Introduction
Lentinan (LNT) is a type of medicinal polysaccharide
isolated from shiitake with various activities including, immune
regulation, anti-tumor, anti-virus and anti-infection effects. LNT
has high efficacy and limited side effects (1). LNT has good curative effects on gastric,
colon, breast and lung cancer, which is able to prolong the
survival time of patients with tumor (2). LNT is often used as an immune enhancer
in clinical application, which can enhance curative effects or
reduce side effects in combination with other drugs (2). Murata et al (3) reported that a combination of cisplatin
and lentinan significantly increased the anti-cancer activity in
the treatment of colon cancer. Drandarska et al (4) demonstrated that treatment with LNT is
able to increase the activation of Bacillus Calmette-Guérin
(BCG)-induced pulmonary macrophages in guinea pigs and reduce
systemic adverse reactions of BCG vaccine.
Oridonin is an ent-kaurene diterpenoid compound
mainly isolated from R. rubescen (5). Previous studies have reported that
oridonin is able to promote tumor cell apoptosis (3,6). Apoptotic
rate has been hypothesized to have an effect on the sensitivity of
tumors to radiation (7). A previous
study also indicated that the sensitization effect of oridonin may
enhance the efficacy of radiotherapy in liver cancer cell lines
(8).
An ideal cancer therapeutic would be a drug that can
induce differentiation and apoptosis of tumor cells. Previous
clinical studies have focused on Chinese medicine preparations
based on anti-proliferative effects, while in recent years the
focus has shifted to the development of preparations which can
induce differentiation and apoptosis of cancer cells (9,10). Drugs
that induce apoptosis can selectively target cancer cells and
therefore normal cells are unaffected by treatment (9).
A number of active chemical substances in natural
plants have strong apoptotic-inducing effects on cancer cells
(8–10), and studies have demonstrated that
lentinan and oridonin are active substances with cancer cell
apoptosis-inducing effects (3,8).
Substances that can induce apoptosis in cancer
cells, which are extracted from plants have a low level of toxicity
and can be safely used. These substances are also able to relieve
pain in the process of treatment. However, the efficacy of numerous
cancer inhibitor components in plants is much lower compared with
synthetic drugs (11). Therefore, a
combination of different natural substances can increase the
inhibitory and therapeutic effects (11).
Finding a reasonable combination is an important
aspect of the research in the anti-cancer activities of natural
products. By studying how oridonin is able to increase the
anticancer effect of lentinan in vitro, the present study
aimed to verify the effects of a novel combination of anti-cancer
substances. By using MTT assay, flow cytometry, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blotting, the present study investigated the effect of
oridonin treatment on growth inhibition hepatoblastoma cells in
vitro. The study also investigated the effect of lentinan
treatment on the apoptosis of hepatoblastoma cells, in order to
accumulate further data that may enable future animal experiments
and even clinical application.
Materials and methods
Cell lines and treatments
Human normal liver L02 cell lines and human
hepatoblastoma HepG2 cells were purchased from the Conservation
Genetics CAS Kunming Cell Bank (Kunming, China). Oridonin and
lentinan were purchased from Shanghai Shamrock Imp and Exp Trading
Co., Ltd. (Shanghai, China). Lentinan-Low (LNL-L, 100 µg/ml) and
Lentinan-High (LNL-H, 200 µg/ml) treatment groups were generated
for the two cell lines.
Cell culture
The normal human liver L02 cell lines (control
group) and human hepatoblastoma HepG2 cells were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.) and cultured in an incubator under
humidified atmosphere of 5% CO2 at 37°C. The medium was
changed 2–3 times a week and sub-cultured for 6–7 days.
Subsequently, the cells were seeded in a 96-well culture plate at a
density of 1×104/ml with 180 µl per well, and cultured
for 24 h under humidified atmosphere of 5% CO2 at
37°C.
MTT assay
The L02 (control group) and HepG2 cells were
incubated with Oridonin (20 µg/ml) and Lentinan (100 µg/ml) for 24
h at room temperature, respectively. After 24 h, 20 µl MTT solution
(5 mg/ml; Ameresco Inc., Framingham, MA, USA) was added to each
well and incubated at 37°C for 4 h, following which the culture
medium was replaced with 150 µl dimethyl sulfoxide (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). The absorbance was measured at 540
nm using a microplate spectrophotometer (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The following formula was used to
calculate the inhibitory rate: Percentage cell
viability=[(Absorbance of untreated cells-absorbance of treated
cells)/absorbance of untreated cells] ×100 (12).
Flow cytometry analysis of cell cycle
distribution and apoptosis
HepG2 cells were seeded at a density of
50×104 cells/60-mm dish and incubated overnight at 37°C.
Oridonin was added to a final concentration of 40 µM and cells were
incubated for 24 h at 37°C. The cells were treated as follows: i)
20 µg/ml oridonin + 200 µg/ml lentinan; ii) PBS (negative control);
iii) 100 µg/ml lentinan; and iv) 200 µg/ml lentinan. Detached and
adherent cells were collected and centrifuged at 1,500 × g for 5
min at 4°C. Pellets were rinsed with ice-cold PBS and fixed with
70% ethanol at 4°C overnight. The density of HepG2 cells was
adjusted to 5×105 cells/ml, and the cells were washed
with PBS three times. Cells were subsequently stained with staining
buffer (PBS containing 20 µg/ml of propidium iodide, 100 µg/ml
RNase A and 0.1% Triton X-100; BD Biosciences, Franklin Lakes, NJ,
USA) for 15 min at 4°C in the dark. The cells were subsequently
labeled with Annexin V-fluorescein isothiocyanate (FITC) and
propidium iodide (Annexin V-FITC apoptosis detection kit; BD
Biosciences; cat. no. 556547). Samples were analyzed using a flow
cytometer (BD Biosciences) and Cell Quest acquisition software
(version 2.9; BD Biosciences) (13).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Cells from different treatment groups (20 µg/ml
oridonin + 200 µg/ml lentinan; PBS as negative control; 100 µg/ml
lentinan; and 200 µg/ml lentinan) were collected, and total RNA was
extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. Total
RNA was reverse transcribed into cDNA using a cDNA reverse
transcription kit (cat. no., 1708840; Bio-Rad Laboratories, Inc.)
