Introduction
Exosomes are vesicles of 30–100 nm in diameter,
which are secreted from eukaryotic cells. Exosomes contain a
variety of cell membrane molecules and associated proteins, which
occupy a lipid bilayer structure, and they are transferred into the
extracellular space through fusion of membrane intracellular
multivesicular bodies (MVBs) with cell membrane. Many types of
cells can release exosomes in this mode of exocytosis (1,2).
Tumor-derived exosomes (Texos), released from tumor cells into the
extracellular environment, are enriched with tumor-associated
antigens, major histocompatibility complex (MHC) molecules,
tetraspanin [cluster of differentiation (CD) 9 and CD63] and
costimulatory molecules (3). They
induce antitumor immune responses of cytotoxic T lymphocytes (CTLs)
(4). However, the low efficiency of
this process renders it difficult to create an effective cancer
treatment exploiting this process. Therefore, it would be
beneficial to enhance Texo-induced anti-tumor effects and develop
an efficient Texo-based tumor vaccination.
Tumor cells exhibit tumor surface antigens, which
cause specific antitumor immunity when immunological cells identify
antigens. Dendritic cells (DCs) are the most potent antigen
presenting cells (APC), and their primary function is to process
and present antigens to stimulate naive T cell proliferation, and
activation (5). This is critical for
the execution, control and maintenance of immune responses,
including tumor-specific immune responses (4). A previous study demonstrated that Texos
target DCs in vitro, and the Texo-DCs enhanced antitumor
immunity (6). In recent years, the
incidence of cervical cancer in China has been identified as the
second highest in gynecological malignant solid tumors, and the age
of female patients presenting with cervical cancer has decreased
(7). Radiotherapy and chemotherapy
are associated with numerous side effects; therefore, novel
therapeutic approaches are required for the treatment of cervical
cancer.
In the present study, HeLa-exo was isolated from
HeLa cells, and co-cultured with DCs to achieve HeLa-exo loading of
DCs in vitro. Finally, the present study investigated the
effect of HeLa-exo-loaded DC T lymphocyte proliferation and CTL
responses for inhibition of the growth of cervical cancer cells.
The present study aimed to provide experimental evidence for
cervical cancer immunotherapy.
Materials and methods
Isolation and purification of
HeLa-exo
HeLa cells (American Type Culture Collection,
Manassas, VA, USA) were cultured in RPMI-1640 medium (Hyclone; GE
Healthcare, Chicago, IL, USA) supplemented with 10%
heat-inactivated fetal bovine serum (FBS; Si Ji Qing, Hangzhou,
China) and incubated at 37°C in 5% CO2 for 2 h. A total
of 200 ml cell culture supernatant was collected while cells were
in the logarithmic growth phase, and centrifuged at 300 × g at 4°C
for 10 min to remove cells. The supernatant was then centrifuged at
2,000 × g at 4°C for 30 min to remove dead cells, and again at
10,000 × g at 4°C for 30 min to remove cell debris. The supernatant
was collected and transferred into 100 kD Amicon Ultra-15
ultracentrifuge tubes (Merck KGaA, Darmstadt, Germany) and
centrifuged at 1,500 × g at 4°C for 30 min to the volume of
concentrated solution <5 ml. The concentrated solution, 30 g/l
sucrose/water-cushion and 0.01 mol/l PBS were added sequentially to
Beckman ultracentrifuge tubes (Beckman Coulter, Inc., Brea, CA,
USA) at a ratio of 3:3:4, and centrifuged at 100,000 × g and 4°C
for 60 min. Next, the HeLa-exo loaded sucrose was collected,
diluted ×50 with PBS, and centrifuged at 100,000 × g at 4°C for 60
min in ultracentrifuge tubes to form a HeLa-exo pellet. The pellet
was collected and resuspended in PBS. The exosome protein
concentration was determined using a BCA protein assay kit
(Wanleibio, Co., Ltd., Shanghai, China), according to the
manufacturer's protocol. Finally, the HeLa-exo solution was
filtered using a 0.22 µm-diameter filter membrane (Beckman Coulter,
Inc.) and stored at −80°C until use.
