Introduction
Urothelial carcinoma of the bladder (UCB) is the
fifth most common malignancy worldwide (1). In total, ~70–80% of patients diagnosed
with well-differentiated or moderately-differentiated non-muscle
invasive bladder cancer (2). Despite
treatment, cancer recurs in 60–70% of patients, of which, 10–30%
eventually develop muscle-invasive bladder cancer or metastatic
bladder cancer. The lung is one of the most common metastatic sites
and is associated with a high frequency of recurrence and
mortality, which contributes to a poor prognosis for patients
(3,4).
Therefore, to develop better therapeutic strategies and decrease
the morbidity and mortality associated with bladder cancer, it is
imperative to clarify the mechanism of bladder cancer invasion and
metastasis.
Clinical investigations have indicated hypoxia to be
a common feature of most solid tumors (5), owing to rapid proliferation of cancer
cells and/or compression of tumor blood vessels. As a cancer
progresses, cancer cells acquire the ability to adapt to hypoxic
environments while also resist to apoptosis, increase angiogenesis,
enhance the invasive and metastatic potential which makes them more
aggressive. A previous study suggests that one of the key factors
regulating the response to hypoxia is the heterodimer
hypoxia-inducible factor-1 (HIF-1) (6). Under hypoxic conditions, the alpha
subunit is not destroyed, and will activate transcription more than
100 gene products that take part in the tumor aggressiveness
(6). Its expression is associated to
an increased metastatic potential that has been demonstrated in
both animal models and human tumors by promotes a perpetual
epithelial to mesenchymal transition (EMT) (7).
The transcription factor Zinc-finger E-box-binding
homeobox 1 (ZEB1) is a known driver of EMT, and our previous
reports (8) confirmed that ZEB1 is an
important regulatory factor of bladder cancer invasion and
metastasis in vitro and in vivo. Furthermore, ZEB1
high expression is closely associated with markers associated with
invasion and metastasis in clinical tumor specimens (9). However, little is known about the
association between HIF-1α and ZEB1 protein in bladder cancer, and
whether there is an interaction between HIF-1α and ZEB1 in the
process of invasion and metastasis of bladder cancer.
To address these issues, an orthotopic animal model
was established by injecting human bladder cancer T24-tumorigenic
(T24-t) cells into a mouse bladder. Subsequently, the primary tumor
and lung metastases were excised and plated on tissue culture
dishes with G418 (400 µg/ml) to derive the sublines T24-parental
(T24-P) and T24-t-lung (T24-L), which were outlined in our previous
study (10,11). In the present study, the previously
described sublines T24-P and T24-L, were used to investigate the
role of downstream gene regulation of HIF-1α and EMT by mimicking
human bladder cancer metastasis (10). In addition, the molecular mechanisms
of the lung metastasis of bladder cancer were explored, focusing on
the effect of HIF-1α expression changes in bladder cancer cells on
ZEB1 expression, and the invasion and metastasis of bladder cancer
cells.
Materials and methods
Human tissue specimens
All the paraformaldehyde-fixed and paraffin-embedded
primary bladder cancer tissues (n=79) and adjacent histologically
normal tissues (n=11) were obtained from the Department of Urology,
The First Affiliated Hospital of Xi'an Jiaotong University, (Xi'an,
China). All the tissues were either obtained from transurethral
resection of bladder tumor (TURBT) or Radical cystectomy between
2,006.1 and 2,011.9. The histopathology of the specimens was
examined and classified by pathologists of Medical School, Xi'an
Jiaotong University. Sixty-six patients were men and thirteen were
women. Mean patient age was 63 years (range, 35–82 years). Bladder
carcinomas were staged according to the tumor-node-metastasis
system based on the International Union against Cancer (12). Genitourinary pathologists determined
tumor stage as: Ta (n=1); T1 (n=41); T2 (n=17); T3 (n=17); T 4
(n=3), and according to World Health Organization (1973) standard
for pathological grade (13): grade I
(n=26), grade II (n=32), grade III (n=21). This study was approved
by the Committee for the Protection of Human Subjects of the First
Affiliated Hospital of Xi'an Jiaotong University, and informed
consent was obtained from all patients.
Reagents and antibodies
A HIF-1α siRNA transfection kit was purchased from
Shanghai GenePharma Co, Ltd (Shanghai, China). Matrigel was
purchased from BD Transduction Laboratories (Franklin Lakes, NJ,
USA). Antibodies used for western blot were as follows: mouse
monoclonal antibodies to cytokeratin18 (1:1,000; cat. no. 4546;
Cell Signaling Technology, Beverly, MA, USA), goat polyclonal
antibody to Vimentin (1:500; cat. no. sc-7557; Santa Cruz
Biotechnology, Santa Cruz, CA, USA), GAPDH (1:1,2000; cat. no.
KC-5G4; Kang Chen Bio-technology, Shanghai, China), rabbit
polyclonal antibody to N-cadherin (1:1,000; cat. no. 13116; Cell
Signaling Technology), HIF-1α (1:1,000; cat. no. ab113642; Abcam,
Cambridge, UK) and rabbit monoclonal antibody to ZEB1 (1:1,000;
cat. no. 3396; Cell Signaling Technology). Rabbit polyclonal
antibody to HIF-1α for immunohistochemical staining (1:500; cat.
no. 04-006; Millipore Corporation, Billerica, MA, USA), rabbit
monoclonal antibody to ZEB1 for immunohistochemical staining
(1:500; cat. no. A301-922A; Bethyl Laboratories, Montgomery, TX,
USA) were used.
Immunohistochemical (IHC)
staining
The standard two-step Envision method of IHC
staining was used to assess the expression of HIF-1α and ZEB1.
Briefly, 5 µm sections were deparaffinized, rehydrated and
subjected to antigen retrieval in citrate buffer (10 mM, pH 6.0)
for 5 min at high temperature (121°C), and then endogenous
peroxidase and alkaline phosphatase activity were and blocked by
incubating in 0.3% H2O2 for 30 min. Slides
were then incubated overnight at 4°C with IHC-specific HIF-1α and
ZEB1 antibodies (dilution, 1:200) in a moist chamber. Following a
wash with PBS, the slides were incubated with horseradish
peroxidase-labelled anti-rabbit (dilution, 1:100; cat. no. K4002;
Dako; Agilent Technologies, Inc.) for 30 min at room temperature.
