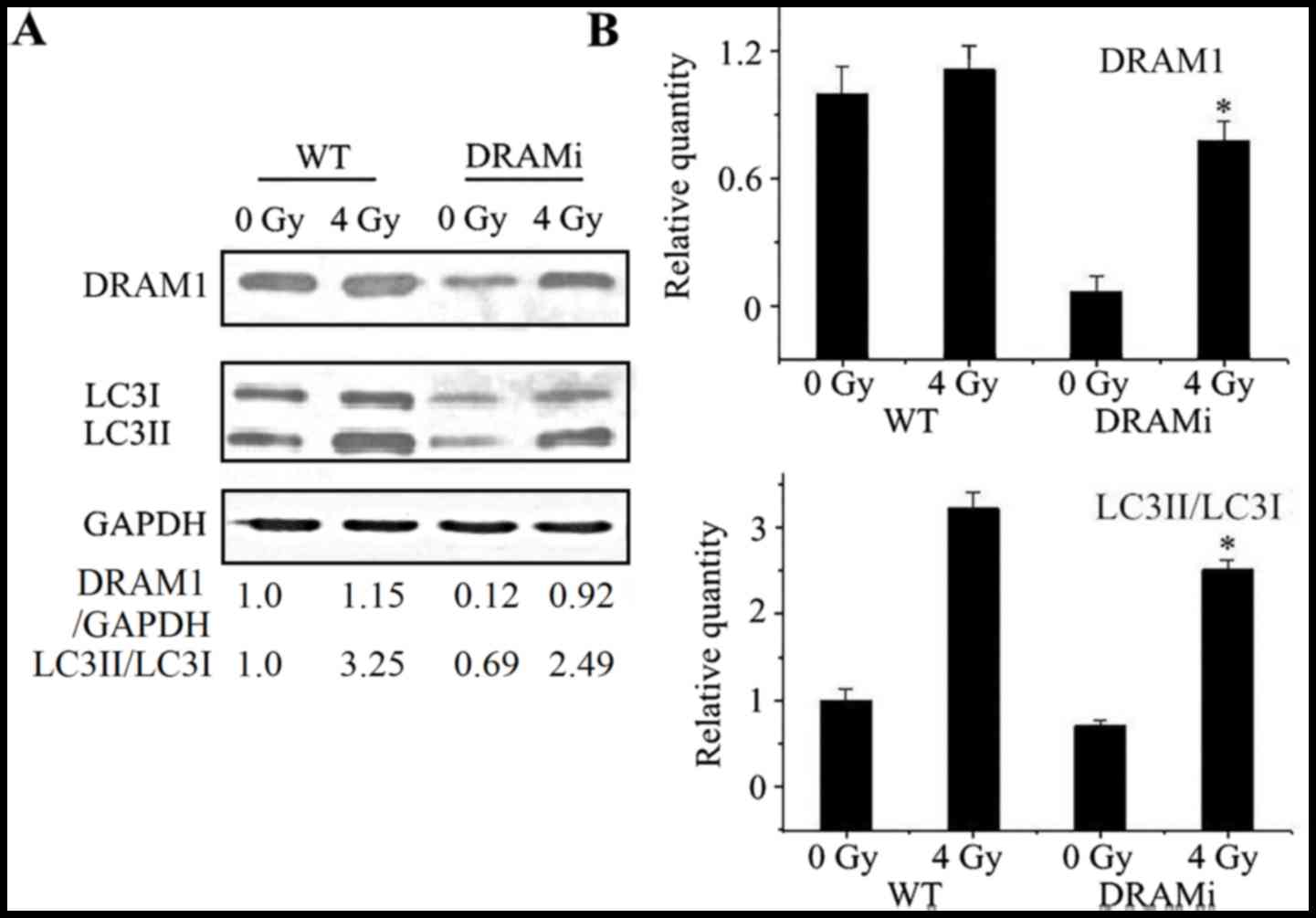
Autophagy was then analyzed in MCF7 cells that were
transfected with siRNA against DRAM1. Compared with negative
controls, the LC3-II to LC3-I ratio in cells transfected with siRNA
against DRAM1 increased following irradiation, whereasthe ratio in
MCF7 cells transfected with DRAM1 remained unchanged. Following a
4-Gy IR exposure, the LC3-II to LC3-I ratio in cells transfected
with DRAM1 was significantly lower than the control cells
(P<0.05; Fig. 4A and B),
suggesting that DRAM1 may inhibit IR-induced autophagy.
Discussion
miRNAs are differentially expressed in normal tissue
compared with cancer tissue; there may be a critical role for
dysregulated miRNA expression in tumorigenesis (17–20).
However, the mechanisms by which miRNAs regulate autophagy are only
starting to be explored. A number of miRNAs have been demonstrated
to modulate autophagy in human cancer cells by targeting
autophagy-associated genes (21–23). These
studies have enriched the understanding of the autophagy signaling
process and provided novel therapeutic perspectives. A range of
previous studies have investigated the role that microRNAs serve in
the cellular response to radiation therapy by examining the process
of autophagy (16,24–29).