according to manufacturer's protocol. The resultant DNA (10 µl) was
subjected to a 25 µl PCR conducted in an iCycler thermal cycler
(Bio-Rad Laboratories, Inc.) using iQ SYBR Green Supermix (Bio-Rad
Laboratories, Inc.). The thermocycling conditions that were used
were as follows: Initial denaturation at 95°C for 5 min, followed
by 35 cycles at 95°C for 20 sec, 58°C for 20 sec and 72°C for 20
sec, with a final extension of 72°C for 5 min. β-actin was used as
the internal reference gene. The relative expression levels were
calculated using the 2−ΔΔCq method (14) and gene expression was normalized to
β-actin. The primers used in the present study were as follows:
B-cell lymphoma (Bcl)-2 forward, 5′-CAAAGGTGGATCAGATTCAAG-3′; Bcl-2
reverse, 5′-GGTGAGCATTATCACCCAGAA-3′; Bcl-2-associated protein X
(Bax) forward, 5′-TGGCAGCAGTGACAGCAGCG-3′; Bax reverse,
5′-TACGGAGGTGGAGTGGGTGT-3′; caspase-3 forward,
5′-AAAGTTTTCAATGACCAAGC-3′; caspase-3 reverse,
5′-TCTGACGAATCTCCTCCAC-3′; caspase-9 forward,
5′-AGTCTATTTTATTATGGGCTCG-3′; caspase-9 reverse,
5′-TGGATGTTTATGTCACCTTTTC-3′; p21 forward,
5′-ATGGAGAACACTGAAAACTC-3′; p21 reverse,
5′-TGTGAGCATGGAAACAATAC-3′; p53 forward,
5′-ACTCCCATTCTTCCACCTTTG-3′; p53 reverse,
5′-CCCTGTTGCTGTAGCCATATT-3′; nuclear factor κB (NF-κB) forward,
5′-GCTATTCAGGCTGTGCTGTC-3′; NF-κB reverse,
5′-GGTAGTCGGTGAGATCTCGG-3′; nuclear factor κB inhibitor α (IκB-α)
forward, 5′-CCAACTATTGCTTCAGCTCCA-3′ IκB-α reverse,
5′-GTGTCCAGGCTCCAAATGT-3′; β-actin forward,
5′-AGCCTTCTCCATGGTCGTGA-3′; and β-actin reverse,
5′-CGGAGTCAACGGATTTGGTC-3′. Primers were synthesized by Invitrogen;
Thermo Fisher Scientific, Inc.
Western blot analysis
HepG2 cells treated with oridonin and/or lentinan as
aforementioned were homogenized and lysed with
radioimmunoprecipitation assay lysis buffer (Invitrogen; Thermo
Fisher Scientific, Inc.; 100 mM NaCl, 50 mM Tris-HCl pH 7.5, 1%
Triton X-100, 1 mM EDTA, 10 mM β-glycerophosphate, 2 mM sodium
vanadate and protease inhibitor). Lysates were sonicated for 5 sec
on ice and centrifuged at 6,000 × g for 5 min at 4°C. Supernatants
were collected and the protein concentration was detected using a
Bio-Rad protein assay kit (cat. no. 500–0002; Bio-Rad Laboratories,
Inc.). A total of 20 µg protein/well was loaded to SDS-PAGE (10%
gel; GE Healthcare Life Sciences, Shanghai, China) and transferred
onto polyvinylidene difluoride membranes, which were activated by
methanol. The membrane was blocked using 10% skimmed milk at room
temperature for 1 h. Subsequently, the membranes were incubated
with the primary antibodies against caspase-3 (1:1,000; cat. no.
ab13847), caspase-9 (1:1,000; cat. no. ab18571), Bcl-2 (1:1,000;
cat. no. ab194583), Bax (1:1,000; cat. no. ab32503), p38 (1:1,000;