Rinsed with PBS, signals were detected by adding substrate hydrogen
peroxide using diaminobenzidine as a chromogen followed by
hematoxylin counterstaining, dehydrated, air-dried, and mounted.
Negative control slices were prepared by omitting the primary
antibody. HIF-1α and ZEB1 expression in human TCC tumors was
semiquantitatively evaluated according to the intensity of the
staining (0, 1+, 2+ and 3+) and the percentage of positive cells [0
(≤10%), 1 (10–25%), 2 (25–50%), 3 (50–75%) and 4 (≥75%)]. The
staining result was considered higher expression when intensity was
2+ or 3+ and the percentage category was 2–4, and lower expression
when intensity was 0 or 1+, or if intensity was >1 and the
percentage category was 0 or 1. All sections were evaluated blindly
by 2 of the authors.
Cell line and cell culture
All components for cell culture were purchased from
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
T24-parental (P) and T24-lung (L) bladder cancer cells were
obtained from primary tumors and lung metastases according to a
protocol previously described (10,11). Cells
were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (FBS; Invitrogen; Thermo Fisher Scientific Inc.) and 400 mg/l
G418 in a humidified incubator with 5% CO2, 95% air at
37°C. While, for mimicking the hypoxia conditions, the cells were
cultured in the atmosphere with 1% O2 and 99% N2 in 37°C (10,11).
Small interfering RNA (siRNA)
transfection
The sequence of siRNA for HIF-1α was as follows:
Sense 5′-CCAGCAGACUCAAAUACAATT-3′, antisense
5′-UUGUAUUUGAGUCUGCUGGTT-3′ (Shanghai GenePharma, Shanghai, China).
A total of 5×105 cells were seeded in a 6-well plate and
grown to 70–80% confluence prior to transfection. Cells were
transfected, according to the manufacturer's protocol, with
oligonucleotide duplexes (200 nM) premixed with Oligofectamine
(Invitrogen; Thermo Fisher Scientific, Inc.) in Opti-MEM-I
(Invitrogen; Thermo Fisher Scientific, Inc.) for 4 h. Cells were
treated with oligofectamine and scrambled siRNA served as a
negative control (NC-siRNA sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and
anti-sense, 5′-ACGUGACACGUUCGGAGAATT-3′; Dharmacon, Lafayette, CO,
USA). Transfection studies were performed in duplicates according
to the manufacturer's protocol.
RNA extraction and quantitative
RT-PCR
Total cellular RNA was extracted using the Highly
Pure RNA Isolation kit (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the protocol provided by the manufacturer, and was
quantified by absorbance at 260 nm. Total RNA (2 µg) was reverse
transcribed using a Revert Aid™ First Strand cDNA Synthesis kit
(Fermentas; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Primers for the amplification of HIF-1α
and ZEB1 were constructed with the following sequences: HIF-1α
forward, 5′-TCAAAGTCGGACAGC-CTCA-3′, reverse,
5′-CCCTGCAGTAGGTTTCT-GCT-3′, 460 bp product; ZEB1 forward,
5′-TTCAAACCCATAGTGGTTGCT-3′, reverse, 5′-TGGGAGATACCAAACCAACTG-3′,
151 bp product; β-actin forward, 5′-ATCATGTTTGAGACCTTCAACA-3′,
reverse, 5′-CATCTCTTGCTCGAAGTCCA-3′, 318 bp product.
For qPCR, the SYBR Premix Ex Taq™ II system (Takara
Biotechnology Co., Ltd., Dalian, China) was used with the CFX96TM
Real-time system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The reaction mix tubes contained SYBR Premix Ex Taq II (12.5 µl), 1
µl primer (10 µM,), 200 ng cDNA and 9.5 µl ddH2O. There
was one cycle of pre-degeneration at 95°C for 30 sec, then 35
repeats of 95°C for 5 sec followed by 60°C for 30 sec, then a final
stage of 95°C for 15 sec followed by 60°C for 30 sec, and 15 sec at
95°C. β-actin was used as an internal control. All experiments were
repeated at least twice in duplicate.
Invasion and migration assays
The invasion and migration capability of cells in
vitro was determined using a Boyden chamber assay. For the
invasion assay, 50 µl of Matrigel was applied to 8 µm pore
polycarbonate membrane filters (BD Biosciences, San Jose, CA, USA)
in a 24-well plate, and allowed to solidify overnight. Then,
5×104 cells, detached using trypsin-EDTA and resuspended
in 200 µl FBS-free DMEM were added to the upper chamber, and 800 µl
FBS-free DMEM was added to the lower chamber. Subsequent to
incubating the plates at 37°C in 5% CO2 for 24 h, cells
on top of the membrane were scraped away using a cotton swab. Cells
at the bottom surface of the membrane were fixed with 4%
paraformaldehyde for 10 min, stained with crystal violet solution
(0.01% in the ethanol) for 15 min at room temperature, then washed
three times with PBS (pH 7.4). The cells were counted using an
inverted microscope; 5 fields of view were randomly selected at
×100 magnification, and the mean number of cells was determined.
For determining cell migration, the Boyden chamber assay was
performed as described above, without Matrigel. Presented data are
representative of three individual wells.
Wound healing assay
Cells were cultured to a monolayer of 100%
confluence in a 6-well plate and washed three times with PBS (pH
7.4) to remove residual FBS. Subsequent to incubating the cells at
37°C in 5% CO2 for 12 h with FBS-free DMEM, the plate
was scratched to remove a 400–450 µm strip of cells across the well
with a standard 200 µl pipette tip. Wounded monolayers were washed
twice with PBS to remove non-adherent cells, and the cells were
incubated at 37°C in 5% CO2. The width of the scratches
was photographed and measured at 0, 6 and 12 h after scratching.
Presented data are representative of three individual wells.
