Introduction
Retinoblastoma is a type of embryonic malignant
tumor of the retina of the eye, which originates from primitive
stem cells (1). Retinoblastoma
primarily occurs in childhood, with an average incidence of between
1/15,000 and 1/20,000 live births, typically arising in infants
<6 years of age, often prior to the age of 2 years (2). Advanced retinoblastoma is able to
rapidly cover the eye, invade the optic nerve and eventually spread
to the central nervous system. Previous evidence from molecular,
cellular and cytogenetic studies has indicated that retinoblastoma
is typically caused by a mutation in the RB transcriptional
co-repressor 1 (RB1) gene on chromosome 13 (3–5).
RB1 inactivation results in mitotic defects, contributing to
genomic instability, which manifests as aneuploidy and chromosomal
rearrangements (6,7). At present, non-metastatic retinoblastoma
is not considered a fatal childhood cancer and is effectively
treated by enucleation, dependent on an early diagnosis and
accurate prognosis of the disease (8). Dalgard et al (9) observed that the retinoblastoma cell
lines Y79 and Weri-Rb1, and retinoblastoma tumor samples, presented
with aberrant microRNA (miR)-34a and miR-34b expression. In
addition, this study provided indicated that knockdown of miR-34a
may result in increased retinoblastoma cell proliferation and
chemotherapeutic resistance.
The aim of the present study was to investigate long
non-coding RNA (lncRNA) H19-mediated regulation of the
development of retinoblastoma in clinical samples and cell lines.
LncRNAs are non-protein-coding transcripts, which are >200
nucleotides in length, and regulate gene expression
transcriptionally or post-transcriptionally. These intergenic
transcripts are involved in diverse cellular processes, including
proliferation, migration, invasion, apoptosis and the reprogramming
of stem cell pluripotency (10–13).
LncRNA H19 has been considered as an oncogenic lncRNA in
multiple types of cancer, including hepatocellular, bladder
carcinoma, breast cancer, bladder tumor and glioma (14–17).
Furthermore, previous studies reported that H19 regulates
the development of gliomas via interactions with miR-675, which in
addition contributes to the proliferation of gastric cancer cells
and was associated with tumor metastasis (18–20).
Emerging evidence has also demonstrated that H19 expression
is upregulated, and is involved, in carcinogenesis and cancer
metastasis via the promotion of cell cycle progression (21). However, the function of H19 in
retinoblastoma remains unclear.
In the present study, the clinical characteristics,
biological function and potential underlying molecular mechanisms
of lncRNA H19 in retinoblastoma were investigated. The
results indicated that H19 levels were markedly increased in
retinoblastoma cells and tissues. Furthermore, patients with
retinoblastoma with increased lncRNA H19 expression
experienced poorer survival time compared with patients with lower
levels of lncRNA H19 expression. Furthermore, lncRNA
H19 expression was also associated with several proteins,
including cyclin-dependent kinase 1 (CDK1), B-cell lymphoma
2-associated X, apoptosis regulator (Bax), tumor protein p53 (p53),
vimentin, cadherin 13 (CDH13) and matrix metalloproteinase 9
(MMP9). The oncogenic function of H19 suggests that it may
serve as a potential target for retinoblastoma therapy and
prognostic prediction.
Materials and methods
Cell lines and tumor tissues
The present study was approved by the Research
Ethics Committee of Tianjin Eye Hospital (Tianjin, China). Written
informed consent for all biological procedures was obtained from
each patient, or their parents, and specimens for the present study
were anonymized. Patients who had received treatment prior to
surgery were excluded from the present study. The tumor samples
were collected between June 2011 until November 2015 and were
extracted from enucleated eyes and immediately snap-frozen in
liquid nitrogen and stored at −80°C. A total of 80 freshly frozen
retinoblastoma tissue samples (44 males and 36 females; the age
distribution: 40 patients ≥2 years and 40 patients <2 years),
and five normal retina samples (3 males and 2 females; the age
distribution: 2 patients ≥2 years and 3 patients <2 years)
obtained from ruptured globes, were collected at Tianjin Eye
Hospital (Tianjin, China).
Cell culture
The cell lines used in the present study were
purchased from the Institute of Biochemistry and Cell Biology of
the Chinese Academy of Sciences (Shanghai, China). The human
retinal pigment epithelial cell line ARPE-19 was cultured in
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin (Invitrogen, Thermo Fisher Scientific, Inc.), and 100
mg/ml streptomycin (Invitrogen, Thermo Fisher Scientific, Inc.).