cat. no. ab31828), p53 (1:1,000; cat. no. ab1431), NF-κB (1:1,000;
cat. no. ab32360), IκB-αα (1:1,000; cat. no. ab7217) and β-actin
(1:5,000; cat. no. ab8226), all purchased from Abcam (Cambridge,
UK), overnight at 4°C. The following day, the membranes were washed
with TBST for 10 min, prior to incubation with the horseradish
peroxidase-conjugated goat anti-mouse IgG secondary antibody
(1:1,000; cat. no. ab6728; Abcam) for 1 h at room temperature and
washed three times with TBST (10 min per wash). The membranes were
visualized using ECL chemiluminescence agent, and β-actin was used
as a control for normalization. Immunoreactivity was determined
using enhanced chemiluminescent reagent (Thermo Fisher Scientific,
Inc.) using an ImageQuant Las4000 digital imager (Thermo Fisher
Scientific, Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical evaluation was performed using Student's t-test or
one-way analysis of variance followed by Student-Newman-Keuls test
using SAS (version 9.2; SAS Institute Inc., Cary, NC, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of oridonin and lentinan
treatment on L02 and HepG2 cells
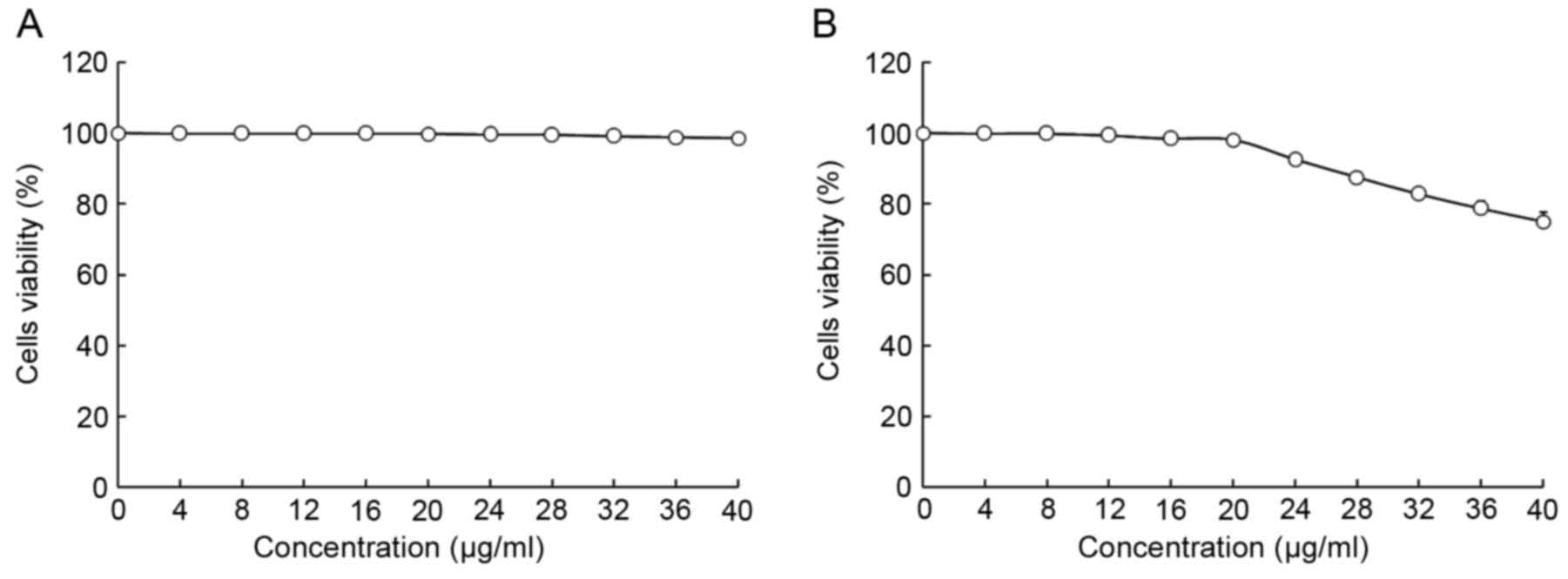
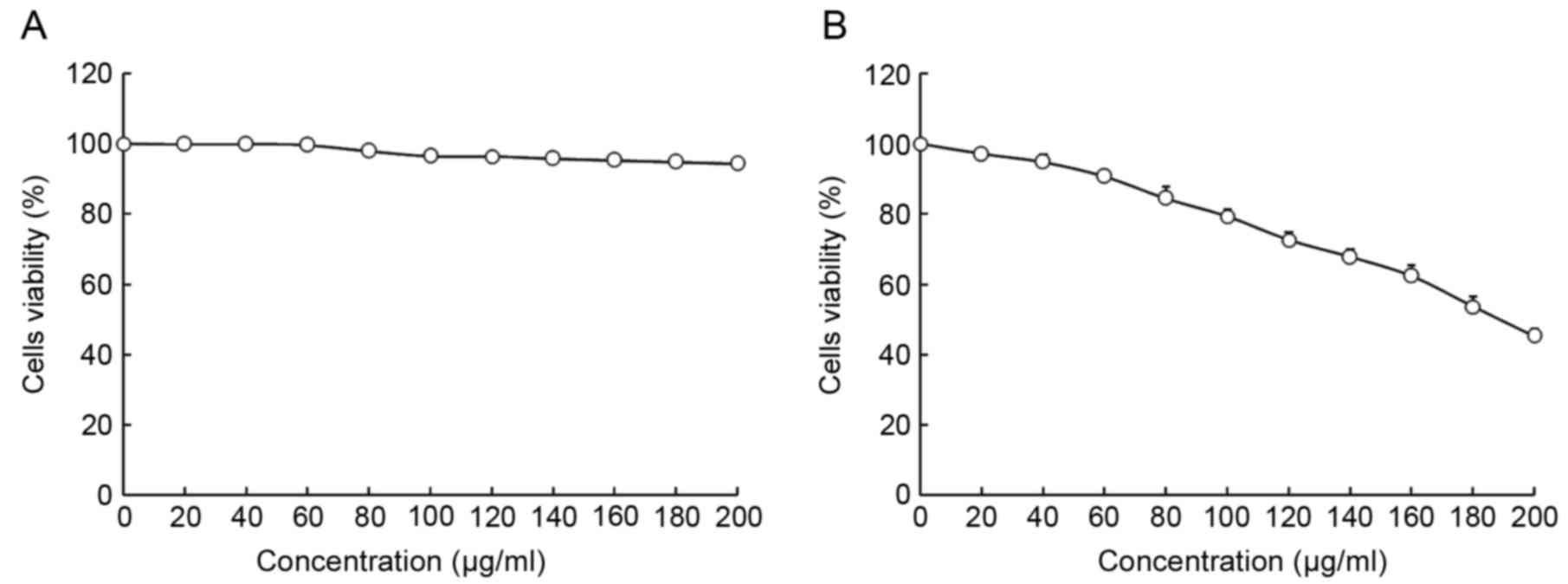
The viability of HepG2 and L02 cells following
treatment with oridonin or LNT was determined using MTT assay
(Figs. 1 and 2). Treatment with 0–20 µl/ml oridonin did
not result in a decrease in viability in L02 or HepG2 cells
(Fig. 1A and B). It was observed that
LNT treatment was able to decrease the viability of HepG2 cells at
an increased concentration. Treatment with 0–200 µl/ml LNT was able
to decrease the viability of HepG2 cancer cells. However, treatment
with the same concentration of LNT did not result in a decrease in
viability in L02 cells.
Based on these results, 20 µl/ml oridonin was
selected for subsequent experiments to investigate whether oridonin
treatment is able to increase the anti-cancer effect of LNT.
Concentrations of 100 and 200 µl/ml were selected to verify the
anticancer effects of LNT (Fig.
2).
The growth inhibition values of HepG2 human
hepatoblastoma cells by oridonin and lentinan are presented in
Table I. The highest OD540
value was observed in the HepG2 cancer cells; oridonin + LNT-H (20
µg/ml oridonin + 200 µg/ml LNT), LNT-H (200 µg/ml LNT) and LNT-L
(100 µg/ml LNT) treatments were able to decrease the
OD540 value compared with the cells in the control
group. Treatment of HepG2 cells with a combination of 20 µg/ml
oridonin and 200 µg/ml LNT increased the growth inhibitory rate
compared with treatment with 200 µg/ml LNT alone (Table I).
 | Table I.Growth inhibition of HepG2 human
hepatoblastoma cells by oridonin and lentinan as assessed by MTT
assay. |
Table I.