Protein extraction and western blot
analysis
Cells were harvested at 70–80% confluence and washed
with 4°C PBS three times. Total cellular protein lysates were
prepared with radio immunoprecipitation assay buffer [50 mM Tris
(pH 8.0), 150 mM NaCl, 0.1% SDS, 1% NP40 and 0.5% sodium
deoxycholate] containing proteinase inhibitors 1% cocktail and 1 mM
PMSF, (Sigma Aldrich; Merck KGaA, Darmstadt, Germany). A total of
20–40 µg of protein (Pierce bicinchoninic acid assay protein assay
kit; cat. no. 23225; Thermo Fisher Scientific, Inc.) was separated
by SDS-PAGE (10% gel) and transferred to nitrocellulose membranes.
Following blocking at room temperature for 1 h with 5% skimmed milk
in TBS (pH 7.6), the membranes were incubated with primary
antibodies (HIF-1α, ZEB1 and N-Cadherin, 1:1,000; CK18, Vimentin,
1:300; GAPDH, 1:15,000) at 4°C overnight, then washed with TBST
with Tween-20 (pH 7.6). Membranes were incubated with goat
anti-rabbit (1:5,000; cat. no. A0545; Sigma Aldrich; Merck KGaA) or
anti-mouse secondary antibody (1:30,000; cat. no. A5278; Sigma
Aldrich; Merck KGaA) coupled to the first antibody at room
temperature in the dark for 1 h, followed by washing as above in
the dark, drying with neutral absorbent paper and scanning by
Odyssey detection system (LI-COR Biosciences, Lincoln, NE, USA).
MG-132 (Sigma Aldrich, Merck KGaA) was used to inhibit
proteasome-dependent degradation if necessary (10 µM, 4 h prior to
protein harvesting). Loading differences were normalized using a
monoclonal GAPDH antibody.
Statistical analysis
All statistical analyses were performed using SPSS
19.0 (IBM Corp., Armonk, NY, USA). Quantitative data are presented
as the mean ± standard deviation from ≥3 independent experiments.
The statistical significance of differences among multiple groups
was one-way analysis of variance followed by Bonferroni's multiple
comparisons test. When the comparison involved only 2 groups, a
2-sided Student's t-test was used. IHC statistical analysis was
performed with the χ2 test. The analysis of HIF-1α and
ZEB1 association was performed using Spearman's correlation
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of HIF-1α and ZEB1 in human
bladder cancer tissues and normal bladder tissues
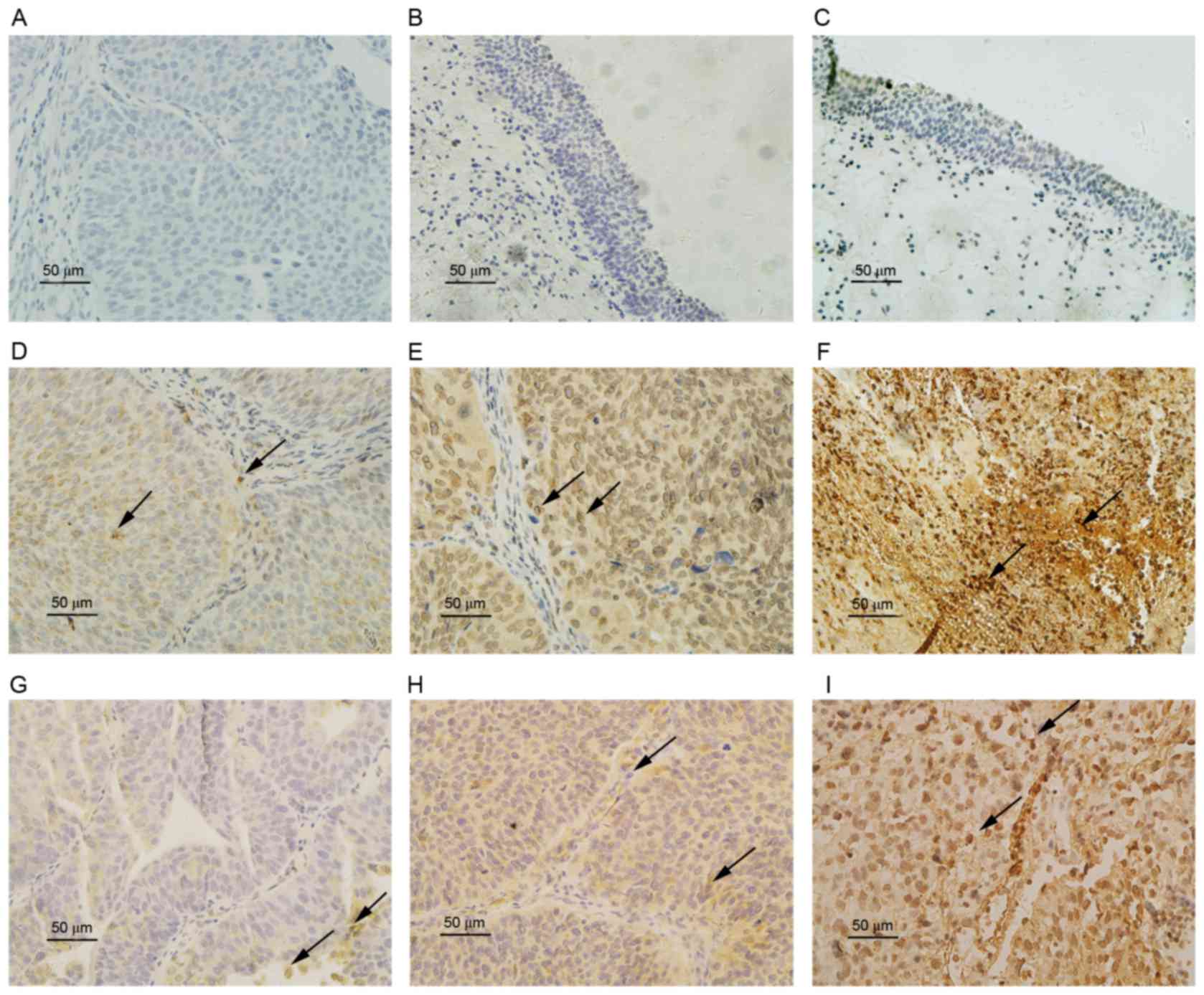
To investigate the association between the
expression of HIF-1α and ZEB1 in bladder carcinoma, IHC staining
for HIF-1α and ZEB1 was performed on 79 UCB tissues and 11 normal
bladder epithelial control tissues. The staining results (Fig. 1) indicated that HIF-1α and ZEB1
expression occurred in the cytoplasm and nucleolus in bladder
cancer tissues. The expression of HIF-1α (P=0.005) and ZEB1
(P=0.007) in UCB tissues was significantly higher than in normal
bladder epithelium (Table I). The
expression score and rate of HIF-1α and ZEB1 were also
significantly higher in the high-grade, invasive and metastatic UCB
than in low-grade, superficial and non-metastatic UCB (P<0.05).