Observation of morphological features
of HeLa-exo by TEM
Samples of HeLa-exo protein were fixed in 2.5%
glutaraldehyde and rinsed with PBS. The samples were then mounted
with 1% osmium tetroxide, and washed twice with PBS. The samples
were then dehydrated using a gradient of acetone and ethanol (30%
ethanol, 50% ethanol, 70% ethanol, 80% acetone, 90% acetone and
100% acetone), impregnated with epoxy resin, embedded and
polymerized with paraffin. Finally, the samples were sliced into 70
nm-ultrathin sections for 3% uranyl acetate-lead citrate staining
at 37°C for 30 min, and observed with a JEM-1200EX transmission
electron microscope (magnification, ×20,000) (JEOL, Ltd., Tokyo,
Japan).
Western blot analysis
The concentration of HeLa cells was diluted in
RPMI-1640 medium (Hyclone; GE Healthcare, Chicago, IL, USA) and
adjusted to 1×108 cells/ml, and after 4 rapid
freeze-thaw cycles at −20°C, the cells and media were centrifuged
at 1,000 × g at 4°C for 30 min, providing a supernatant containing
the cell lysate. A BCA protein assay was used to calculate the
protein concentration of the supernatant (Wanleibio, Co., Ltd.),
according to the manufacturer's protocol. A total of 40 µg HeLa-exo
or untreated HeLa cell lysate were subjected to 12% SDS-PAGE, and
the separated proteins were transferred onto cellulose acetate
membranes. Next, the membranes was blocked in 5% skim milk at room
temperature for 2 h, and incubated with anti-CD63 (cat. no. PB0236;
dilution, 1:500) and anti-β-actin (cat. no. BM0627; dilution,
1:500) (both Boster Biological Technology, Pleasanton, CA, USA) at
4°C overnight. The membranes were then washed thrice with Tris-PBS,
prior to incubation with a horseradish peroxidase-labeled goat
anti-rabbit secondary antibody (cat. no. BA1050; dilution, 1:5,000;
Boster Biological Technology) at 37°C for 30 min. The membranes
were washed thrice with Tris-PBS, and ECL luminescent liquid (cat.
no. 34577; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was
used for exposure and development, according to the manufacturer's
protocol.
Induction of DCs from monocytes
All the samples were obtained from 18 patients with
cervical cancer, who were admitted to the Department of Obstetrics,
Xinxiang Central Hospital (mean age, 53.8±3.6 years) between
January 2015 and June 2015 who provided informed consent, and the
following experiments were approved according to Xinxiang Central
Hospital (Xinxiang, China) guidelines. A total of 30 ml each fresh
human peripheral blood sample from 18 patients was divided evenly
and placed into separate 50 ml-centrifuge tubes. PBS was added to
reach a volume of 35 ml, then 15 ml Ficoll separating medium
(Dingguo, Beijing, China) was added to the bottom of the tubes. The
solutions were centrifuged at 300 × g at room temperature for 20
min. The lymphocyte-containing second layer was collected and
placed into a centrifuge tube. Following the addition of 5 ml PBS,
it was further centrifuged at 300 × g at room temperature for 25
min, and then the supernatant was discarded. The remaining cells
were rinsed in PBS and counted on a hemocytometer. D14+ cells were
isolated using a CD14 cell magnetic activated cell sorting system
(Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), according to
the manufacturer's protocol. CD14-cells were stored at −80°C. CD14+
cells were placed in complete RPMI-1640 medium (Hyclone; GE
Healthcare, Chicago, IL, USA, Logan, Utah, USA) supplemented with
10% heat-inactivated FBS (Si Ji Qing, Hangzhou, China) and counted.
Recombinant human granulocyte-macrophage colony stimulating factor,
recombinant human interleukin 4 and tumor necrosis factor α were
all added at 1,000 IU/ml (all from PeproTech, Inc., Rocky Hill, NJ,
USA). Cells were then seeded in 12-well cell culture plates at
1×106 cells/well, and the same concentrations of growth
factors were added after 3 days. After being cultured for 7 days,
1×105 cells were collected for analysis of CD1a
expression by flow cytometry. Finally, 2 wells were selected for
loading of HeLa-exo (10 µg). The cells were cultured with HeLa-exo
for 48 h, prior to harvesting of HeLa-exo-DCs.
T cell proliferation assay
Frozen CD14-cells were recovered and resuspended in
RPMI-1640 medium prior to being filtered through a nylon mesh to
separate the T cells. After 1 h in conventional culture, DCs and T
lymphocytes (Ts) were cultured at a ratio of 1:3. This assay
included 3 groups: A, HeLa-exo-DC+T; B, DC+T; and C, T (negative
control). The cells were plated at 100 µl (1×105
cells)/well in a 96-well culture plate, with six replicates for
each group. The plate was kept at 37°C in 5% CO2. After
3 days, 20 µl MTT was added to each well and the plate was
incubated for another 4 h. A total of 150 µl DMSO (Sigma-Aldrich;
Merck KGaA) was then added into each well and shaken gently to
dissolve the formazan crystals. Absorbance (A) values were measured
by a spectrophotometer at 490 nm.