The downregulation of miR-26b expression in breast
cancer tissue compared with noncancerous tissue was previously
identified, as well as in breast cancer cells compared with normal
breast cells (10). In the present
study, upregulated miR-26b expression levels were associated with a
reduction in the levels of IR-induced autophagy in MCF7 cells.
Additionally, DRAM1 mRNA was identifiedas a potential target for
miR-26b. Previous studies have demonstrated that the DRAM1 gene
codes for a number of splice variant proteins that are induced by
p53, and that these proteins localize to autophagosomes and
peroxisomes, where they may be required for the process of
p53-induced autophagy (30,31). The luciferase and western blot assays
of the present study identified DRAM1 as a target of miR-26b. MCF7
cells transfected with an miR-26b mimic exhibited the reduced
expression of DRAM1, which may be due to the 3′UTR of the DRAM1
mRNA being targeted. Additionally, transfection with the miR-26b
mimic suppressed the expression of DRAM1 protein in MCF7 cells,
regardless of whether they had been exposed to IR. When taken
together, the data suggest that miR-26b, at least partially,
inhibits IR-induced autophagy in MCF7 cancer cells by inhibiting
DRAM1. Overall, the results of the present study may improve the
understanding of the various mechanisms by which miRNAs influence
cells
It was also demonstrated that IR downregulated
miR-26b expression in MCF7 cells. MCF7 cells transfected with an
miR-26b inhibitor exhibited reduced miR-26b expression prior to IR
and enhanced miR-21b expression following IR; however, the
underlying mechanism for this response remains unclear. Previous
studies have demonstrated that miRNA modulation may allow an
improvement of the clinical effect of radio- or chemotherapy
(32–34). However, the mechanisms by which miRNAs
produce their effects are not fully understood. Additional studies
are required to identify the miRNAs that modulate the effects of
chemo- or radiotherapy, as well as their effect on cellular
metabolic processes. It will also be necessary to confirm that
patients can be safely and effectively treated by a clinical
regimen that targets miRNAs.
In summary, it was demonstrated that miR-26b may
regulate the basal level of autophagy in MCF7 cells, as well as
autophagy resulting from IR exposure. DRAM1 was identified as a
target gene through which miR-26b may modulate autophagy. The
present study may provide evidence of a specific role for miR-26b
in carcinogenesis and cancer therapy effectiveness. This result may
enhancethe understanding of the miRNA-modulated networks associated
with autophagy, ultimately facilitating improvement of the methods
for treating cancer.
Acknowledgements
The present study was supported by NSFC grants
(grant nos. 31500682 and 81570897).
References
|
1
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen CZ: MicroRNAs as oncogenes and tumor
suppressors. N Engl J Med. 353:1768–1771. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gottardo F, Liu CG, Ferracin M, Calin GA,
Fassan M, Bassi P, Sevignani C, Byrne D, Negrini M, Pagano F, et
al: Micro-RNAs profiling in kidney and bladder cancers. Urol Oncol.
25:387–392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hsu CM, Lin PM, Wang YM, Chen ZJ, Lin SF
and Yang MY: Circulating miRNA is a novel marker for head and neck
squamous cell carcinoma. Tumour Biol. 33:1933–1942. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ji J, Shi J, Budhu A, Yu Z, Forgues M,
Roessler S, Ambs S, Chen Y, Meltzer PS, Croce CM, et al: MicroRNA
expression, survival, and response to interferon in liver cancer. N
Engl J Med. 361:1437–1447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kozubek J, Ma Z, Fleming E, Duggan T, Wu
R, Shin DG and Dadras SS: In-depth characterization of microRNA
transcriptome in melanoma. PLoS One. 8:e726992013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang LP, Zhu ZT and He CY: Expression of
miRNA-26b in the diagnosis and prognosis of patients with
non-small-cell lung cancer. Future Oncol. 12:1105–1115. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo M, Shen D, Wang W and Xian J: Aberrant
expression of microRNA-26b and its prognostic potential in human
cervical cancer. Int J Clin Exp Pathol. 8:5542–5548.
2015.PubMed/NCBI
|
|
9
|
Cao J, Guo T, Dong Q, Zhang J and Li Y:
miR-26b is downregulated in human tongue squamous cell carcinoma
and regulates cell proliferation and metastasis through a
COX-2-dependent mechanism. Oncol Rep. 33:974–980. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, Kong X, Zhang J, Luo Q, Li X and
Fang L: Correction: MiRNA-26b inhibits proliferation by targeting
PTGS2 in breast cancer. Cancer Cell Int. 13:172013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX,
Cao DX, He M, Chen GQ, He JR and Zhao Q: MicroRNA-26b is
underexpressed in human breast cancer and induces cell apoptosis by
targeting SLC7A11. FEBS Lett. 585:1363–1367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao L, Sun Y, Hou Y, Peng Q, Wang L, Luo
H, Tang X, Zeng Z and Liu M: MiRNA expression analysis of
cancer-associated fibroblasts and normal fibroblasts in breast
cancer. Int J Biochem Cell Biol. 44:2051–2059. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Crighton D, Wilkinson S, O'Prey J, Syed N,
Smith P, Harrison PR, Gasco M, Garrone O, Crook T and Ryan KM:
DRAM, a p53-induced modulator of autophagy, is critical for
apoptosis. Cell. 126:121–134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Crighton D, Wilkinson S and Ryan KM: DRAM
links autophagy to p53 and programmed cell death. Autophagy.