However, a Mann-Whitney U test identified no significant
differences (P>0.05) in HIF-1α and ZEB1 expression levels
according to age (<60 and ≥60 years) and sex (Table II).
 | Table I.HIF-1α and ZEB1 expression in bladder
tumor and normal tissues as determined with immunohistochemistry
analysis. |
Table I.
HIF-1α and ZEB1 expression in bladder
tumor and normal tissues as determined with immunohistochemistry
analysis.
|
| HIF-1α expression
score | ZEB1 expression
score |
|---|
|
|
|
|
|---|
| Tissue type | Mean | SD | P-value | Mean | SD | P-value |
|---|
| Tumor | 3.75 | 0.44 | 0.005 | 2.81 | 0.39 | 0.007 |
| Normal | 2.02 | 0.81 |
| 2.01 | 0.79 |
|
 | Table II.Association between the IHC expression
of HIF-1α and ZEB1 with clinical characteristics. |
Table II.
Association between the IHC expression
of HIF-1α and ZEB1 with clinical characteristics.
|
|
| HIF-1α
expression | ZEB1 expression |
|---|
|
|
|
|
|
|---|
| Characteristic | n | (−), n | (+), n | R value | P-value | (−), n | (+), n | R value | P-value |
|---|
| Age |
|
|
| −0.143 | 0.825 |
|
| −0.117 | 0.753 |
| ≤60 | 31 | 14 | 17 |
|
| 16 | 15 |
|
|
|
>60 | 48 | 18 | 30 |
|
| 24 | 24 |
|
|
| Sex |
|
|
| −0.132 | 0.439 |
|
| 0.115 | 0.557 |
|
Male | 66 | 26 | 40 |
|
| 36 | 30 |
|
|
|
Female | 13 | 6 | 7 |
|
| 4 | 9 |
|
|
| Grade |
|
|
| 0.462 | 0.003 |
|
| 0.381 | 0.004 |
| G1 | 26 | 21 | 5 |
|
| 22 | 4 |
|
|
|
G2-3 | 53 | 11 | 42 |
|
| 18 | 35 |
|
|
| Stage |
|
|
| 0.387 | 0.002 |
|
| 0.452 | <0.001 |
|
Ta-1 | 42 | 30 | 12 |
|
| 32 | 10 |
|
|
|
T2-4 | 37 | 2 | 35 |
|
| 8 | 29 |
|
|
| Lymphatic
metastasis |
|
|
| 0.213 | <0.001 |
|
| 0.314 | 0.003 |
|
Yes | 10 | 0 | 10 |
|
| 1 | 9 |
|
|
| No | 69 | 32 | 37 |
|
| 39 | 30 |
|
|
In the 79 UCB tissues, the positive expression rate
of HIF-1α was 59.49% (47/79), and that of ZEB1 was 49.37% (36/79).
The positive expression rate of ZEB1 was 76.6% (36/47) in HIF-1α
positive expression group. In the HIF-1α-negative group, the ZEB1
positive expression rate was 9.38% (3/32). Spearman's rank
correlation analysis indicated a significant positive association
between the expression of HIF-1α and ZEB1 (r=0.337, P<0.05,
Table II).
HIF-1α promotes bladder cancer cell
migration and invasion in vitro
It was reported in our previous study that the T24-P
and T24-L sublines had a similar growth rate; however, the growth
rate of orthotopic and metastatic xenograft bladder tumors of T24-L
cells was slightly higher than that of T24-P cells. In addition,
the incidences of distant metastasis observed in the T24-P and
T24-L group were 9 and 36%, respectively.