Cytotoxicity assay
This assay included four groups: Experimental group
[HeLa-exo-DCs+T (1:3)], effector cell control group (T), target
cell control group (HeLa-exo+T), DC negative control group (DCs+T),
with T lymphocytes regarded as effector cells. Next, cells in the
experimental group were plated at 100 µl (1×105
cells)/well in a 96-well culture plate containing HeLa cells.
Experiments were performed with ratios of effector:target cells of
12.5:1, 25:1 and 50:1, and each group had three replicates. The
plate was incubated with interleukin-2 (400 U/ml) (Boster
Biological Technology) at 37°C in 5% CO2. After 48 h, 20
µl MTT was added to each well, and the plate was incubated for
another 4 h. A total of 150 µl DMSO (Sigma-Aldrich; Merck KGaA) was
then added to each well, and the plate was gently agitated at 37°C
for 10 min. Absorbance (A) values were measured by a
spectrophotometer at 570 nm, and used to calculate the percentages
of cytotoxicity according to the following formula: [A value of
HeLa-exo+T group-(A value of HeLa-exo-DCs+T group-A value of T
group)/A value of HeLa-exo+T group] ×100.
Statistical analysis
All statistical analyses were performed using SPSS
17 software (SPSS, Inc., Chicago, IL, USA). Measurement data is
expressed as mean ± standard deviation. Comparisons between 2
groups were performed using Student's t-test, while comparisons
among ≥3 groups were analyzed using one-way analysis of variance
followed by the post hoc test with Student-Newman-Keuls test.
P<0.05 was considered to indicate a statistically significant
difference. A χ2 test was also performed on cytotoxicity
data, and the significance threshold of pairwise comparison was
adjusted to P<0.01 with Bonferroni correction method.
Results
Identification of HeLa-exo
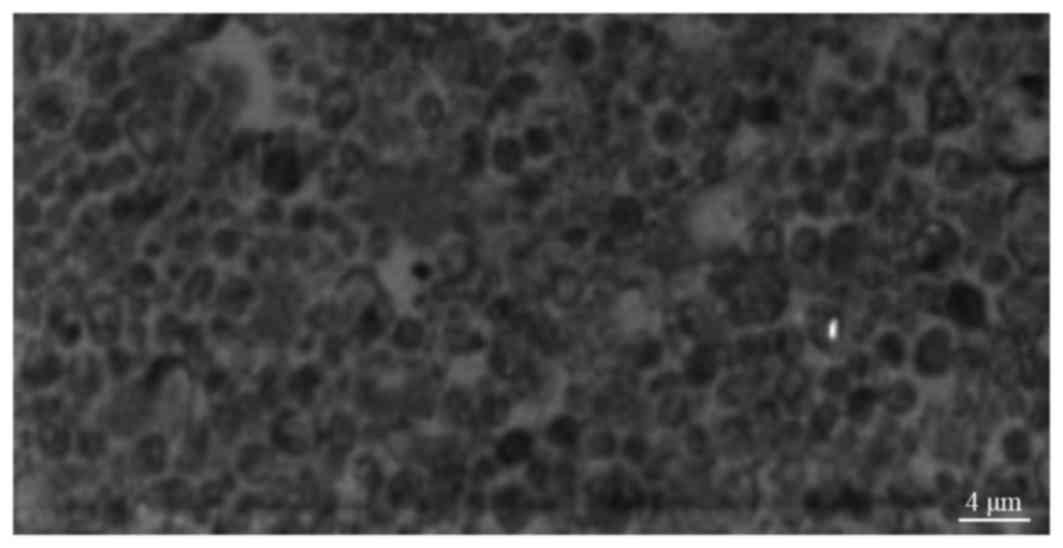
As demonstrated in Fig.
