Introduction
Abiraterone is a potent CYP17 inhibitor that blocks
androgen synthesis and improves survival in castration-resistant
prostate cancer (CRPC), which is generally administered in
association with a daily dose of 10 mg prednisolone to prevent
mineralocorticoid excess (1). Recent
novel therapies for metastatic CRPC (mCRPC), including abiraterone
and taxane-based chemotherapy, require concomitant corticosteroids
(1,2).
Recent studies have suggested a survival advantage with the
addition of cytotoxic docetaxel chemotherapy with prednisolone for
newly diagnosed patients with high-volume metastatic disease
(3). More recently, abiraterone
acetate plus prednisone in addition to initial androgen-deprivation
therapy also demonstrated increased overall survival in men with
newly diagnosed metastatic prostate cancer (4). Currently, corticosteroids are also
frequently used in palliative care (5). Therefore, corticosteroids are more
frequently used for prolonged periods than previously for treating
mCRPC.
Previously, it had been assumed that antitumor
effects of corticosteroids are caused by reduced synthesis of
adrenal androgens due to suppression of pituitary
adrenocorticotropic hormone (ACTH) production (6,7).
Accordingly, any corticosteroids, including prednisolone,
hydrocortisone and dexamethasone, were all thought to be equally
effective (6). However, current
clinical trials suggested that dexamethasone would be more potent
compared with prednisolone for treating mCRPC for monotherapy and
combination therapy (8,9). In addition, a ‘steroid switch’, that is,