HIF-1α is known to serve an important role in tumor
metastasis. To identify the roles of HIF-1α in bladder cancer
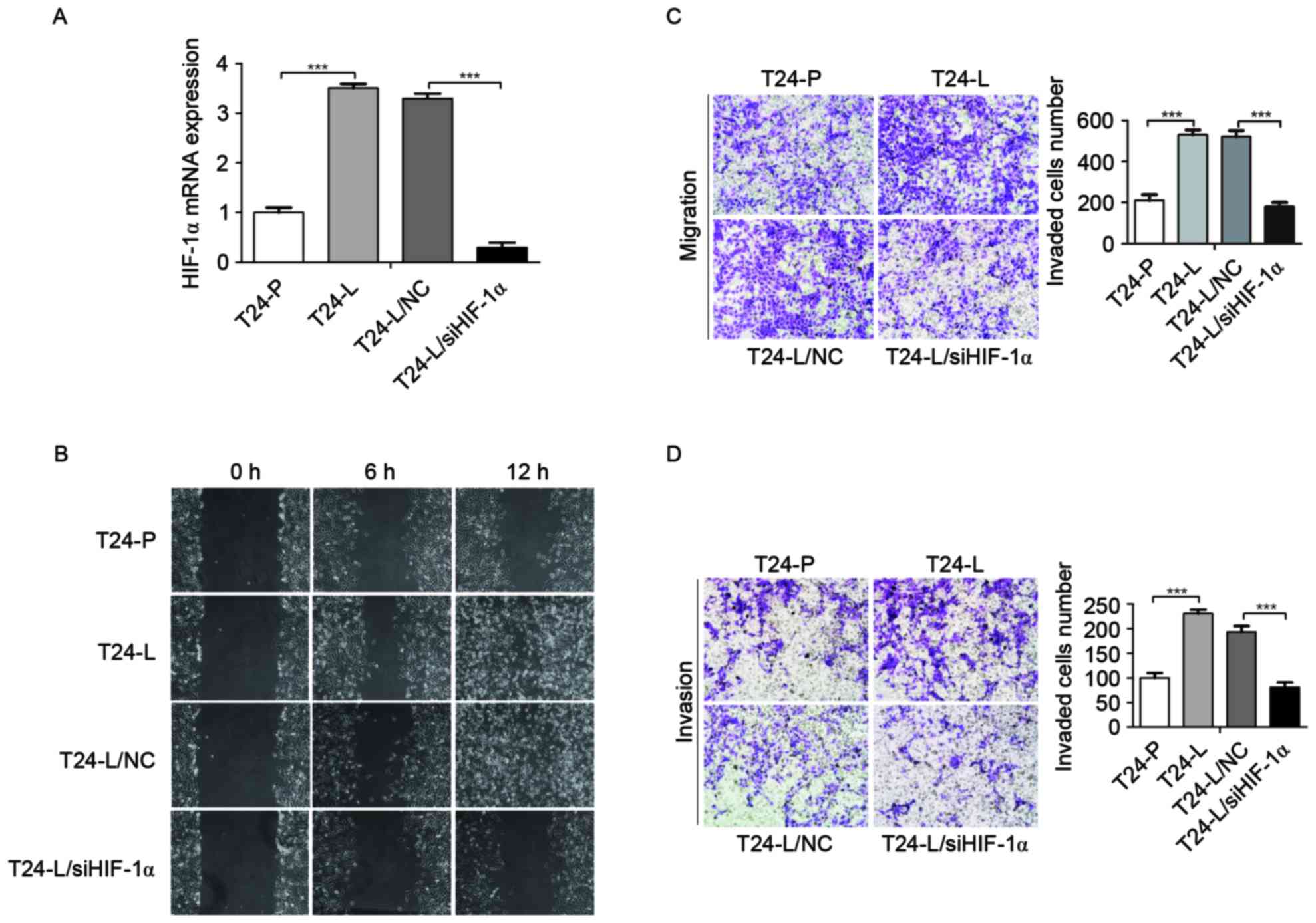
invasion and metastasis, the expression of HIF-1α in T24-P and
T24-L cells was examined; it was identified that HIF-1α mRNA level
was higher in T24-L cells than that in T24-P cells using an RT-qPCR
assay (Fig. 2A). In addition, to
further identify the function of HIF-1α in T24-L cells, HIF-1α
expression was knocked down by transfecting HIF-1α and scrambled
siRNA into T24-L cells. As demonstrated in Fig. 2A, the HIF-1α expression level was
significantly reduced by transfection with siRNA against HIF-1α
compared with the negative control group in T24-L cells, as
determined by RT-qPCR analysis (P<0.001).
To determine whether HIF-1α knockdown affects the
migration and/or invasion of bladder cancer cell lines,
wound-healing and transwell assays were performed. It was
identified that the migration and invasion ability of T24-L cells
were greater than T24-P cells (Fig. 2B, C
and D; P<0.001); in addition, HIF-1α knockdown by siRNA
significantly reduced the cell motility and invasion of T24-L cells
(P<0.001). These results indicated that HIF-1α promoted the
migration and invasion of bladder cancer cells.
HIF-1α increases ZEB1 expression and
promotes EMT in bladder cancer cells
EMT may be a crucial step in the initiation of the
metastatic spread of tumor cells into distal organs (7); ZEB1 may be a key driver of bladder
cancer invasion and metastasis (14).
Therefore, it was examined whether the expression of HIF-1α
modulates ZEB1 expression or the process of EMT in bladder cancer
cells. As demonstrated in Fig. 3A,
ZEB1 mRNA expression in T24-L cells was significantly higher than
that in T24-P cells, as detected by RT-qPCR, and significantly
reduced in T24-L cells transfected with HIF-1α siRNA. Furthermore,
as determined by western blot analysis, HIF-1 α, ZEB1, N-cadherin
and vimentin protein expression was higher in T24-L cells than in
T24-P cells, whereas cytokeratin-18 protein level was lower in
T24-L cells. In T24-L cells transfected with HIF-1α siRNA, HIF-1α,
ZEB1, N-cadherin and vimentin protein was downregulated whereas the
expression of cytokeratin-18 was upregulated (Fig. 3B). These results indicate that HIF-1α
expression increased the expression of ZEB1 and promoted the
process of EMT in bladder cancer cells.