The human retinal microvascular endothelial cell line (HRMEC) was
maintained in Endothelial-Cell Growth medium (EGM-2 SingleQuot Kit
Supplement & Growth Factors; Lonza Group, Ltd., Basel,
Switzerland), supplemented with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin (Invitrogen,
Thermo Fisher Scientific, Inc.), and 100 mg/ml streptomycin
(Invitrogen, Thermo Fisher Scientific, Inc.). The retinoblastoma
cell lines Weri-Rb1 and Y79 were maintained in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS,
100 U/ml penicillin (Invitrogen, Thermo Fisher Scientific, Inc.),
and 100 mg/ml streptomycin (Invitrogen, Thermo Fisher Scientific,
Inc.). All cell cultures were incubated at 37°C in a humidified
atmosphere containing 5% CO2.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. RNA was purified and then
reverse-transcribed into cDNA using a PrimeScript RT Reagent kit
(Takara Biotechnology Co., Ltd., Dalian, China) according to the
manufacturers protocol. qPCR was performed using SYBR Premix Ex Taq
(Takara Biotechnology Co., Ltd.), according to the manufacturer's
protocol, and run on an ABI Prism 7000 Sequence Detection System.
Relative expression of lncRNA H19 (primer: Forward,
5′-ATCGGTGCCTCAGCGTTCGG-3′; and reverse, 5′-CTGTCCTCGCCGTCACACCG-3)
was normalized to the expression of GAPDH (primer: Forward,
5′-AGCCACATCGCTCAGACAC-3′ and reverse, 5′-GCCCAATACGACCAAATCC-3′).
Relative gene expression levels were quantified using the
2−ΔΔCq method (22). The
thermocycling conditions for lncRNA H19 quantification were
as follows: 95°C for 10 min; 40 cycles of 95°C for 15 sec and 60°C
for 1 min. Each sample was examined in triplicate.
Small interfering (si)RNA
transfection
RNA interference was conducted using synthetic siRNA
duplexes. Two synthetic siRNA duplexes (si-H19) corresponding to
the H19 mRNA sequences, 5′-CCCACAACAUGAAAGAAAU-3′ (forward)
and 5′-GCUAGAGGAACCAGACCUU-3′ (reverse), were used to inhibit
H19 RNA expression. si-H19 and si-negative control (NC) were
purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
Cells were cultured in 6-well plates and maintained until they
reached ~60% confluence, prior to transfection with siRNA duplexes
(25 nM) using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Transfection efficiency to assess the inhibition of H19
after 48 h of transfection in Y79 cells was performed using RT-qPCR
as previously outlined.
Flow cytometric analysis
Cell apoptosis was examined by flow cytometry.
Weri-Rb1 or Y79 cells were seeded at a density of 1×104
cells/well in 96-well plates. In brief, cells were transfected with
si-H19 or si-NC as aforementioned and, 2 days post-transfection,
cells were trypsinized by 0.25% Trypsin (Gibco; Thermo Fisher
Scientific, Inc.), followed by centrifugation at 350 × g for 2 min.
and washed with PBS. Cells were then resuspended in PBS and fixed
with 100% ethanol at 4°C overnight, and apoptosis was detected
using a dual-staining method with annexin V-fluorescein
isothiocyanate in combination with propidium iodide (D Pharmingen,
San Diego, CA, USA). Apoptosis was analyzed using a FACScan flow
cytometer and Cell Quest analysis software version 5.2 (BD
Biosciences, Franklin Lakes, NJ, USA). Each experiment was run in
quadruplicate for each condition.
Cell proliferation analysis
Weri-Rb1 or Y79 cells were transfected with si-H19
or si-NC for 24 h, and then seeded in 6-well plate with a density
of 0.5×103 cells per well and cultured for 7–10 days
with RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS, 100 U/ml penicillin (Invitrogen, Thermo
Fisher Scientific, Inc.), and 100 mg/ml streptomycin (Invitrogen,
Thermo Fisher Scientific, Inc.). The medium was replenished every
two days. Colonies were then fixed for 5 min at room temperature
with 10% formaldehyde, stained with 1.0% crystal violet for 30 sec
at room temperature and counted under a light microscope (Olympus,
Tokyo, Japan) in five predetermined fields of interest at ×200
magnification. An MTT assay was performed using the Cell
Proliferation kit I (Roche Diagnostics, Basel, Switzerland)
according to the manufacturer's protocol. Briefly, 0.5 mg/ml MTT
and 100% dimethylsulfoxide reagent were added to the cells at the
indicted times. The optical density was measured at 590 nm using
the Tecan SpectraFluor Microplate Reader (Tecan Group, Ltd.,
Mannedorf, Switzerland). Experiments were performed three
times.