3:72–74. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Criollo A, Dessen P and Kroemer G: DRAM: A
phylogenetically ancient regulator of autophagy. Cell Cycle.
8:2319–2320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yi H, Liang B, Jia J, Liang N, Xu H, Ju G,
Ma S and Liu X: Differential roles of miR-199a-5p in
radiation-induced autophagy in breast cancer cells. FEBS Lett.
587:436–443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rutnam ZJ and Yang BB: The involvement of
microRNAs in malignant transformation. Histol Histopathol.
27:1263–1270. 2012.PubMed/NCBI
|
|
18
|
Banno K, Yanokura M, Kisu I, Yamagami W,
Susumu N and Aoki D: MicroRNAs in endometrial cancer. Int J Clin
Oncol. 18:186–192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mayne GC, Hussey DJ and Watson DI:
MicroRNAs and esophageal cancer-implications for pathogenesis and
therapy. Curr Pharm Des. 19:1211–1226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iorio MV and Croce CM: microRNA
involvement in human cancer. Carcinogenesis. 33:1126–1133. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Frankel LB and Lund AH: MicroRNA
regulation of autophagy. Carcinogenesis. 33:2018–2025. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang P, Zhang L, Chen Z and Meng Z:
MicroRNA targets autophagy in pancreatic cancer cells during cancer
therapy. Autophagy. 9:2171–2172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu LL, Wen X, Bao JK and Liu B:
MicroRNA-modulated autophagicsignaling networks in cancer. Int J
Biochem Cell Biol. 44:733–736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Comincini S, Allavena G, Palumbo S, Morini
M, Durando F, Angeletti F, Pirtoli L and Miracco C: microRNA-17
regulates the expression of ATG7 and modulates the autophagy
process, improving the sensitivity to temozolomide and low-dose
ionizing radiation treatments in human glioblastoma cells. Cancer
Biol Ther. 14:574–586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dickey JS, Zemp FJ, Martin OA and
Kovalchuk O: The role of miRNA in the direct and indirect effects
of ionizing radiation. Radiat Environ Biophys. 50:491–499. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gwak HS, Kim TH, Jo GH, Kim YJ, Kwak HJ,
Kim JH, Yin J, Yoo H, Lee SH and Park JB: Silencing of microRNA-21
confers radio-sensitivity through inhibition of the PI3K/AKT
pathway and enhancing autophagy in malignant glioma cell lines.
PLoS One. 7:e474492012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Metheetrairut C and Slack FJ: MicroRNAs in
the ionizing radiation response and in radiotherapy. Curr Opin
Genet Devel. 23:12–19. 2013. View Article : Google Scholar
|
|
28
|
Qased AB, Yi H, Liang N, Ma S, Qiao S and
Liu X: MicroRNA-18a upregulates autophagy and ataxia telangiectasia
mutated gene expression in HCT116 colon cancer cells. Mol Med Rep.
7:559–564. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang P, Zhang J, Zhang L, Zhu Z, Fan J,
Chen L, Zhuang L, Luo J, Chen H, Liu L, et al: MicroRNA 23b
regulates autophagy associated with radioresistance of pancreatic
cancer cells. Gastroenterology. 145:1133–1143.e1112. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lorin S, Pierron G, Ryan KM, Codogno P and
Djavaheri-Mergny M: Evidence for the interplay between JNK and
p53-DRAM signalling pathways in the regulation of autophagy.
Autophagy. 6:153–154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yen ML, Jim OP, Baudot AD, Attje H and
Ryan KM: DRAM-1 encodes multiple isoforms that regulate autophagy.
Autophagy. 8:18–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jin Q, Li XJ and Cao PG: MicroRNA-26b
enhances the radiosensitivity of hepatocellular carcinoma cells by
targeting EphA2. Tohoku J Exp Med. 238:143–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shi L, Yin W, Zhang Z and Shi G:
Down-regulation of miR-26b induces cisplatin resistance in
nasopharyngeal carcinoma by repressing JAG1. FEBS Open Bio.
6:1211–1219. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Arora H, Qureshi R, Park AK and Park WY:
Coordinated regulation of ATF2 by miR-26b in γ-irradiated lung
cancer cells. PLoS One. 6:e238022011. View Article : Google Scholar : PubMed/NCBI
|