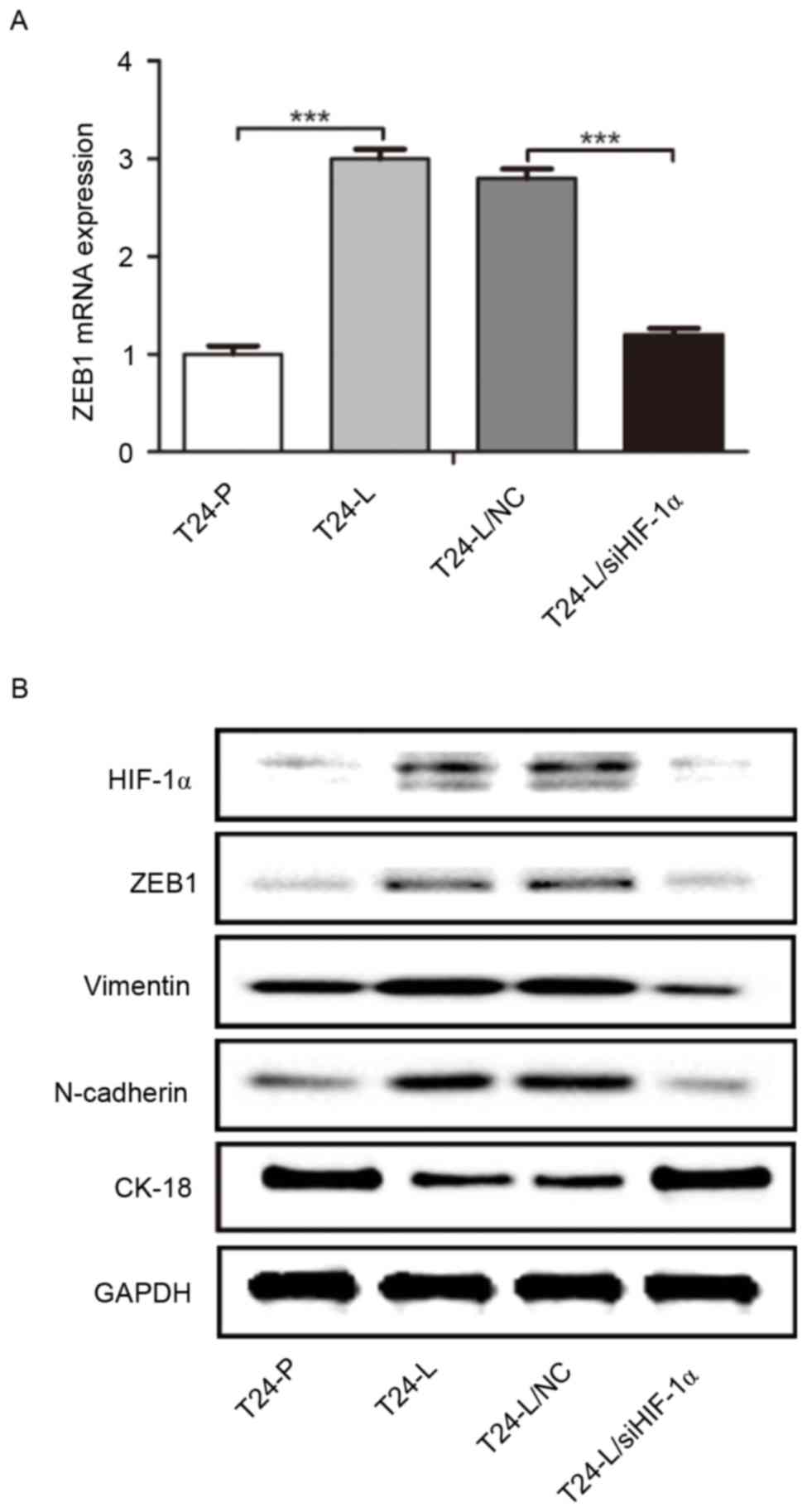 | Figure 3.ZEB1 expression is regulated by
HIF-1α, and promotes epithelial-mesenchymal transition. (A) Reverse
transcription-quantitative polymerase chain reaction was used to
determine the level of ZEB1 mRNA expression in
T24-P/T24-L/T24-NC/T24-L/siHIF-1α. ZEB1 was significantly elevated
in T24-L, and transfection with siHIF-1α downregulated ZEB1
expression in T24-L cells, compared with the control. (B) Western
blot analysis indicated that the protein level of HIF-1α, ZEB1,
vimentin and N-cadherin was higher in T24-L cells than T24-P cells,
whereas CK-18 expression was lower in T24-L cells. siHIF-1α
transfection in T24-L led to the downregulation of ZEB1, Vimentin
and N-Cadherin, but the upregulation of CK-18. ***P<0.001. ZEB1,
zinc-finger E-box-binding homeobox 1; HIF-1α, hypoxia inducible
factor-1α; T24-L, T24 lung metastasis cells; siHIF-1α, small
interfering RNA against HIF-1α; T24-P, T24 parental cells; CK-18,
cytokeratin-18; NC, negative control. |
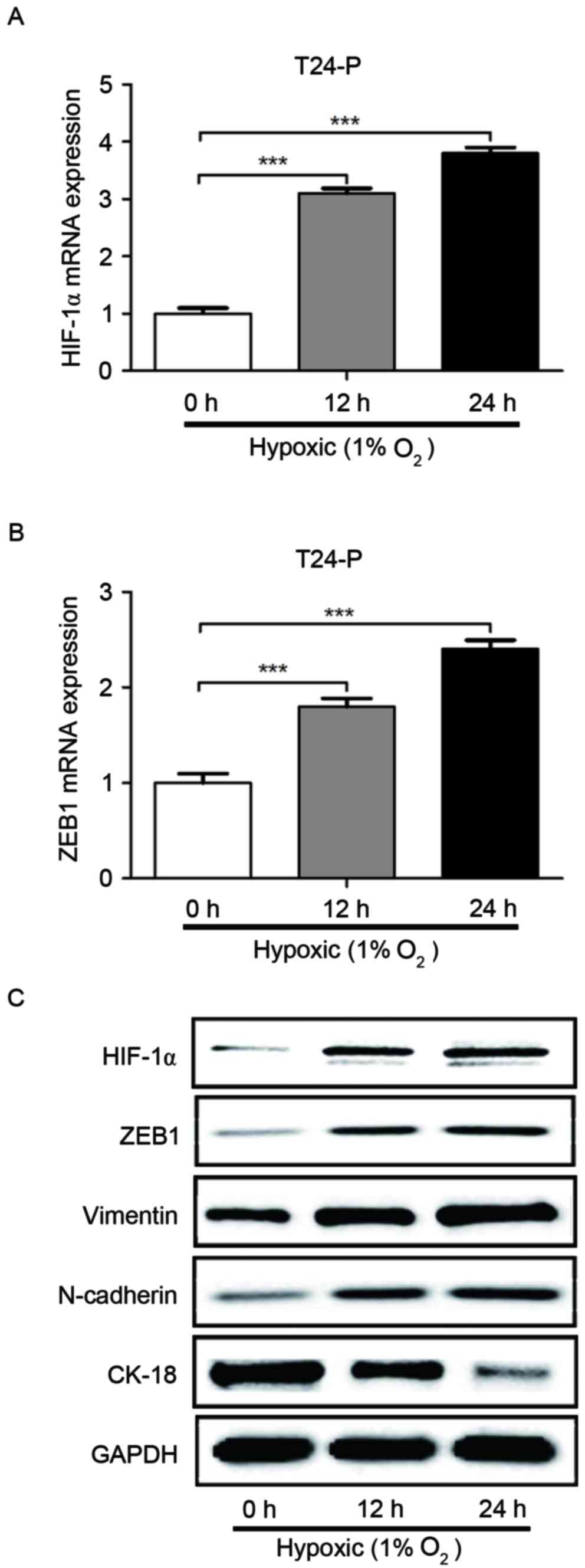
It was then determined whether the induction of
HIF-1α expression by hypoxia also regulated ZEB1 expression. As
displayed in Fig. 4, hypoxia
significantly increased the mRNA level of HIF-1α and ZEB1 in T24-P
cells, as detected by RT-qPCR. Furthermore, hypoxia increased the
protein expression of HIF-1α, ZEB1, N-cadherin and vimentin in
T24-P cells, whereas cytokeratin18 protein expression was reduced,
as identified by western blot analysis. These results confirm that
HIF-1α can increase the expression of ZEB1 and suggest that hypoxia
promotes cell migration and invasion through HIF-1α and ZEB1
expression in bladder cancer.