1, HeLa-derived exosomes exhibited a circular or elliptical
shape, were relatively uniform in size and had a diameter of 30–100
(57.3±4.2) nm. HeLa-exo were surrounded by a lipid membrane and had
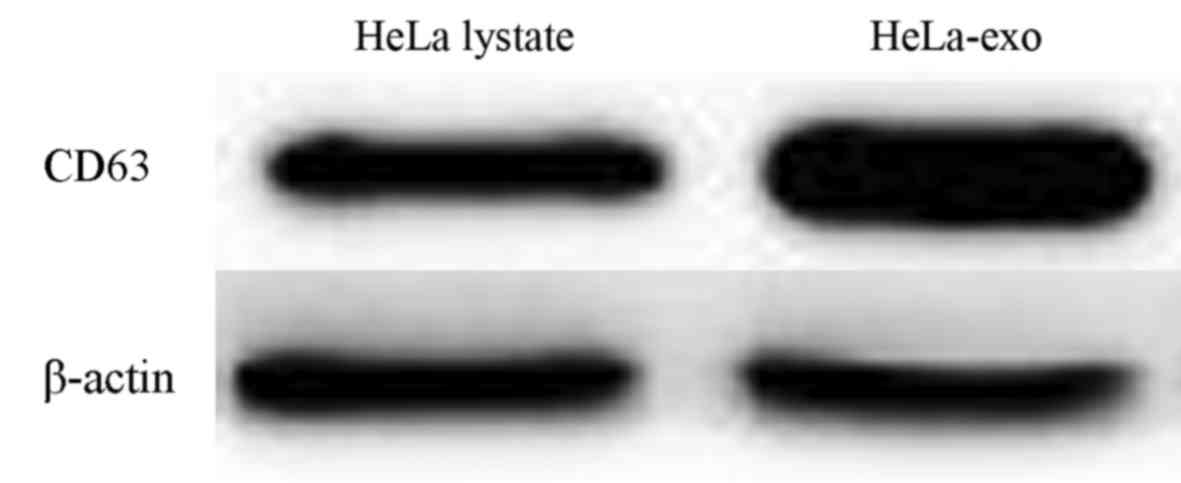
an inner low electron density membrane. Both HeLa-exo and HeLa cell
lysates expressed CD63; however, CD63 expression of the
tumor-derived exosomes was markedly higher compared with that of
tumor cell lysates (Fig. 2).
DC phenotyping
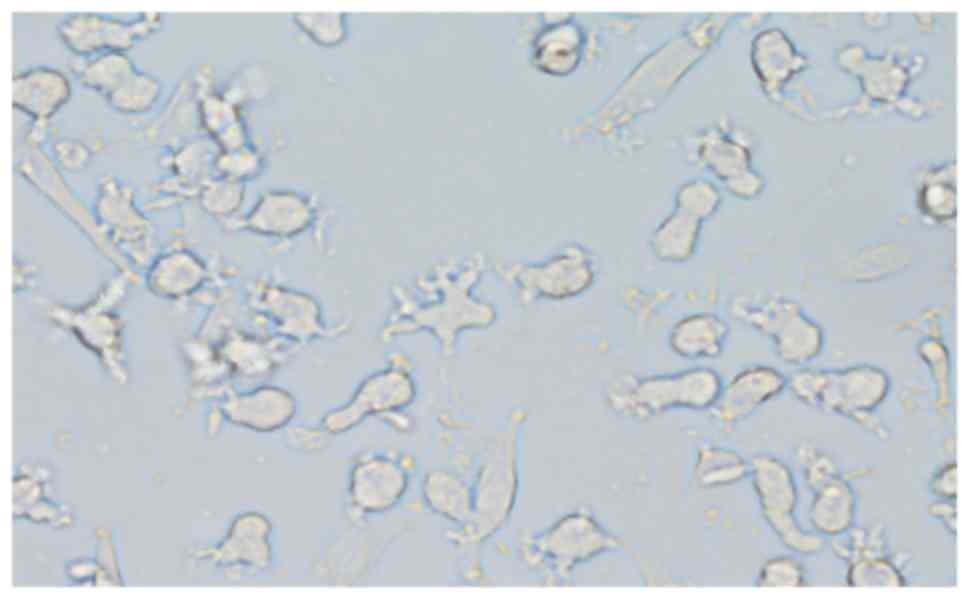
After 7 days of culture, adherent CD14+ cell
aggregates were visible under a light microscope, presenting
typical dendritic processes of different sizes, and some of the
cells were forming colonies (Fig. 3).