switching concomitant corticosteroids from prednisone to
dexamethasone, is known to have antitumoral effects on patients
with mCRPC (10,11).
In this manuscript, a patient with mCRPC is
reported. The patient had a drastic decrease in the level of
prostate-specific antigen (PSA) following a ‘steroid switch’ during
abiraterone treatment. A review of the recent literature is
provided, and potential explanations of the mechanism underlying
this phenomenon are described.
Case report
A 69-year-old man presented initially with back pain
and incomplete paralysis of the bilateral lower extremities. The
level of PSA in the patient elevated to 167.0 ng/ml. Further
evaluation revealed multiple bone metastases involving thoracic
spinal compression. Thoracic laminectomy was immediately performed
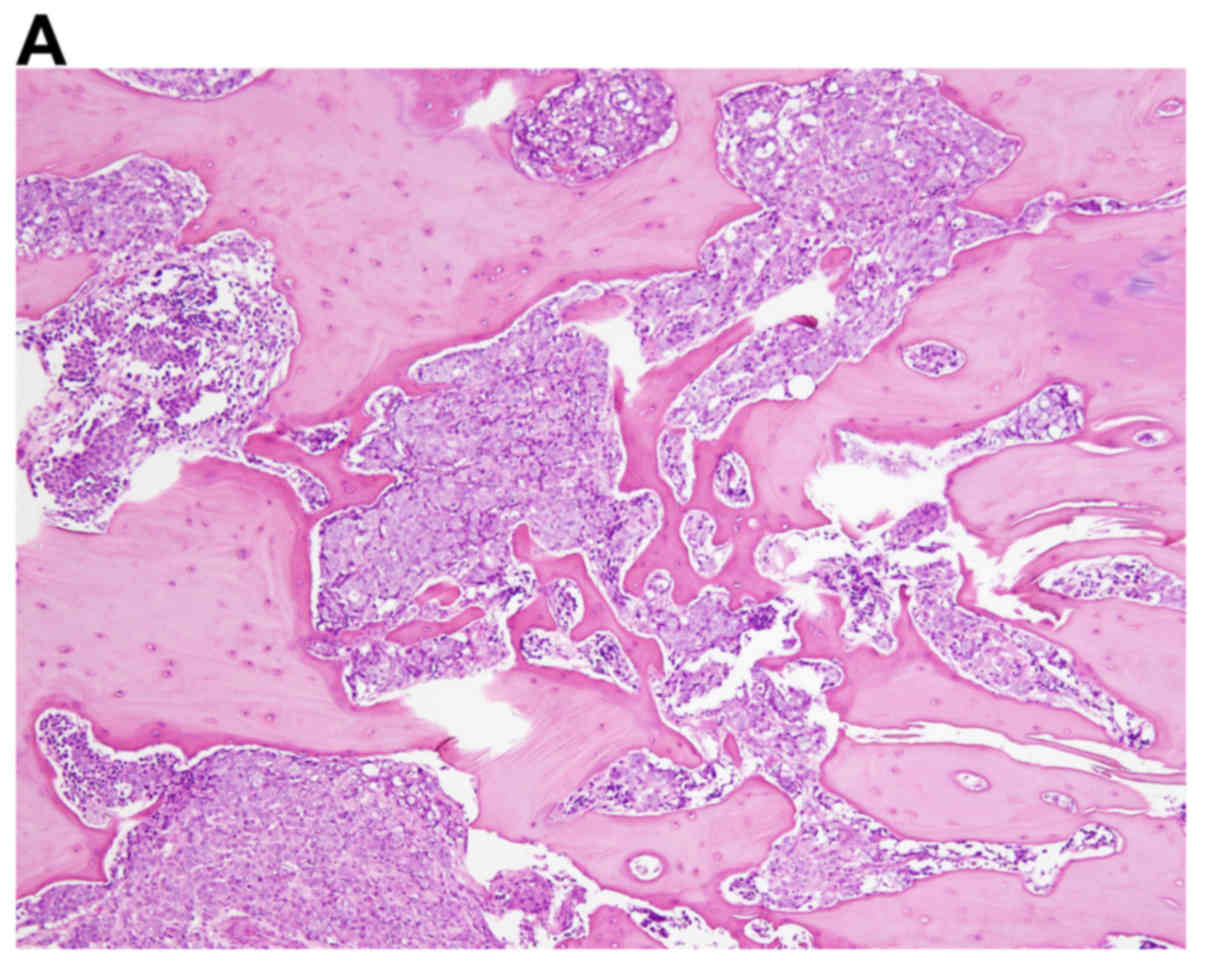
for spinal decompression. The resected lesion was pathologically
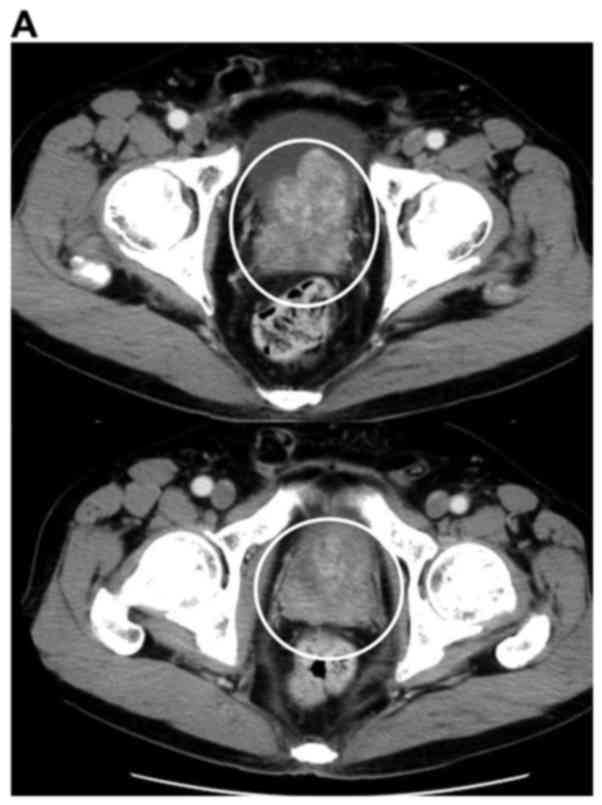
consistent with metastatic adenocarcinoma of the prostate (Fig. 1). The patient was diagnosed with
prostate cancer T3bN0M1b (Fig.
2).
Combined androgen blockade with degarelix and
bicalutamide was started in October 2013. Monthly infusions of
zoledronic acid were simultaneously initiated. The kinetics of PSA
during treatment is displayed in Fig.
3. The serum level of PSA decreased to <1.0 ng/ml, however
thereafter gradually increased. Subsequent anti-androgen withdrawal
response was not observed following the discontinuation of
bicalutamide. Then, anti-androgen switch to flutamide was
attempted, which also resulted in a poor response.