Discussion
Owing to incomplete blood vessel networks and the
imbalance between proliferation and angiogenesis, hypoxia is a
common feature of the microenvironment in various solid tumors,
including bladder cancer (15,16).
Hypoxia serves a critical role in various cellular and physiologic
events, including cell proliferation, survival, angiogenesis
(17), immune-surveillance,
metabolism, and tumor invasion and metastasis, and it is often
associated with a poor prognosis (18). Investigating the biology of tumor
cells in hypoxic conditions may be important for improving
therapeutic efficacy and for eradication of cancer. Hypoxia
activates relevant gene expression through HIFs, transcription
factors from a family that includes HIF-1, HIF-2 and HIF-3. HIF is
a heterodimer composed of an alpha and a beta subunit, in which the
HIF-1α protein is a master regulator of the hypoxic response in
bladder urothelial carcinomas (6).
HIF-1α may be associated with clinicopathological parameters with
prognostic value, including tumor stage, grade, proliferation index
and microvessel density (19). Our
previous study provided novel insights into the mechanism of
HIF-1α/matrix metalloproteinase-1 in the process of distant
metastasis of bladder cancer, offering a potential therapeutic
target for metastatic bladder cancer therapy (10). In the present study, it was identified
that HIF-1α promotes cell migration and cell invasion, and that
hypoxia may induce EMT through HIF-1α in bladder cancer, suggesting
that HIF-1α serves an important role in bladder cancer
metastasis.
As a potent suppressor of epithelial marker,
transcription factor ZEB1 is one of the key inducers of EMT; its
expression promotes the tumorigenesis and metastasis of various
types of carcinoma (20). Our
previous study (21) revealed a novel
mechanism facilitating metastatic bladder cancer cell
re-colonization into bone, in which phosphoinositide 3-kinase/Akt
targeted glycogen synthase kinase 3β/β-catenin and regulated the
expression of ZEB1. Furthermore, the results provided a molecular
and clinicopathological basis for the role of ZEB1 in bladder
cancer invasion and metastasis. Therefore, ZEB1 could be a
potential prognostic marker and a drug target for muscle-invasive
or metastatic bladder cancer (21).
It was previously reported that dysregulated HIF-1
activity resulting from VHL loss of function in RCC4 cells induces
the expression of multiple known repressors of CDH1 (ZEB1) gene
transcription directly or indirectly, so as to promote tumor
progression, invasion and metastasis (17). However, there is limited data
regarding the association between HIF-1α and ZEB1 protein in
bladder cancer tissue, and whether there is interaction between
HIF-1α and ZEB1 in the process of invasion and metastasis of
bladder cancer. In the present study, the expression of HIF-1α and
ZEB1 in bladder transitional cell carcinoma tissues were
significantly increased compared with normal bladder epithelium
tissues. Furthermore, the expression score and rate of both HIF-1α
and ZEB1 were significantly higher in the high-grade, invasive and
metastatic bladder cancer compared with low-grade, superficial and
non-metastatic bladder cancer (P<0.05) and expression of both
proteins were positively associated with each other in bladder
carcinoma tissues. To our knowledge, this is the first study
involved in the association between HIF-1α and ZEB1 protein
expression in bladder cancer tissue.
To further investigate the interaction between HIF-1
α and ZEB1 in the process of invasion and metastasis of bladder
cancer, a novel model of TCC metastasis was adopted, consisting of
two isogenetic T24-t sublines, T24-P and T24-L we adopt a novel
model of bladder cancer metastasis consisting of two isogenetic
T24-t sublines, designated T24-P and T24-L, generated through
successive in vivo passaging and in vitro subculture
(11).
The sublines were previously demonstrated to exhibit
a similar in vitro growth rate, and share several of the
same numerical and structural abnormalities of karyotypes.
Cytogenetic evaluation of T24-P cells (having the same molecular
features of bladder cancer in situ) and T24-L cells (having
the same molecular features with cells of bladder cancer lung
metastasis) revealed that these cell lines are indeed associated;
however, exhibit specific cytogenetic abnormalities (10,11). For
example, T24-L cells exhibited more invasive and metastatic
abilities than orthotopic T24-P cells in vitro; the T24-L
subline acquired more mesenchymal-like characteristics and the
T24-P subline was more epithelial-like.
Consistently, in the present study, HIF-1α, ZEB1,
vimentin, and N-cadherin expression were higher, but cytokeratin-18
expression was lower in T24-L cells compared with T24-P cells.
Furthermore, knockdown endogenous HIF-1α expression significantly
downregulated the expression of ZEB1, accompanied with a decrease
in invasive and metastatic ability in vitro and EMT-related
protein changes in T24-L cells. When T24-P cells were cultured
under hypoxic conditions, HIF-1α expression was induced
responsively; meanwhile, simultaneously, the expression level of
ZEB1, N-cadherin, and vimentin increased, and the expression level
of cytokeratin 18 decreased. The data from the present study
suggests that the promotion of EMT by HIF-1α-mediated induction of
ZEB1 may serve a crucial role in the process of lung
metastasis.