Cell migration and invasion
assays
Cell migration and invasion assays were performed
using Transwell insert chambers (Costar; Corning Incorporated,
Corning, NY, USA), In brief, Weri-Rb1 or Y79 cells were seeded at a
density of 1×104 cells/100 ml RPMI-1640 medium, without
FBS, on a fibronectin-coated polycarbonate membrane insert in a
Transwell apparatus (Costar; Corning Incorporated) and the lower
chamber was filled with 500 µl RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 20% FBS, 100 U/ml
penicillin (Invitrogen, Thermo Fisher Scientific, Inc.), and 100
mg/ml streptomycin (Invitrogen, Thermo Fisher Scientific, Inc.).
Cells were incubated for 12 h at 37°C in a humidified atmosphere
containing 5% CO2. And then the cells adhering to the
lower surface were fixed with 100% methanol for 10 min at room
temperature, stained with Giemsa solution at room temperature for
10 min and washed with PBS three times. The cells colonies were
then counted under a light microscope (Olympus Corporation, Tokyo,
Japan) in five predetermined fields of interest at ×200
magnification. The cell invasion assay was performed as
aforementioned, with the exception that the Transwell membranes
were precoated with 24 mg/ml Matrigel (R&D Systems, Inc.,
Minneapolis, MN, USA). Cells were counted as aforementioned.
Experimental data are representative of a minimum of three
independent repeats.
In vivo growth assay
A total of 40 female nude mice (18–20 g,
Balb/C-nu/nu athymic; 4–5 weeks old) were purchased from the Animal
Center of the Cancer Institute of Chinese Academy of Medical
Science (Beijing, China). The mice were maintained in a facility
under specific pathogen-free conditions and an allowed free access
to pathogen free laboratory chow, allowed free access to autoclaved
water in an air-conditioned room with a 12 h light/dark cycle. Mice
were divided into an si-H19 group and an si-NC group (with each
group containing 20 mice). Suspensions of 1×107 cells
(Y79 cells transfected with siRNA duplexes) were subcutaneously
injected into the upper back of the female nude mice. Mice were
monitored daily. Tumor samples were carefully removed 21 days after
injection and the size was calculated on the basis of width
(x) and length (y): x2y/2,
where x<y. The humane endpoints for the present
study was that the mean diameter of the tumor did not exceed 1.2 cm
in mice. Ethical approval for the animal experiments was granted by
the Animal Ethical and Welfare Committee of Tianjin Medical
University (Tianjin, China).
Western blotting
Total protein was extracted from transfected cells
with radioimmunoprecipitation lysis buffer (http://www.keygentec.com.cn/; KenGEN, Nanjing,
Jiangsu, China) for 30 min at 4°C, and centrifuged at 12,000 × g
for 10 min at 4°C. The supernatant fraction was collected and then
quantified using a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology, Haimen, China). Protein lysates (30 mg)
were separated by 12% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes (Merck KGaA, Darmstadt, Germany) at 30 V
overnight. The membranes were blocked with 5% non-fat milk in TBST
for 1 h at room temperature and gentle shaking, and then probed
with antibodies against epithelial (E-)cadherin (cat. no. ab15148,
dilution 1:1,000; Abcam, Cambridge, UK), MMP9 (cat. no. sc-10737,
dilution 1:200; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
CDH13 (cat. no. sc-7940, dilution 1:200; Santa Cruz Biotechnology,
Inc.), vimentin (cat. no. sc-5565, dilution 1:300; Santa Cruz
Biotechnology, Inc.) and GAPDH (cat. no. sc-25778, dilution
1:2,500; Santa Cruz Biotechnology, Inc.) at 4°C overnight.
Following washing 3 times with TBS 0.1% Tween 20, the membranes
were incubated with goat anti-rabbit horseradish
peroxidase-conjugated secondary antibody (cat. no. 7074, Cell
Signaling Technology, Danvers, MA, USA) at a dilution of 1:5,000
for 1 h at room temperature. Band detection was performed using an
enhanced chemiluminescence kit (Pierce; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol.
Statistical analysis
Statistical analyses were performed using the SPSS
software package 17.0 (SPSS, Inc., Chicago, IL, USA). Results are
expressed as the mean ± standard deviation. Data analysis was
performed using Student's t-test (two-tailed) and one-way-analysis
of variance followed by Tukey's multiple comparisons test as a
post-hoc test. Univariate and multivariate relative risks were
calculated using the Cox proportional hazards regression. P<0.05
was considered to indicate a statistically significant
difference.