Growth inhibition of HepG2 human
hepatoblastoma cells by oridonin and lentinan as assessed by MTT
assay.
| Treatment | OD540
value | Inhibitory rate
(%) |
|---|
| Oridonin + LNT-H |
0.076±0.006a | 84.3±2.5a |
| Control |
0.484±0.005a | – |
| LNT-L |
0.384±0.010a | 20.7±1.9a |
| LNT-H |
0.219±0.011a | 54.8±2.2a |
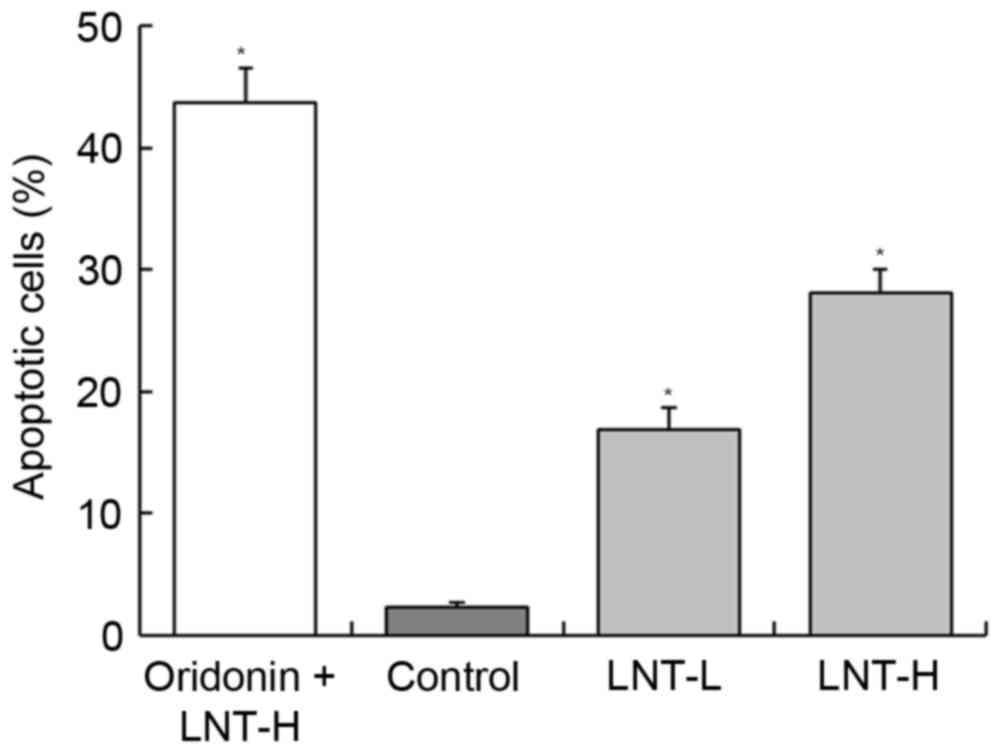
DNA content of sub-G1 HepG2 cells
Following treatment of HepG2 cancer cells with LNT,
the percentage of apoptotic cells (percentage of sub-G1DNA content)
was increased compared with the control cells (2.3±0.4%; Fig. 3). The percentage of apoptotic HepG2
cells treated with a high concentration of LNT (LNT-H, 200 µg/ml)
was 28.1±1.9%, and the percentage of cells treated with a low
concentration of LNT (LNT-L, 100 µg/ml) was 16.8±1.8%. A nontoxic
concentration (20 µg/ml) of oridonin was able to increase the
percentage of apoptoticHepG2 cells that were also treated with
LNT-H (oridonin + LNT-H vs. LNT-H, 43.7±2.8 vs. 28.1±1.9%; Fig. 3).
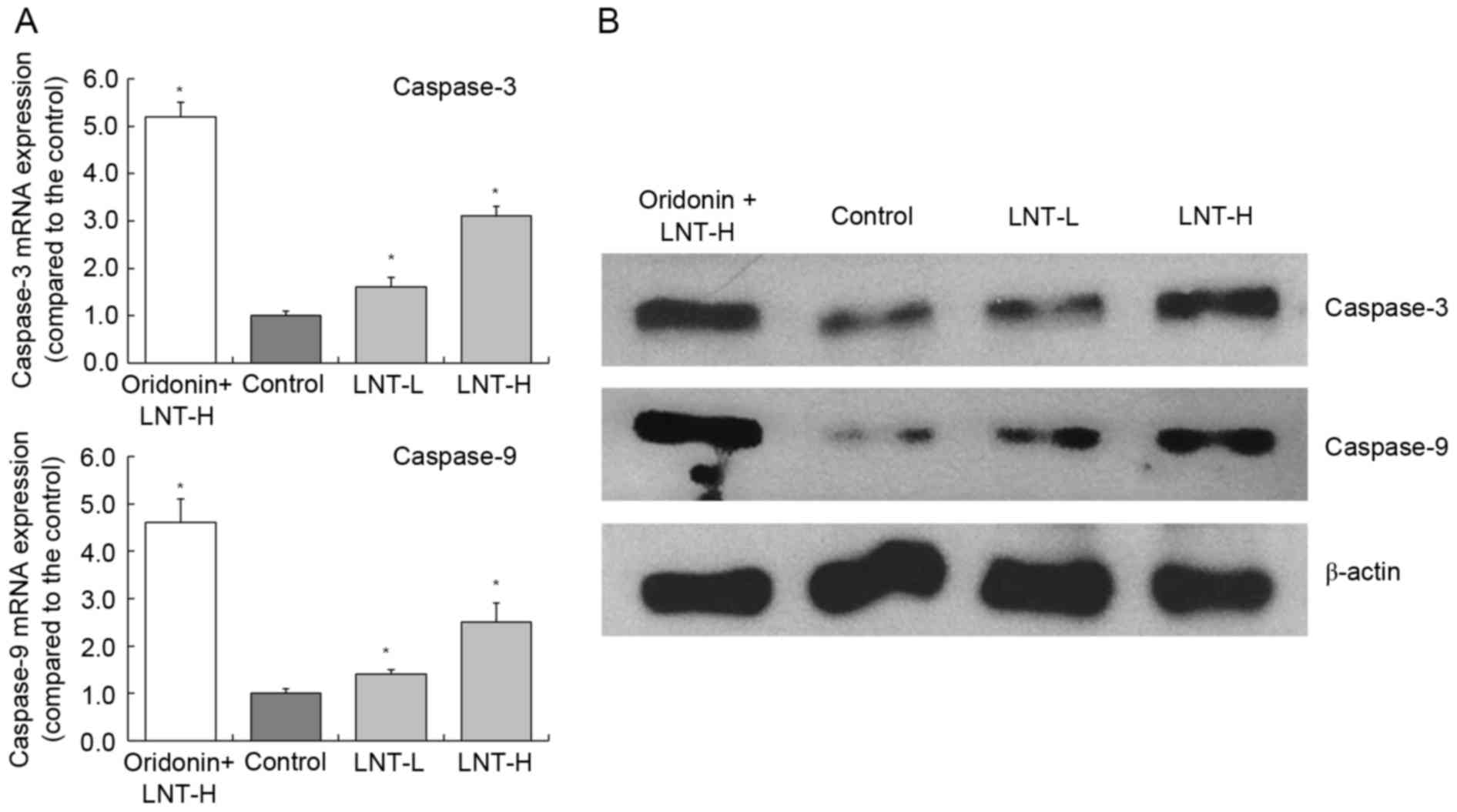
mRNA and protein expression of
caspase-3 and −9 in HepG2 cells
The highest levels of caspase-3 and −9 mRNA
expression were observed in HepG2 cells treated with oridonin and
LNT-H, with a 3.10 and 2.51-fold increase compared with the
control, respectively (Fig. 4). The
oridonin + LNT-H treated HepG2 cells also exhibited higher
caspase-3 and caspase-9 protein expression compared with the cells
in the other groups (control, LNT-L and LNT-H).