In the present study, it was identified that the
expression of HIF-1α and ZEB1 were associated with each other in
bladder cancer tissue. Furthermore, HIF-1α expression increased the
expression of ZEB1 and promoted EMT, cell migration and invasion in
a bladder cancer cell spontaneous lung metastases model. These
results indicate that HIF-1α serves an important role in the
metastasis of bladder cancer, and that HIF-1α and ZEB1 may be
potential targets for inhibiting bladder metastasis in the
future.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81572520),
the Key Science and Technology Program of Shaanxi Province, China
(grant no. 2015SF176), and the Long-term Scheduled Medical Student
Research Fund (grant no. 15YB05; The First Affiliated Hospital of
Xi'an Jiaotong University, Xi'an, Shaanxi).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Raghavan D, Shipley WU, Garnick MB,
Russell PJ and Richie JP: Biology and management of bladder cancer.
N Engl J Med. 322:1129–1138. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stenzl A, Cowan NC, De Santis M, Jakse G,
Kuczyk MA, Merseburger AS, Ribal MJ, Sherif A and Witjes JA: The
updated EAU guidelines on muscle-invasive and metastatic bladder
cancer. Eur Urol. 55:815–825. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kurian A, Lee J and Born A: Urothelial
bladder cancer with cavitary lung metastases. Can Respir J.
18:e46–e47. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hill RP, Marie-Egyptienne DT and Hedley
DW: Cancer stem cells, hypoxia and metastasis. Semin Radiat Oncol.
19:106–111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adams JM, Difazio LT, Rolandelli RH, Luján
JJ, Haskó G, Csóka B, Selmeczy Z and Németh ZH: HIF-1: A key
mediator in hypoxia. Acta Physiol Hung. 96:19–28. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Joseph JV, Conroy S, Pavlov K, Sontakke P,
Tomar T, Eggens-Meijer E, Balasubramaniyan V, Wagemakers M, den
Dunnen WF and Kruyt FA: Hypoxia enhances migration and invasion in
glioblastoma by promoting a mesenchymal shift mediated by the
HIF1α-ZEB1 axis. Cancer Lett. 359:107–116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu K, Ning Z, Zeng J, Fan J, Zhou J, Zhang
T, Zhang L, Chen Y, Gao Y, Wang B, et al: Silibinin inhibits
β-catenin/ZEB1 signaling and suppresses bladder cancer metastasis
via dual-blocking epithelial-mesenchymal transition and stemness.
Cell Signal. 25:2625–2633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kenney PA, Wszolek MF, Rieger-Christ KM,
Neto BS, Gould JJ, Harty NJ, Mosquera JM, Zeheb R, Loda M, Darling
DS, et al: Novel ZEB1 expression in bladder tumorigenesis. BJU Int.
107:656–663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang T, Fan J, Wu K, Zeng J, Sun K, Guan
Z, Wang X, Hiesh JT and He D: Roles of HIF-1α in a novel optical
orthotopic spontaneous metastatic bladder cancer animal model. Urol
Oncol. 30:928–935. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karam JA, Huang S, Fan J, Stanfield J,
Schultz RA, Pong RC, Sun X, Mason RP, Xie XJ, Niu G, et al:
Upregulation of TRAG3 gene in urothelial carcinoma of the bladder.
Int J Cancer. 128:2823–2832. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Witjes JA, Compérat E, Cowan NC, De Santis
M, Gakis G, Lebret T, Ribal MJ, Van der Heijden AG and Sherif A;
European Association of Urology, : EAU guidelines on
muscle-invasive and metastatic bladder cancer: Summary of the 2013
guidelines. Eur Urol. 65:778–792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schned AR, Andrew AS, Marsit CJ, Kelsey
KT, Zens MS and Karagas MR: Histological classification and stage
of newly diagnosed bladder cancer in a population-based study from
the Northeastern United States. Scand J Urol Nephrol. 42:237–242.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Murai T, Yamada S, Fuchs BC, Fujii T,
Nakayama G, Sugimoto H, Koike M, Fujiwara M, Tanabe KK and Kodera
Y: Epithelial-to-mesenchymal transition predicts prognosis in
clinical gastric cancer. J Surg Oncol. 109:684–689. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pouysségur J, Dayan F and Mazure NM:
Hypoxia signalling in cancer and approaches to enforce tumour
regression. Nature. 441:437–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Denko NC: Hypoxia, HIF1 and glucose
metabolism in the solid tumour. Nat Rev Cancer. 8:705–713. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krishnamachary B, Zagzag D, Nagasawa H,
Rainey K, Okuyama H, Baek JH and Semenza GL: Hypoxia-inducible
factor-1-dependent repression of E-cadherin in von Hippel-Lindau
tumor suppressor-null renal cell carcinoma mediated by TCF3,
ZFHX1A, and ZFHX1B. Cancer Res. 66:2725–2731. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rohwer N, Lobitz S, Daskalow K, Jöns T,
Vieth M, Schlag PM, Kemmner W, Wiedenmann B, Cramer T and Höcker M:
HIF-1alpha determines the metastatic potential of gastric cancer
cells. Br J Cancer. 100:772–781. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deniz H, Karakök M, Yagci F and Güldür ME:
Evaluation of relationship between HIF-1alpha immunoreactivity and
stage, grade, angiogenic profile and proliferative index in bladder
urothelial carcinomas. Int Urol Nephrol. 42:103–107. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng X, Kong DH, Li N, Zong ZH, Liu BQ, Du
ZX, Guan Y, Cao L and Wang HQ: Knockdown of BAG3 induces
epithelial-mesenchymal transition in thyroid cancer cells through
ZEB1 activation. Cell Death Dis. 5:e10922014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu K, Fan J, Zhang L, Ning Z, Zeng J, Zhou
J, Li L, Chen Y, Zhang T, Wang X, et al: PI3K/Akt to
GSK3β/β-catenin signaling cascade coordinates cell colonization for
bladder cancer bone metastasis through regulating ZEB1
transcription. Cell Signal. 24:2273–2282. 2012. View Article : Google Scholar : PubMed/NCBI
|