Results
lncRNAH19 is overexpressed in
retinoblastoma tissues and cell lines
To explore whether lncRNA H19 had an effect
on the malignant phenotype of retinoblastoma, the expression
pattern of H19 in retinoblastoma tissues and cell lines was
analyzed. The results from the present study indicated that
H19 expression was significantly increased in retinoblastoma
tissues compared with non-tumor retinal tissues (P<0.001;
Fig. 1A). In addition, lncRNA
H19 expression was upregulated in Weri-Rb1 and Y79
retinoblastoma cells compared with ARPE-19 and HRMECs (P<0.05;
Fig. 1B).
lncRNA H19 is associated with
retinoblastoma progression
To investigate the association between lncRNA
H19 and retinoblastoma further, the overall survival times
of patients with retinoblastoma were analyzed in accordance with
H19 expression levels. According to classification by
Motoyama et al (23), 80
retinoblastoma tissue samples were divided into the high-expression
group (n=38) or the low-expression group (n=42). As presented in
Table I, the associations between
lncRNA H19 expression and tumor size (<10 vs. ≥10 mm;
P=0.007), optic nerve invasion (positive vs. negative; P=0.013) and
choroidal invasion (positive vs. negative; P=0.043) were
statistically significant. However, no significant associations in
age (P=0.263), sex (P=0.750), laterality (P=0.626) or pathological
grade (P=0.282) were observed. Overall survival analysis
demonstrated that the level of lncRNA H19 expression was
significantly associated with the overall survival rate of
retinoblastoma patients, as patients with high lncRNA H19
levels had a poorer survival rate compared with those with low
lncRNA H19 levels (P<0.001; Fig. 1C). Simultaneously, multivariate
analysis was applied to evaluate the association between lncRNA
H19 expression and prognostic factors for overall survival
rates. The results presented in Table
II demonstrated that increased lncRNA H19 expression was
a poor independent prognostic factor for patients with
retinoblastoma (P<0.040).
 | Table I.Association between lncRNA H19
expression and clinicopathological characteristics in
retinoblastoma. |
Table I.
Association between lncRNA H19
expression and clinicopathological characteristics in
retinoblastoma.
|
|
| lncRNA H19
expression |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Number | High | Low | P-value |
|---|
| Age, years |
|
|
|
|
|
<2 | 40 | 22 (55) | 18 (45) | 0.263 |
| ≥2 | 40 | 16 (40) | 24 (60) |
|
| Sex |
|
|
|
|
|
Male | 44 | 18 (40.9) | 26 (59.1) | 0.750 |
|
Female | 36 | 16 (44.4) | 20 (55.6) |
|
| Tumor size, mm |
|
|
|
|
|
<10 | 33 | 11 (33.3) | 22 (66.7) | 0.007a |
|
≥10 | 47 | 30 (63.8) | 17 (36.2) |
|
| Choroidal
invasion |
|
|
|
|
|
Negative | 45 | 18 (40) | 27 (60) | 0.043a |
|
Positive | 35 | 22 (62.9) | 13 (37.1) |
|
| Optic nerve
invasion |
|
|
|
|
|
Negative | 47 | 26 (55.3) | 31 (44.7) | 0.013a |
|
Positive | 43 | 24 (72.7) | 9 (27.3) |
|
| Laterality |
|
|
|
|
|
Unilateral | 56 | 27 (48.2) | 29 (51.8) | 0.626 |
|
Bilateral | 24 | 13 (54.2) | 11 (45.8) |
|
| Pathological
grade |
|
|
|
|
|
Well-differentiated | 43 | 24 (55.8) | 19 (44.2) | 0.282 |
|
Poorly | 37 | 25 (67.6) | 12 (32.4) |
|
 | Table II.Univariate and multivariate Cox's
regression of prognostic factors for overall survival in
retinoblastoma. |
Table II.
Univariate and multivariate Cox's
regression of prognostic factors for overall survival in
retinoblastoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years |
|
|
|
|
|
|
| <2
vs. ≥2 | 1.046 | 0.425–2.433 | 0.875 |
|
|
|
| Sex |
|
|
|
|
|
|
| Male
vs. female | 1.204 | 0.569–2.364 | 0.640 |
|
|
|
| Size, mm |
|
|
|
|
|
|
| <10
vs. ≥10 | 5.012 | 2.011–12.04 | 0.001a | 2.01 | 0.715–7.140 | 0.249 |
| Choroidal
invasion |
|
|
|
|
|
|
|
Negative vs. positive | 4.497 | 1.978–10.013 |
<0.001a | 1.848 | 0.695–4.827 | 0.213 |
| Optic nerve
invasion |
|
|
|
|
|
|
|
Negative vs. positive | 4.098 | 1.457–5.98 | 0.001a | 3.126 | 1.321–6.015 | 0.038a |
| Laterality |
|
|
|
|
|
|
|
Bilateral vs. unilateral | 1.654 | 0.871–2.923 | 0.397 |
|
|
|
| Pathological
grade |
|
|
|
|
|
|
|
Well-differentiated vs.
poorly | 0.818 | 0.353–1.857 | 0.270 |
|
|
|
|
LncRNA-H19 |
|
|
|
|
|
|
| Low vs.