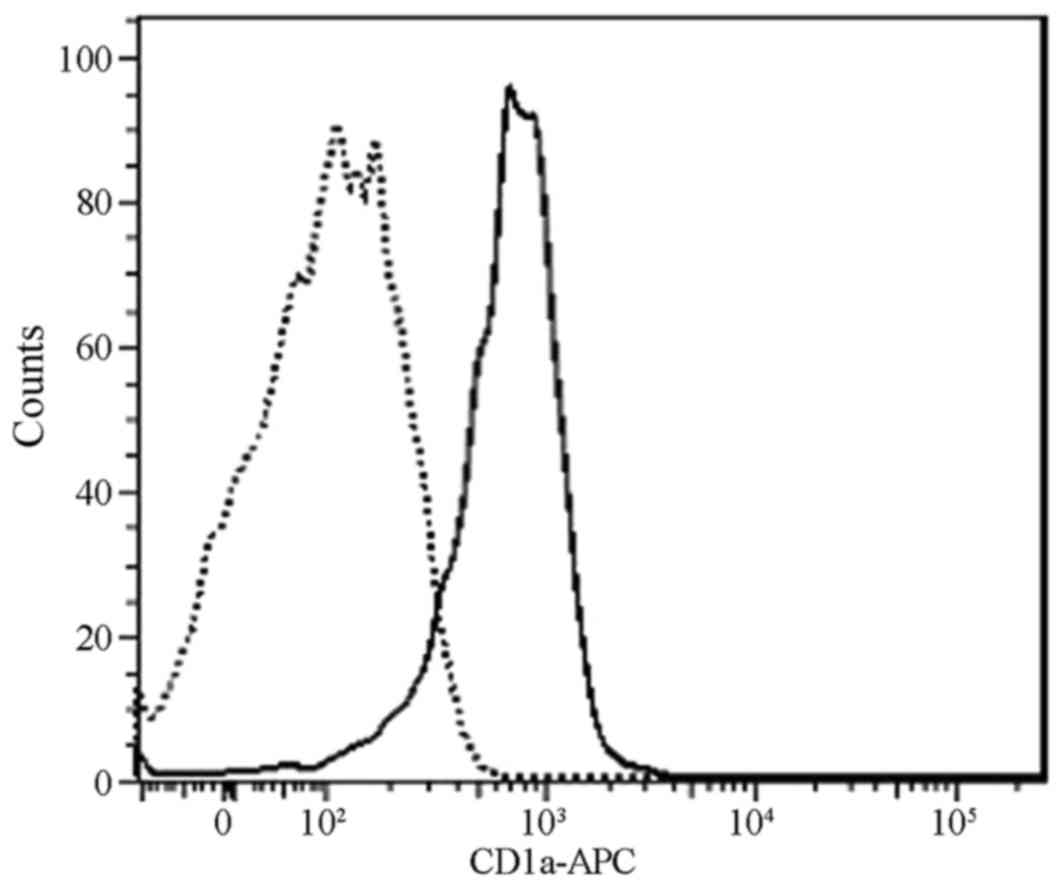
As demonstrated in Fig. 4, flow
cytometry analysis detected CD1a marker expression on the surface
of the cells, indicating that the DC induction was successful.
DCs loaded with HeLa-exo stimulate T
cell proliferation
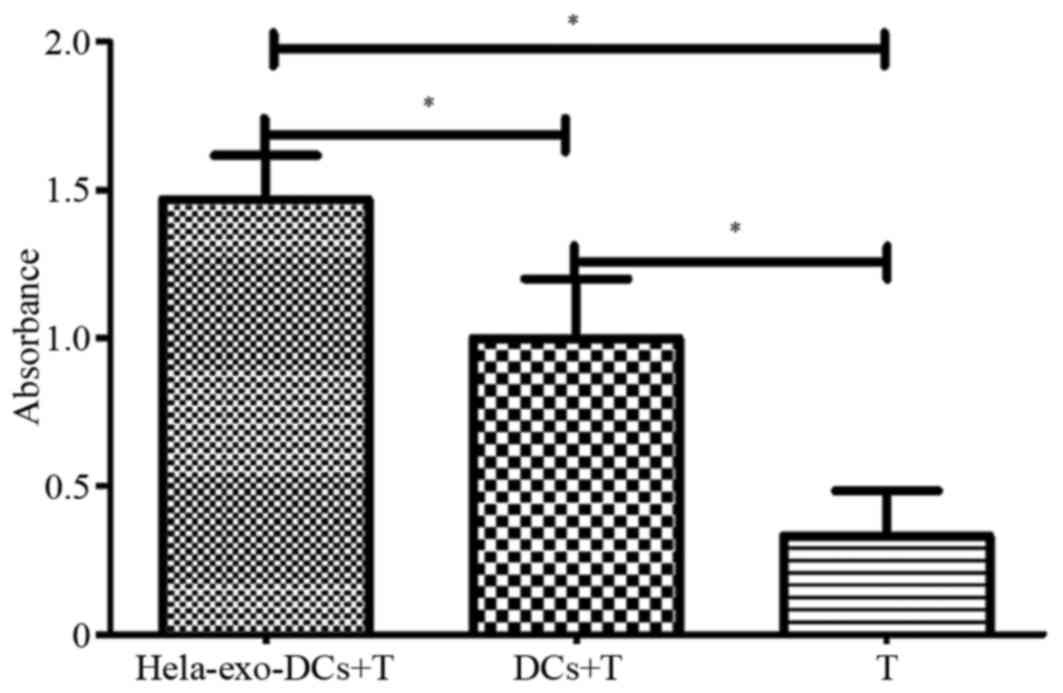
As presented in Fig.