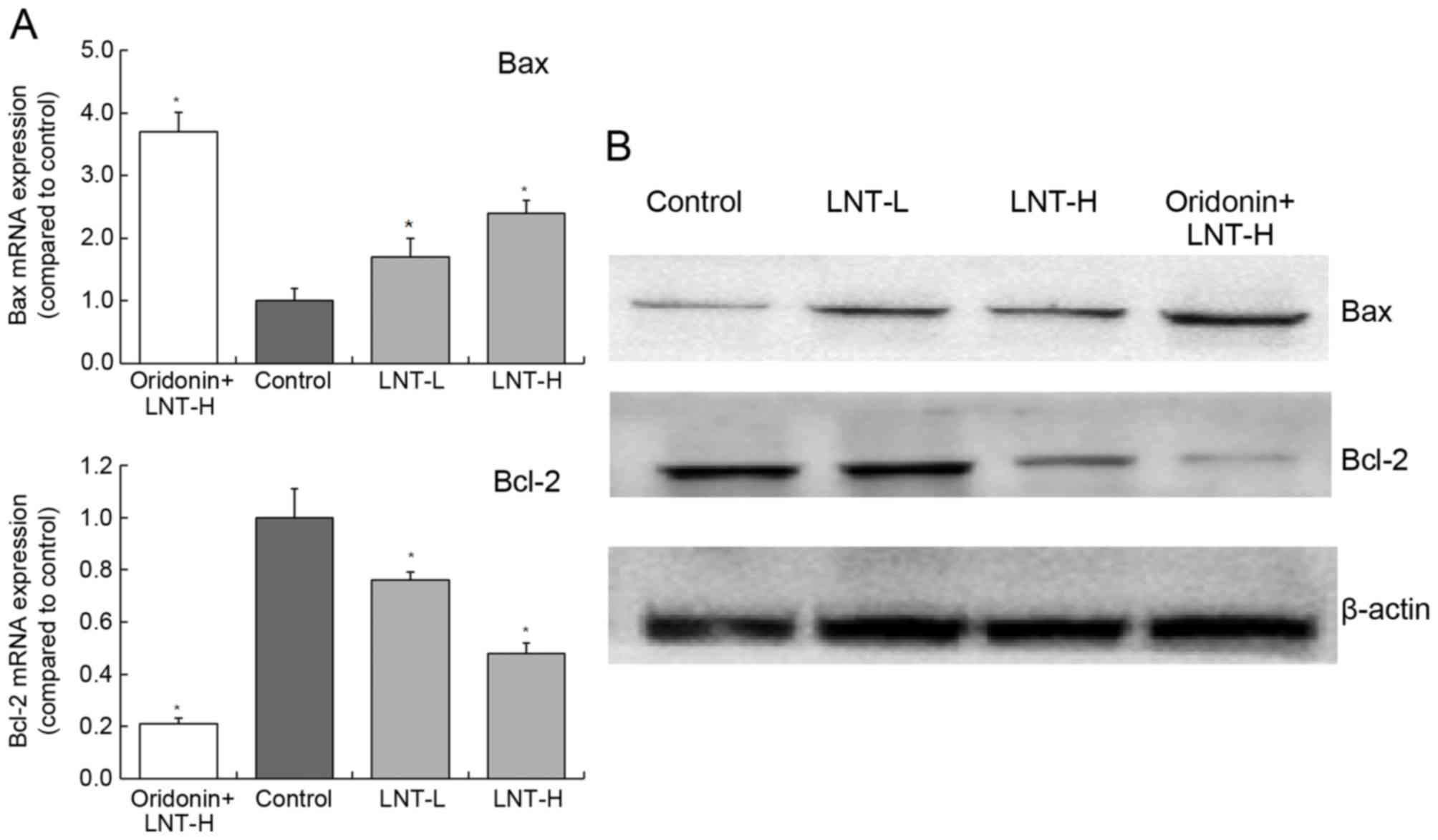
Gene and protein expression of Bax and
Bcl-2 in HepG2 cells
The levels of Bax mRNA expression in cells treated
with oridonin + LNT-H, LNT-H and LNT-L demonstrated a3.71-, 1.70-
and 2.42-fold increase compared with the control (Fig. 5). The levels of Bcl-2 mRNA expression
in cells treated with oridonin + LNT-H, LNT-H and LNT-L
demonstrated a0.21-, 0.48- and 0.76-fold decrease compared with the
control. The highest protein expression of Bax was observed in the
oridonin + LNT-H treatment group, and the lowest Bcl-2 protein
expression was also observed in the oridonin + LNT-H group
(Fig. 5).