high | 5.124 | 2.324–11.532 |
<0.001a | 2.912 | 1.021–8.452 | 0.039a |
Knockdown of lncRNA H19 decreases
retinoblastoma cell proliferation
To elucidate the function of H19 on
retinoblastoma cell proliferation, loss-of-function analysis using
retroviral transduction of H19 in Weri-Rb1 and Y79 cells was
performed. qPCR analysis was performed to confirm that H19
expression was significantly inhibited following knockdown
(Fig. 2A). The MTT assay results
revealed that the viability of cells (Weri-Rb1 and Y79 cells)
transfected with si-H19 was significantly decreased compared with
controls, at 48- and 72 h post-transfection (Fig. 2B and C). In addition, the results of
the cell colony assay demonstrated that inhibition of H19
expression resulted in decreased cell proliferation in Weri-Rb1 and
Y79 cells compared with controls (Fig.
2D).
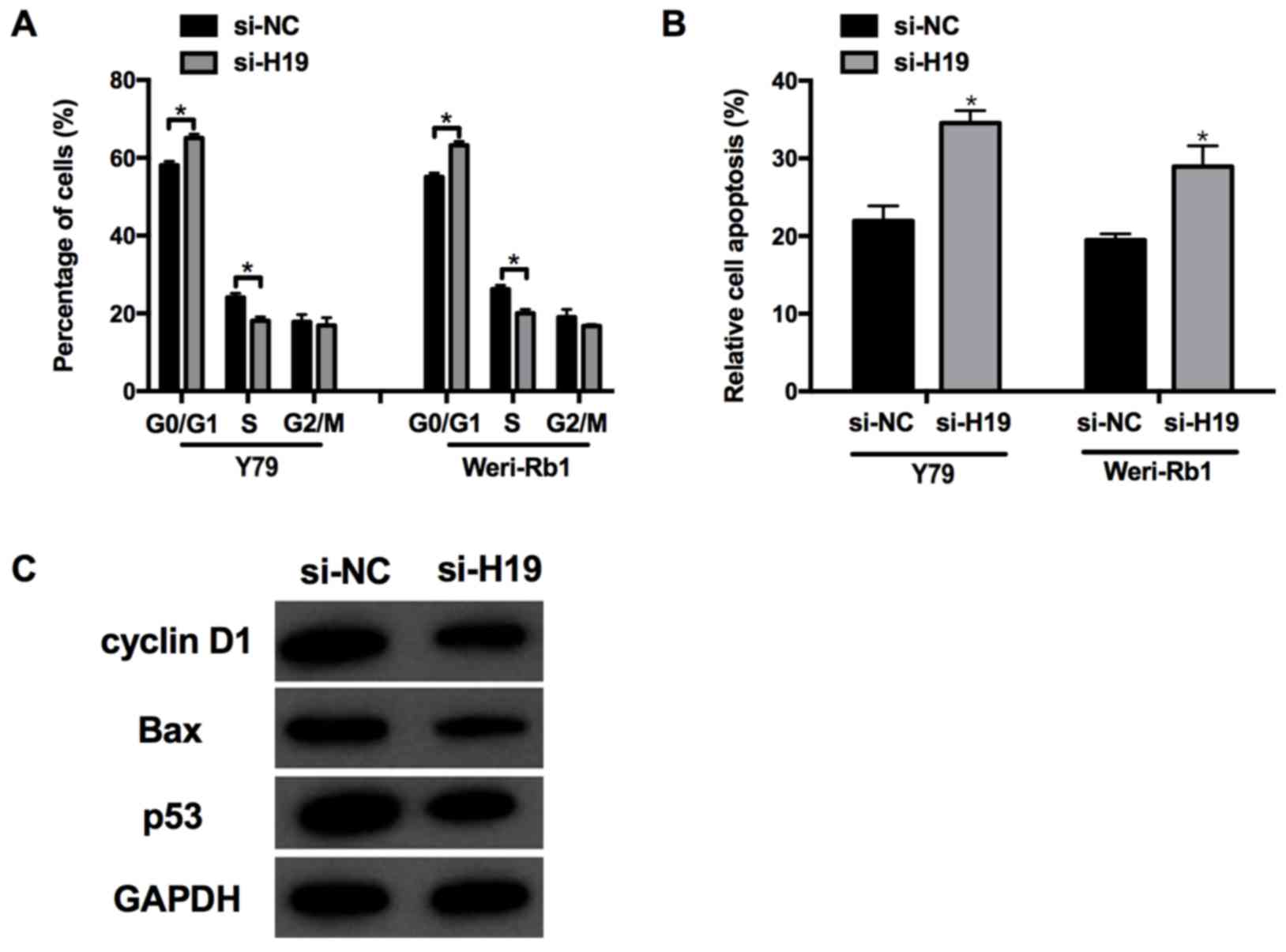
Knockdown of lncRNA H19 promotes cell
apoptosis
As aforementioned, knocking down H19
significantly decreased retinoblastoma cell proliferation, and
therefore the subsequent effects on cell cycle and apoptosis were
investigated further. In accordance with the aforementioned
results, cells transfected with si-H19 displayed an increased
proportion of G1 phase cells and a decreased proportion
of S phase cells, indicating that silencing H19 may induce
G1/S phase arrest in Weri-Rb1 and Y79 cells (Fig. 3A). Furthermore, analysis of cell
apoptosis in the si-H19 group was markedly increased compared with
si-NC (P<0.05; Fig. 3B).