5, the number of T cells in the HeLa-exo-DC+T and DC+T groups
increased significantly compared with the T group (P<0.05).
Furthermore, the number of T cells identified in the HeLa-exo-DC+T
group was significantly increased compared with the DC+T group
(P<0.05). This indicates that loading DC with HeLa-exo
significantly enhanced the ability to stimulate T cell activation
compared with DCs without HeLa-exo loading (Fig. 5).
HeLa-exo-DCs induce CTL specific
cytotoxicity
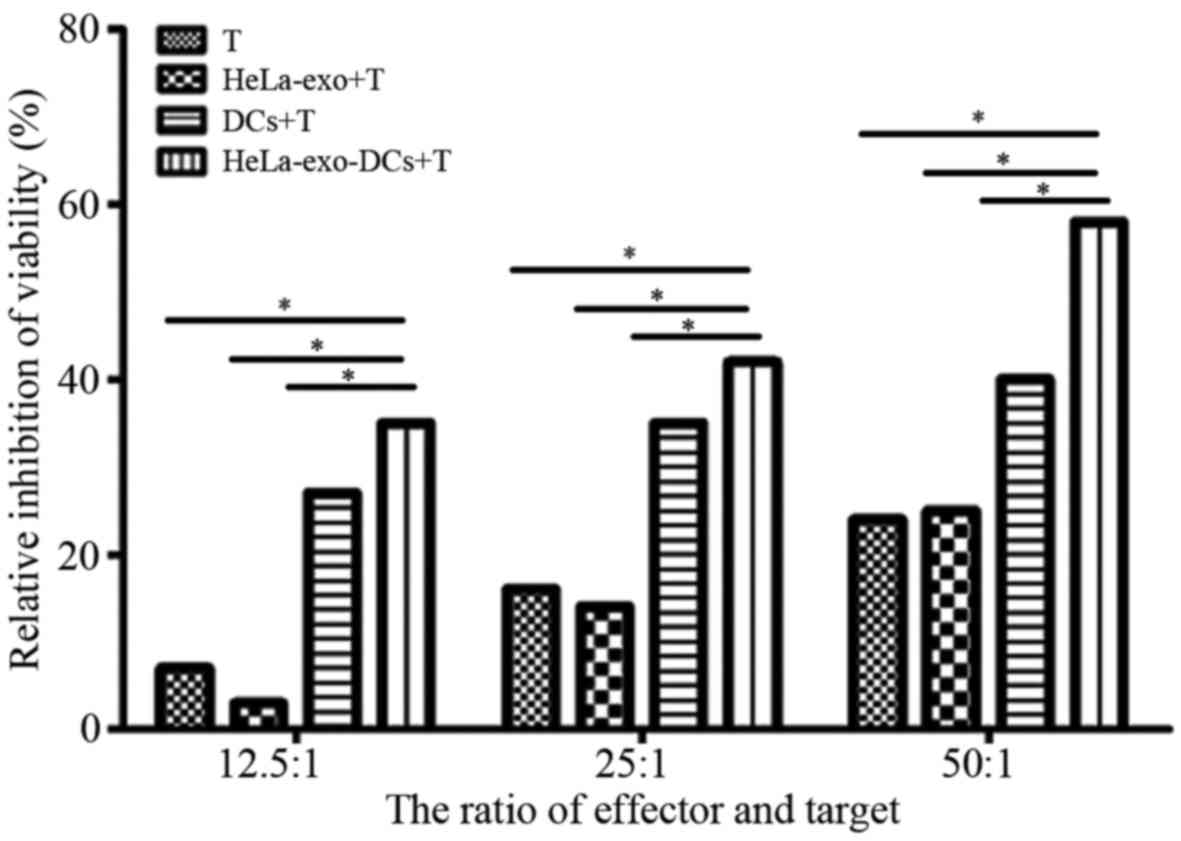
As demonstrated in Table
I, with an effector:target ratio of 12.5:1, 25:1 or 50:1, the
anti-HeLa activity induced by DCs loaded with HeLa-exo in
HeLa-exo-DCs+T group was significantly increased compared with all
other groups (P<0.01; Table I;
Fig. 6).
 | Table I.Percentage inhibition of HeLa cell
growth following induction of CTL activity by Hela-exo loaded
DCs. |
Table I.