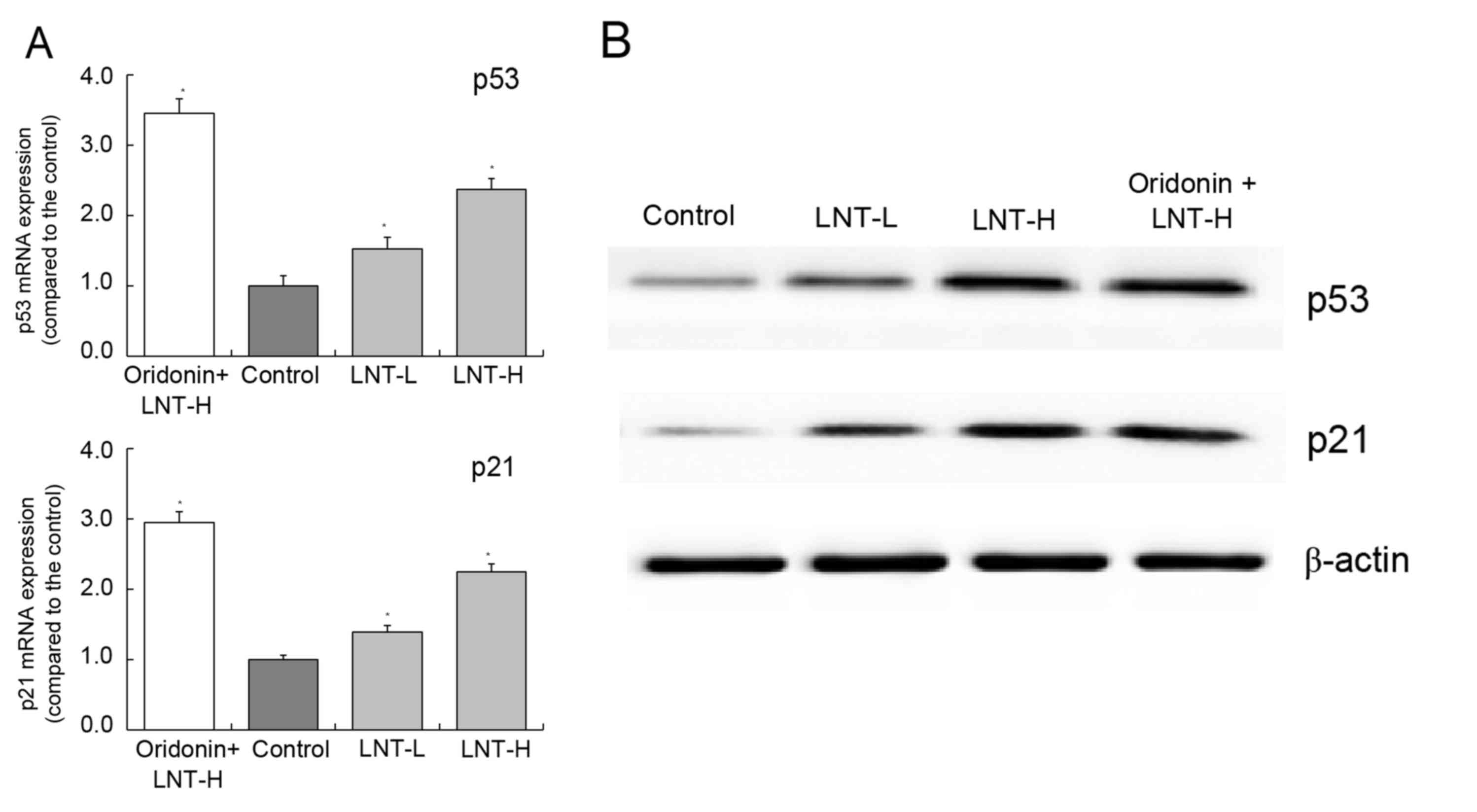
mRNA and protein expression of p53 and
p21 in HepG2 cells
The highest levels of p53 and p21 mRNA and protein
expression were observed in the oridonin + LNT-H group. The levels
of p53 and p21 were observed to be higher in the oridonin + LNT-H
group compared with the LNT-L group (Fig.
6A and B). The levels of p53 and p21 mRNA expression in the
oridonin + LNT-H group were 2.37 and 2.25 times higher compared
with the control, respectively (Fig.
6A).
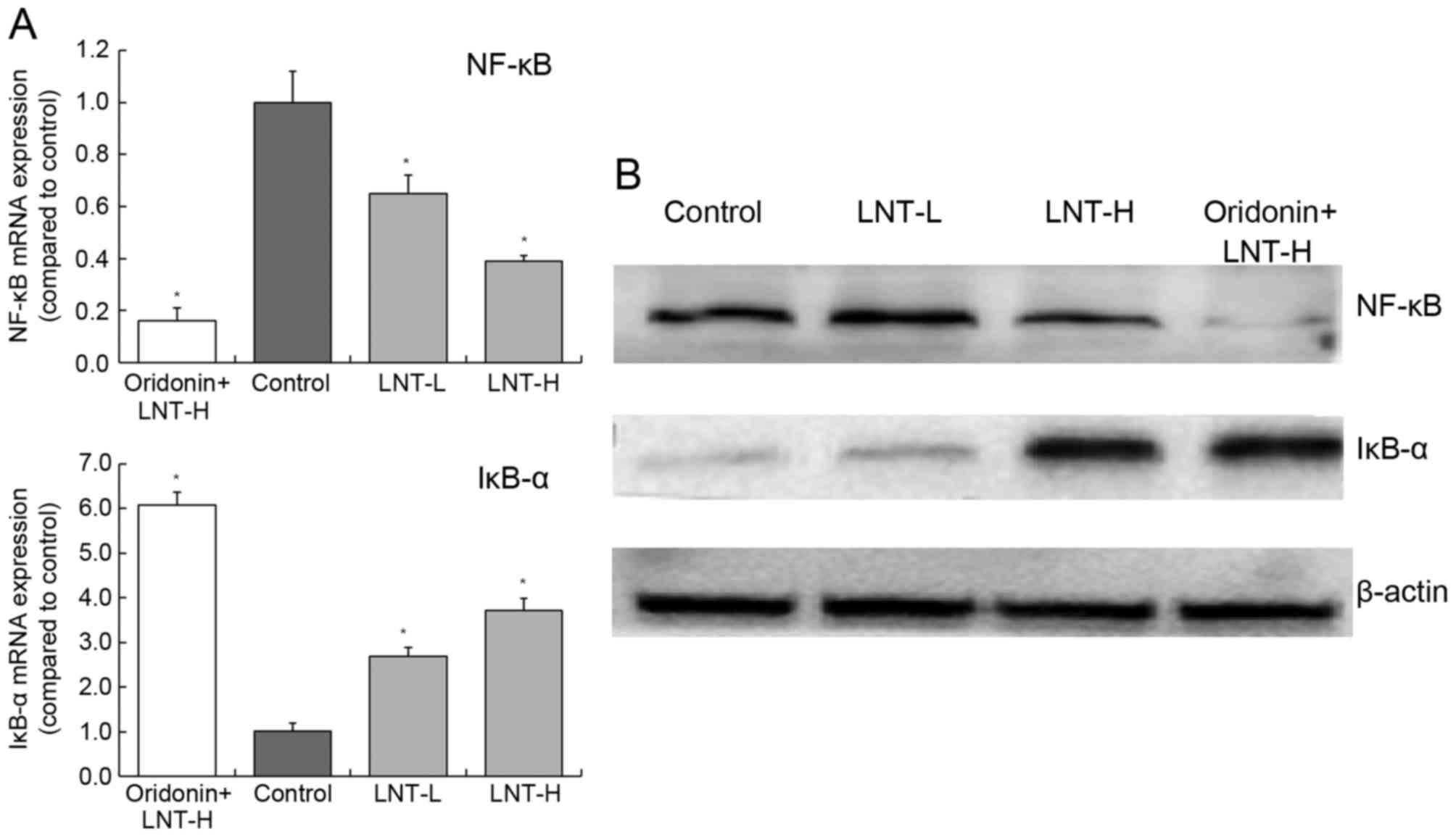
mRNA and protein expression of NF-κB
and IκB-α in HepG2 cells
Treatment of cells with oridonin and LNT-H was able
to reduce NF-κB expression and increase IκB-α expression compared
with the control (Fig. 7). The levels
of NF-κB mRNA was decreased 0.39-fold and the levels of IκB-α mRNA
were increased 3.72-fold in cells treated with oridonin and LNT-H,
compared with control cells (Fig.