Consistent with these changes, the expression of
apoptosis-associated proteins, including cyclin D1, Bax and p53 was
decreased in the si-H19 group compared with that in the si-NC group
(Fig. 3C), which indicated that
H19 may influence cell apoptosis in Weri-Rb1 and Y79
cells.
Knockdown of lncRNAH19 inhibits cells
migration and invasion in vitro
The effect of lncRNA H19 on cell migration
and invasion in Y79 cells was investigated further. The results of
the present study of the Matrigel invasion assay revealed that the
number of migratory cells in the si-H19 group was significantly
decreased compared with that in the si-NC group (P<0.05;
Fig. 4A). As presented in Fig. 4B, the migration of cells transfected
with si-H19 was also significantly suppressed in Y79 cells
(P<0.05; Fig. 4B). Taken together,
these results support the hypothesis that silencing of lncRNA
H19 inhibited the migration and invasion of Y79 cells. In
addition, the effects of lncRNA H19 on the expression of the
migration- and invasion-associated proteins MMP9, CDH13, E-cadherin
and vimentin were analyzed. As presented in Fig. 4C, lncRNA H19 downregulation
decreased the expression of MMP9, CDH13, vimentin and E-cadherin
compared with that in the si-NC group.
Knockdown of lncRNA H19 suppresses the
proliferation of retinoblastoma cells in vivo
To validate the effects of lncRNA H19 on the
proliferation and invasion of Y79 cells in vivo, the volume
of tumors that grew in a nude mice xenograft model, 3 weeks after
subcutaneous injection of Y79/si-H19 cells, were analyzed. After 3
weeks, subcutaneous tumors were established in the right groin of
20 nude mice injected with Y79/si-H19 or Y79/si-NC cells.
Furthermore, a significant decrease in tumor volume was only
observed in the si-H19 treatment group, compared with corresponding
controls (Y79/si-NC cells). The results of the present study
demonstrated that lncRNA H19 inhibited the proliferation of
retinoblastoma cells in vivo.
Discussion
LncRNAs, >200 nucleotides in length, are
intergenic transcripts, which, although they do not exhibit a
protein-coding function, have been identified to mediate structural
and regulatory functions in the development and pathogenesis of
cancer (24–26). It is well-documented that lncRNAs
exert regulatory functions via interactions with target genes, cell
cycle regulators, transcription factors or chromatin-modifying
complexes, including p53 (27), E2Fs
(28), Enhancer of Zeste homolog 2
(29) and sex-determining region
y-related high-mobility group-box-2 (30). It has been identified that aberrant
lncRNA expression is associated with multiple diverse cancer
processes, indicating that lncRNAs may have a marked function in
cancer development. A well-documented lncRNA, namely E2F1-regulated
inhibitor of cell death (ERIC) was transcriptionally
upregulated upon activation, and inhibition of ERIC resulted
in increased apoptotic cell death (31).
Previous studies have demonstrated that
lncRNA-H19 is involved in the development of cancer
pathogenesis and may serve as a potential tumor biomarker. For
example, Luo et al (32)
observed that the H19 expression pattern was markedly
increased in patients with invasive bladder cancer and demonstrated
pro-tumorigenic properties. Furthermore, the level of H19
was significantly upregulated in patients with gastric cancer (GC),
and the increased expression promoted the differentiation of
early-stage GC (33). Shi et
al (18) also revealed that
H19 expression was associated with tumor grade, and was able
to mediate glioma cell invasion by directly regulating CDH13.
Consistent with previous studies, the present study demonstrated
that H19 levels were markedly increased in retinoblastoma
cells and tissues, and may serve a crucial function in
retinoblastoma migration and invasion, as a potential novel target
for therapeutic strategy and prognostic prediction.
In order to investigate the function of H19
further, an in vivo murine model in which female nude mice
were injected with Y79/si-H19 cells was established. At 3 weeks
post-injection, mice exhibited subcutaneous tumors and tumor
volumes were significantly decreased in the si-H19 treatment group
compared with controls, indicating that H19 may be involved
in the development of retinoblastoma. Furthermore, the survival
rates of patients with retinoblastoma with varying H19
expression levels were determined, and it was revealed that
patients with high H19 levels had a poorer survival rate
compared with those with low H19 levels. Therefore, the
results of the present study indicated that patients with high
H19 levels had a shorter survival time compared with
patients with low levels. Collectively, the results support the
hypothesis that H19 knockdown significantly increased the
proliferation and inhibited the apoptosis of retinoblastoma
cells.