Percentage inhibition of HeLa cell
growth following induction of CTL activity by Hela-exo loaded
DCs.
|
| Percentage
inhibition |
|
|---|
|
|
|
|
|---|
| Effector:target
ratio | A: T | B: Hela-exo+T | C: DCS+T | D:
Hela-exo-DCs+T | χ2
(P-value) |
|---|
| 12.5:1 | 7.3 | 3.1a | 27.2a,b | 38.1a–c | 53.556
(<0.001) |
| 25:1 | 16.3 | 12.5a | 38.3a,b | 41.6a,b | 33.626
(<0.001) |
| 50:1 | 22.2 | 23.8 | 39.3a,b | 58.5a-c | 37.193
(<0.001) |
Discussion
Cervical cancer is one of the most common types of
malignant tumor identified in women (8). Although conventional surgical treatment
may effectively remove visible tumor masses, surgery does not
ensure the removal of all small lesions or metastases. Radiotherapy
and chemotherapy have limited efficacy as adjuvant therapies for
patients who are resistant to chemotherapy drugs or who cannot
tolerate their side effects. Immunotherapy remains as a treatment
option. DC tumor vaccines may efficiently deliver specific antigens
for naïve T cell activation for antigen-specific CTL responses, in
order to induce lasting tumor-specific immune responses in patients
with cervical cancer (9).
Tumor-derived exosomes have been a popular research
topic in recent years due to their potential to be used in
anti-tumor vaccines (3,4). An exosome is a microcapsule membranous
structure secreted from living cells and its functions vary
depending on the cell source (10–12).
Previous studies have primarily focused on exosomes derived from
tumor cells and DCs. Tumor-derived exosomes, containing MHC-I
molecules, tetraspanin (CD9 and CD63) and tumor-associated
antigens, activate T lymphocyte proliferation and CTL responses,
but only at low levels (13,14). However, following DC loading, such
exosomes are able to activate T lymphocyte responses, which serve
anti-tumor immune effects (15,16).
In the present study, ultrafiltration centrifugation
and sucrose density gradient ultracentrifugation were applied to
isolate HeLa-exo from the supernatant of HeLa cells. Morphological
investigation by TEM revealed that HeLa-exo exhibited a circular or
elliptical shape and a relatively uniform size, with a diameter of
30–100-nm. The cells were surrounded by a lipid outer-membrane and
a low electron density inner-membrane. Furthermore, expression of
CD63 molecules was visible on the surface of DCs, which was
consistent with the results of previous literature (17,18).
Subsequent to cytokine DC induction from peripheral blood
mononuclear cells derived from patients with cervical cancer, the
DCs were loaded with HeLa-exo in vitro. The present study
investigated the effects of DCs loaded with HeLa-exo on T cell
activation and proliferation, and the cytotoxic effects of
activated T cells on cervical cancer cells. The results
demonstrated that HeLa-exo alone was not able to stimulate T cell
proliferation, but DCs loaded with HeLa-exo and DCs without
HeLa-exo were able to stimulate T cell proliferation. DCs loaded
with HeLa-exo had an enhanced ability to stimulate T cell
activation compared with DCs without HeLa-exo. Cytotoxicity assays
revealed that DCs loaded with HeLa-exo had a strong cytotoxic
ability compared with the T group and DCs without HeLa-exo had no
significant anti-tumor effect compared with the T group. These
results suggest that HeLa-exo-DC induces T lymphocyte activation,
and that activated CTLs exhibit an anti-tumor effect, which
inhibits the growth of HeLa cells.
HeLa-exo alone are insufficient to induce CTL
responses, because T cell activation required DCs, as well as
MHC-1, and tumor antigens, to provide costimulatory signals. Thus,
only when loaded into DCs could HeLa-exo efficiently induce the CTL
anti-tumor effect.