7A). There was a similar trend in the expression of NF-κB and
IκB-α proteins as the mRNA expression of these proteins (Fig. 7B).
Discussion
Liver cancer cell apoptosis has an important role in
the development of liver cancer. It is now known that there are a
variety of cell signals mediated by receptors involved at the
initiation of liver cancer cell apoptosis. There are also a number
of proteases involved in apoptosis signal conduction as well as
multiple genes involved in the regulation of liver cancer cell
apoptosis. In previous years, it has been demonstrated that
caspase-3 protease contributes a major function in apoptosis
signaling transduction (15,16). The mechanism of caspase-3 protease
activation is highly complex and regulated by various factors, thus
there are different activation pathways. The activation of
caspase-3 protease activation is closely associated with liver
cancer cell apoptosis. Among the different proteases, caspase-3 has
a key role in apoptosis; it is the core protease that causes
caspase cascade reactions leading to apoptosis. Caspase-3 is
activated in apoptosis of liver cancer cell and is induced by a
variety of factors (17,18). Therefore, by inhibiting the activation
and activity of caspase-3, this may inhibit the apoptosis of liver
cancer cells (18).
Studies have reported have reported that Bcl-2
family members exert an important regulatory role in the process of
caspase-3 activation (18,19). Anti-apoptotic member of Bcl-2 family,
Bcl-xL inhibits oligomers of apoptotic protease-activating factor 1
(Apaf-1), which results in the loss-of-function of Apaf-1 molecules
and inhibits Apaf-1-dependent activation of caspase-9 (20). Anti-apoptotic members of the Bcl-2
family, which is primarily present in the outer membrane of the
mitochondria, are able to prevent the release of cytochrome c from
the mitochondria, which therefore inhibits the activation of
pro-caspase-9 (21).
All pro-apoptosis Bcl-2 family members can form
miscellaneous dimers with Bcl-2, Bcl-xL, A1 and Mcl-1 in the BH3
domain, which demonstrates that pro-apoptosis Bcl-2 family members,
at least in part, function by interacting with anti-apoptosis Bcl-2
family members. Pro-apoptosis Bcl-2 family members are also able to
induce the activation of caspases (22). Studies have also demonstrated that p53
is able to cause apoptosis through the activation of caspases
(23,24). Fuchs et al (25) reported that fas-deficient cells, which
induced the expression of wild type p53, are able to
activatecaspase-3 expression and characteristic changes in
apoptosis, indicating that p53-dependent apoptosis can directly
activate caspases, dependent on the involvement of Fas (25). Apoptosis inhibitory factor, Bcl-2,
regulates apoptosis by forming dimer by itself or different dimers
with Bax protein. An increase in the Bcl-2/Bax ratio results in
inhibition of apoptosis, yet if the ratio decreases apoptosis is
promoted. The mechanism of action of Bcl-xL is similar to that of
Bcl-2 (26). Silencing Bcl-2 gene is
also able to induce the activation of p53-dependent apoptotic
signaling pathway. In p53 wild-type cancer cells, following the
activation of p53, Bax expression increases if the concentration of
ultraviolet radiation treatment increases. However, the expression
of Bcl-2 and Bcl-xL decreases with the increase of inhibitor
concentration. These findings indicated that in the process of
killing cancer cells by inhibitors, p53 induces apoptosis primarily
through the Bax/Bcl-2 and Bax/Bcl-xL signaling pathways (27). Another study has indicated that in
cancer cells, ultraviolet radiation may change the expression of
p21 through p53, therefore inducing cell cycle arrest. The increase
in the expression of p21 and p53 is one of the markers that
indicate that ultraviolet radiation is able to induce cancer cell
apoptosis (28).
NF-κB is a type of nuclear transcription regulatory
factor, which is present in the majority of cells. When the cell is
not stimulated, NF-κB and its inhibitor IκB exist in cytoplasm in
an activated form (29). However,
when cells are stimulated by cellular damage or viruses, IκB is
phosphorylated and degraded. This results in the translocation of
NF-κB to the nucleus and consequently activation of NF-κB.
Following activation, NF-κB can promote transcription of cytokines,
chemokines and adhesion factors (30). In previous years, numerous studies
have revealed that NF-κB can control proliferation, regulate cell
cycle and apoptosis, affect differentiation, promote tumor
metastasis and have a close association with the occurrence and
development of tumors (30,31).
In the present study, MTT, flow cytometry, RT-qPCR
and western blot analysis were performed. Treatment with LNT
induced a decrease in cell viability in HepG2 cancer cells in a
dose-dependent manner, and oridonin treatment promoted the
anticancer effects of LNT in vitro. The results from the
present study provide evidence that oridonin may be used to
sensitize cells to LNT in vitro.
References
|
1
|
Kupfahl C, Geginat G and Hof H: Lentinan
has a stimulatory effect on innate and adaptive immunity against
murine Listeria monocytogenes infection. Int Immunopharmacol.
6:686–696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hou XJ and Chen W: Optimization of
extraction process of crude polysaccharides from wild edible BaChu
mushroom by response surface methodology. Carbohyd Polym. 72:67–74.