Previous studies have demonstrated that ectopic
expression of H19 partly contributed to increased cell
proliferation, cancer migration and invasion by targeting miRNA
(18,19). The results of the present study
demonstrated that H19 promoted the proliferation, migration
and invasion of Weri-Rb1 and Y79 cells, whereas silencing
H19 significantly suppressed cell proliferation, migration
and invasion in vitro. Furthermore, H19 regulates
several proteins, including vimentin, CDK1, p53, CDH13 and
E-cadherin. For example, H19 functioned as a competing
endogenous RNA for miR-138 and miR-200a, antagonized their
functions and led to the de-repression of their endogenous targets
vimentin, E-cadherin and CDH13 (18,32,34,35).
In the present study, the expression pattern of several
corresponding proteins was investigated, and it was revealed that
inhibition of H19 repressed MMP9, CDH13, vimentin and
E-cadherin expression. However, the precise association between
H19 and the corresponding proteins requires further
study.
In conclusion, the results of the present study
indicated that H19 may serve an oncogenic function in
retinoblastoma cells and tissues, suggesting that inhibition of
H19 may be a potential target mechanism for retinoblastoma
therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Foundation of
Premature Retinopathy Genetic Screening and Clinical Screening
Study (grant no. 2011KR17).
Availability of data and materials
The data generated in the present study are
available from the corresponding author upon reasonable
request.
Author's contributions
LL and LST performed the experiment. WC and YCW
analyzed the experimental data and patients data. MH designed the
experiment and wrote the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Tianjin Eye Hospital (Tianjin, China) and
written informed consent was obtained from all patients.
Consent for publication
Written informed consent was obtained from all
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Villegas VM, Hess DJ, Wildner A, Gold AS
and Murray TG: Retinoblastoma. Curr Opin Ophthalmol. 24:581–588.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
American Cancer Society: Learn about
cancer. Retinoblastoma. https://www.cancer.org/cancer/retinoblastoma.html
|
|
3
|
Hernando E, Nahlé Z, Juan G,
Diaz-Rodriguez E, Alaminos M, Hemann M, Michel L, Mittal V, Gerald
W, Benezra R, et al: Rb inactivation promotes genomic instability
by uncoupling cell cycle progression from mitotic control. Nature.
430:797–802. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dimaras H, Khetan V, Halliday W, Orlic M,
Prigoda NL, Piovesan B, Marrano P, Corson TW, Eagle RC Jr, Squire
JA and Gallie BL: Loss of RB1 induces non-proliferative retinoma:
Increasing genomic instability correlates with progression to
retinoblastoma. Hum Mol Genet. 17:1363–1372. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Manning AL, Longworth MS and Dyson NJ:
Loss of pRB causes centromere dysfunction and chromosomal
instability. Genes Dev. 24:1364–1376. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amato A, Lentini L, Schillaci T, Iovino F
and Di Leonardo A: RNAi mediated acute depletion of retinoblastoma
protein (pRb) promotes aneuploidy in human primary cells via
micronuclei formation. BMC Cell Biol. 10:792009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iovino F, Lentini L, Amato A and Di
Leonardo A: RB acute loss induces centrosome amplification and
aneuploidy in murine primary fibroblasts. Mol Cancer. 5:382006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin P and O'Brien JM: Frontiers in the
management of retinoblastoma. Am J Ophthalmol. 148:192–198. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dalgard CL, Gonzalez M, deNiro JE and
O'Brien JM: Differential microRNA-34a expression and tumor
suppressor function in retinoblastoma cells. Invest Ophthalmol Vis
Sci. 50:4542–4551. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsai MC, Spitale RC and Chang HY: Long
intergenic noncoding RNAs: New links in cancer progression. Cancer
Res. 71:3–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wapinski O and Chang HY: Long noncoding
RNAs and human disease. Trends Cell Biol. 21:354–361. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carninci P, Kasukawa T, Katayama S, Gough
J, Frith MC, Maeda N, Oyama R, Ravasi T, Lenhard B, Wells C, et al:
The transcriptional landscape of the mammalian genome. Science.
309:1559–1563. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Spizzo R, Almeida MI, Colombatti A and
Calin GA: Long non-coding RNAs and cancer: A new frontier of
translational research? Oncogene. 31:4577–4587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adriaenssens E, Dumont L, Lottin S, Bolle
D, Leprêtre A, Delobelle A, Bouali F, Dugimont T, Coll J and Curgy
JJ: H19 overexpression in breast adenocarcinoma stromal cells is
associated with tumor values and steroid receptor status but
independent of p53 and Ki-67 expression. Am J Pathol.