In conclusion, the present study successfully
isolated exosomes from HeLa cells, and confirmed that DCs loaded
with HeLa-derived exosomes induce CTL activity, enhancing the
anti-tumor immune response. This provides basis for research into
HeLa-exo use for cancer immunotherapy.
References
|
1
|
Lu M, Huang B, Hanash SM, Onuchic JN and
Ben-Jacob E: Modeling putative therapeutic implications of exosome
exchange between tumor and immune cells. Proc Natl Acad Sci USA.
111:pp. E4165–E4174. 2014; View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hannafon BN and Ding WQ: Intercellular
communication by exosome-derived microRNAs in cancer. Int J Mol
Sci. 14:14240–14269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duvallet E, Boulpicante M, Yamazaki T,
Daskalogianni C, Prado Martins R, Baconnais S, Manoury B, Fahraeus
R and Apcher S: Exosome-driven transfer of tumor-associated Pioneer
Translation Products (TA-PTPs) for the MHC class I
cross-presentation pathway. Oncoimmunology. 5:e11988652016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tauro BJ, Greening DW, Mathias RA,
Mathivanan S, Ji H and Simpson RJ: Two distinct populations of
exosomes are released from LIM1863 colon carcinoma cell-derived
organoids. Mol Cell Proteomics. 12:587–598. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shang N, Figini M, Shangguan J, Wang B,
Sun C, Pan L, Ma Q and Zhang Z: Dendritic cells based
immunotherapy. Am J Cancer Res. 7:2091–2102. 2017.PubMed/NCBI
|
|
6
|
Marleau AM, Chen CS, Joyce JA and Tullis
RH: Exosome removal as a therapeutic adjuvant in cancer. J Transl
Med. 10:1342012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li H, Duan D, Xu J, Gong Q, Wang Y, Ji W,
DU L, Han L and Xu G: The incidence and mortality of cervical
cancer in ningbo during 2006–2014, China. Iran J Public Health.
46:1324–1331. 2017.PubMed/NCBI
|
|
8
|
Jiang X, Tang H and Chen T: Epidemiology
of gynecologic cancers in China. J Gynecol Oncol. 29:e72018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Santos PM and Butterfield LH: Dendritic
cell-based cancer vaccines. J Immunol. 200:443–449. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wahlgren J, Karlson Tde L, Glader P,
Telemo E and Valadi H: Activated human T cells secrete exosomes
that participate in IL-2 mediated immune response signaling. PLoS
One. 7:e497232012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
King HW, Michael MZ and Gleadle JM:
Hypoxic enhancement of exosome release by breast cancer cells. BMC
Cancer. 12:4212012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei Y, Li M, Cui S, Wang D, Zhang CY, Zen
K and Li L: Shikonin inhibits the proliferation of human breast
cancer cells by reducing tumor-derived exosomes. Molecules.
21(pii): E7772016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hayoun D, Kapp T, Edri-Brami M, Ventura T,
Cohen M, Avidan A and Lichtenstein RG: HSP60 is transported through
the secretory pathway of 3-MCA-induced fibrosarcoma tumour cells
and undergoes N-glycosylation. FEBS J. 279:2083–2095. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hartman ZC, Wei J, Glass OK, Guo H, Lei G,
Yang XY, Osada T, Hobeika A, Delcayre A, Le Pecq JB, et al:
Increasing vaccine potency through exosome antigen targeting.
Vaccine. 29:9361–9367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marton A, Vizler C, Kusz E, Temesfoi V,
Szathmary Z, Nagy K, Szegletes Z, Varo G, Siklos L, Katona RL, et
al: Melanoma cell-derived exosomes alter macrophage and dendritic
cell functions in vitro. Immunol Lett. 148:34–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bu N, Wu H, Sun B, Zhang G, Zhan S, Zhang
R and Zhou L: Exosome-loaded dendritic cells elicit tumor-specific
CD8+ cytotoxic T cells in patients with glioma. J Neurooncol.
104:659–667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sung BH, Ketova T, Hoshino D, Zijlstra A
and Weaver AM: Directional cell movement through tissues is
controlled by exosome secretion. Nat Commun. 6:71642015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
del Cacho E, Gallego M, Lillehoj HS,
Quilez J, Lillehoj EP and Sánchez-Acedo C: Tetraspanin-3 regulates
protective immunity against Eimeria tenella infection following
immunization with dendritic cell-derived exosomes. Vaccine.
31:4668–4674. 2013. View Article : Google Scholar : PubMed/NCBI
|