2008. View Article : Google Scholar
|
|
3
|
Murata T, Hatayama I, Kakizaki I, Satoh K,
Sato K and Tsuchida S: Lentinan enhances sensitivity of mouse colon
26 tumor to cis-diamminedichloroplatinum (II) and decreases
glutathione transferase expression. Jpn J Cancer Res. 87:1171–1178.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Drandarska I, Kussovski V, Nikolaeva S and
Markova N: Combined immunomodulating effects of BCG and Lentinan
after intranasal application in guinea pigs. Int Immunopharmacol.
5:795–803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang RL: Dong ling
caozhiliaoyuanfaxingganai 31 li linchuang guan cha. Ai Zheng.
3:501984.(In Chinese).
|
|
6
|
Zhang JF, Chen GH, Lu MQ and Liu JJ: Anti
proliferation effects of oridonin on hepatocellular carcinoma
BEL-7402 cells and its mechanism. Chinese Traditional Patent Med.
28:1325–1329. 2006.
|
|
7
|
Huang HL, Weng HY, Wang LQ, Yu CH, Huang
QJ, Zhao PP, Wen JZ, Zhou H and Qu LH: Triggering Fbw7-mediated
proteasomal degradation of c-Myc by oridonin induces cell growth
inhibition and apoptosis. Mol Cancer Ther. 11:1155–1165. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang H, Yu HS and Xue HW: Radio
sensitization effect of oridonin on HepG2 in vitro. Med J Qi lu.
22:339–342. 2007.(In Chinese).
|
|
9
|
Mullard A: Pioneering apoptosis-targeted
cancer drug poised for FDA approval. Nat Rev Drug Discov.
15:147–149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Denisenko TV, Sorokina IV, Gogvadze V and
Zhivotovsky B: Mitotic catastrophe and cancer drug resistance: A
link that must to be broken. Drug Resist Updat. 24:1–12. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O'Connor SE: Plant biochemistry. Fighting
cancer while saving the mayapple. Science. 349:1167–1168. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao X, Wang Q, Li GJ, Chen F, Qian Y and
Wang R: In vitro antioxidant, anti-mutagenic, anti-cancer and
anti-angiogenic effects of Chinese Bowl tea. J Funct Food.
7:590–598. 2014. View Article : Google Scholar
|
|
13
|
Zhao X, Qian Y, Zhou YL, Wang R, Wang Q
and Li GJ: Pu-erh tea has in vitro anticancer activity in TCA8113
cells and preventive effects on buccal mucosa cancer in U14 cells
injected mice in vivo. Nutr Cancer. 66:1059–1069. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao X, Kim SY and Park KY: Bamboo salt
has in vitro anticancer activity in HCT-116 cells and exerts
anti-metastatic effects in vivo. J Med Food. 16:9–19. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O'Donovan N, Crown J, Stunell H, Hill AD,
McDermott E, O'Higgins N and Duffy MJ: Caspase 3 in breast cancer.
Clin Cancer Res. 9:738–742. 2003.PubMed/NCBI
|
|
18
|
Rodríguez-Berriguete G, Galvis L, Fraile
B, de Bethencourt FR, Martínez-Onsurbe P, Olmedilla G, Paniagua R
and Royuela M: Immunoreactivity to caspase-3, caspase-7, caspase-8,
and caspase-9 forms is frequently lost in human prostate tumors.
Hum Pathol. 43:229–237. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsujimoto Y: Role of Bcl-2 family proteins
in apoptosis: Apoptosomes or mitochondria? Genes Cells. 3:697–707.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Swanton E, Savory P, Cosulich S, Clarke P
and Woodman P: Bcl-2 regulates a caspase-3/caspase-2 apoptotic
cascade in cytosolic extracts. Oncogene. 18:1781–1787. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park HJ, Jeon YK, You DH and Nam MJ:
Daidzein causes cytochrome c-mediated apoptosis via the Bcl-2
family in human hepatic cancer cells. Food Chem Toxicol.
60:542–549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Willis SN, Chen L, Dewson G, Wei A, Naik
E, Fletcher JI, Adams JM and Huang DC: Proapoptotic Bak is
sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by
BH3-only proteins. Genes Dev. 19:1294–1305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu SY, Xiao DJ, Luan YZ, Wang YS, Wang L
and Shi K: Regulatory mechanism of cell cycle block and apoptosis
in p53 mutated gastric cancer cells during cisplatin stress. J
Shandong Univ (Health Sci). 46:478–484. 2008.(In Chinese).
|
|
24
|
Moulin M, Carpentier S, Levade T and
Arrigo AP: Potential roles of membrane fluidity and ceramide in
hyperthermia and alcohol stimulation of TRAIL apoptosis. Apoptosis.
12:1703–1720. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fuchs EJ, McKenna KA and Bedi A:
p53-dependent DNA damage-induced apoptosis requires
Fas/APO-1-independent activation of CPP32beta. Cancer Res.
57:2550–2554. 1997.PubMed/NCBI
|
|
26
|
Klostergaard J, Leroux ME, Auzenne E,
Khodadadian M, Spohn W, Wu JY and Donato NJ: Hyperthermia engages
the intrinsic apoptotic pathway by enhancing upstream caspase
activation to overcome apoptotic resistance in MCF-7 breast
adenocarcinoma cells. J Cell Biochem. 98:356–369. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Woo SM, Choi YK, Kim AJ, Cho SG and Ko SG:
p53 causes butein-mediated apoptosis of chronic myeloid leukemia
cells. Mol Med Rep. 13:1091–1096. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hollmann G, Linden R, Giangrande A and
Allodi S: Increased p53 and decreased p21 accompany apoptosis
induced by ultraviolet radiation in the nervous system of a
crustacean. Aquat Toxicol. 173:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shih RH, Wang CY and Yang CM: NF-kappaB
signaling pathways in neurological inflammation: A mini review.
Front Mol Neurosci. 8:772015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carter SL, Centenera MM, Tilley WD, Selth
LA and Butler LM: IκBα mediates prostate cancer cell death induced
by combinatorial targeting of the androgen receptor. BMC Cancer.
16:1412016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Seubwai W, Vaeteewoottacharn K, Kraiklang
R, Umezawa K, Okada S and Wongkham S: Inhibition of NF-κB activity
enhances sensitivity to anticancer drugs in cholangiocarcinoma
cells. Oncol Res. 23:21–28. 2016. View Article : Google Scholar
|