153:1597–1607. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cooper MJ, Fischer M, Komitowski D,
Shevelev A, Schulze E, Ariel I, Tykocinski ML, Miron S, Ilan J, de
Groot N and Hochberg A: Developmentally imprinted genes as markers
for bladder tumor progression. J Urol. 155:2120–2127. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lottin S, Adriaenssens E, Dupressoir T,
Berteaux N, Montpellier C, Coll J, Dugimont T and Curgy JJ:
Overexpression of an ectopic H19 gene enhances the tumorigenic
properties of breast cancer cells. Carcinogenesis. 23:1885–1895.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Biran H, Ariel I, de Groot N, Shani A and
Hochberg A: Human imprinted genes as oncodevelopmental markers.
Tumour Biol. 15:123–134. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi Y, Wang Y, Luan W, Wang P, Tao T,
Zhang J, Qian J, Liu N and You Y: Long non-coding RNA H19 promotes
glioma cell invasion by deriving miR-675. PLoS One. 9:e862952014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang F, Bi J, Xue X, Zheng L, Zhi K, Hua J
and Fang G: Up-regulated long non-coding RNA H19 contributes to
proliferation of gastric cancer cells. FEBS J. 279:3159–3165. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matouk IJ, Raveh E, Abu-lail R, Mezan S,
Gilon M, Gershtain E, Birman T, Gallula J, Schneider T, Barkali M,
et al: Oncofetal H19 RNA promotes tumor metastasis. Biochim Biophys
Acta. 1843:1414–1426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hibi K, Nakamura H, Hirai A, Fujikake Y,
Kasai Y, Akiyama S, Ito K and Takagi H: Loss of H19 imprinting in
esophageal cancer. Cancer Res. 56:480–482. 1996.PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Motoyama K, Inoue H, Nakamura Y, Uetake H,
Sugihara K and Mori M: Clinical significance of high mobility group
A2 in human gastric cancer and its relationship to let-7 microRNA
family. Clin Cancer Res. 14:2334–2340. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mattick JS and Gagen MJ: The evolution of
controlled multitasked gene networks: The role of introns and other
noncoding RNAs in the development of complex organisms. Mol Biol
Evol. 18:1611–1630. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gibb EA, Brown CJ and Lam WL: The
functional role of long non-coding RNA in human carcinomas. Mol
Cancer. 10:382011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gibb EA, Vucic EA, Enfield KS, Stewart GL,
Lonergan KM, Kennett JY, Becker-Santos DD, MacAulay CE, Lam S,
Brown CJ and Lam WL: Human cancer long non-coding RNA
transcriptomes. PLoS One. 6:e259152011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huarte M, Guttman M, Feldser D, Garber M,
Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M,
et al: A large intergenic noncoding RNA induced by p53 mediates
global gene repression in the p53 response. Cell. 142:409–419.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Polager S and Ginsberg D: E2F-at the
crossroads of life and death. Trends Cell Biol. 18:528–535. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao J, Sun BK, Erwin JA, Song JJ and Lee
JT: Polycomb proteins targeted by a short repeat RNA to the mouse X
chromosome. Science. 322:750–756. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Amaral PP, Neyt C, Wilkins SJ,
Askarian-Amiri ME, Sunkin SM, Perkins AC and Mattick JS: Complex
architecture and regulated expression of the SOX2ot locus during
vertebrate development. RNA. 15:2013–2027. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feldstein O, Nizri T, Doniger T, Jacob J,
Rechavi G and Ginsberg D: The long non-coding RNA ERIC is regulated
by E2F and modulates the cellular response to DNA damage. Mol
Cancer. 12:1312013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo M, Li Z, Wang W, Zeng Y, Liu Z and Qiu
J: Long non-coding RNA H19 increases bladder cancer metastasis by
associating with EZH2 and inhibiting E-cadherin expression. Cancer
Lett. 333:213–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou X, Yin C, Dang Y, Ye F and Zhang G:
Identification of the long noncoding RNA H19 in plasma as a novel
biomarker for diagnosis of gastric cancer. Sci Rep. 5:115162015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liang WC, Fu WM, Wong CW, Wang Y, Wang WM,
Hu GX, Zhang L, Xiao LJ, Wan DC, Zhang JF and Waye MM: The lncRNA
H19 promotes epithelial to mesenchymal transition by functioning as
MiRNA sponges in colorectal cancer. Oncotarget. 6:22513–22525.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wan X, Ding X, Chen S, Song H, Jiang H,
Fang Y, Li P and Guo J: The functional sites of miRNAs and lncRNAs
in gastric carcinogenesis. Tumor Biol. 36:521–532. 2015. View Article : Google Scholar
|