Consequently, abiraterone was initiated at a
standard dose of 1,000 mg daily in combination with 10 mg
prednisolone when the level of PSA increased to 35.9 ng/ml in June
2015. Immediately, the level of PSA decreased to the lowest point
of 4.0 ng/ml. However, after a while, the level of PSA gradually
increased again to 21.7 ng/ml in April 2016.
The patient had limited treatment options at that
time; he was considered to be unfit for chemotherapy because he had
developed severe bisphosphonate-related osteonecrosis of the jaw.
Considering the scarcity of the available treatment options, a
‘steroid switch’ was attempted prior to starting further therapy.
The ‘steroid switch’ involved a continuation of abiraterone
therapy, but concomitant steroid was switched from prednisone to
dexamethasone (1.0 mg per day), which may have effects on the
values of PSA (10,11). Fortunately, a good PSA response was
observed, and the serum PSA level promptly decreased to 0.6 ng/ml.
The levels of PSA following the ‘steroid switch’ are displayed in
Fig. 3.
As the level of PSA decreased and became stable
following the ‘steroid switch’ it was decided to continue with
dexamethasone monotherapy and discontinue abiraterone. It was
hypothesized that dexamethasone itself might be effective, so
abiraterone is no longer necessary. However, following the deletion
of abiraterone, the serum level of PSA had slowly started to
increase again, and local radiological progression was
hypothesized. Subsequently, the patient received 72 Gy of external
beam radiation onto the prostate gland, which exhibited a temporary
response. However the level of increased again after a while.
Re-exposure with abiraterone was attempted when the serum PSA level
increased to 13.4 ng/ml in June 2017. Temporally, the level of PSA
once decreased again to 8.1 ng/ml in response to the re-exposure of
abiraterone. However, the levels of PSA continued to increase
thereafter.
In the present study, written informed consent was
obtained from the patient for the publication of the present case
report and any accompanying images. No ethics approval was obtained
as this was a case report with no direct impact on patient
outcome.
Discussion
Corticosteroids have been used in the treatment of
CRPC for over three decades. Although it was used as a monotherapy,
corticosteroids demonstrate substantial biochemical, clinical and
radiologic responses (12,13). Corticosteroids are also used in
combination with cytotoxic chemotherapy to palliate its toxicities,
and with abiraterone to prevent mineralocorticoid excess (1,2). In
addition, corticosteroids are also widely prescribed in palliative
care to improve nonspecific symptoms, including pain, anorexia,
nausea, weakness and general well-being (5). Therefore, patients with mCRPC are more
frequently exposed to corticosteroid therapy than before.
As an antitumoral agent for CRPC, corticosteroids
are generally used as low-dose, continuous and administered orally.
A ‘low dose’ is usually considered to be prednisone or prednisolone
at 5–15 mg/day and dexamethasone at 0.5–3 mg/day (14). On the other hand, intermittent
relatively higher doses of corticosteroids in combination with
chemotherapy have demonstrated insufficient antitumor effects in
CRPC (9,15).
Among corticosteroids, prednisolone is the most
commonly used corticosteroid in combination with abiraterone or
chemotherapy, and it is currently recommended by more than one
guidelines including those from the AUA, EAU and NCCN (14). Corticosteroids are well known to
suppress pituitary ACTH production, which leads to adrenal androgen
suppression (7). Previously, the
adrenal androgen suppression was assumed to be the main cause of
the antitumor effect against CRPC. Accordingly, any corticosteroids
including prednisolone, hydrocortisone and dexamethasone were once
thought to be equally effective (6).
However, current clinical trials suggested that dexamethasone could
be more potent compared with prednisolone in the treatment of mCRPC
(8,9).
Several non-randomized trials have suggested higher response rates
with dexamethasone compared with prednisolone; the PSA response
rate for CRPC was 27–38% for prednisolone (7.5–10 mg/day) and
40–62% for dexamethasone (0.5–1.5 mg/day) (8). Recent randomized phase 2 trial of
dexamethasone 0.5 mg daily vs. prednisolone 10 mg daily as
monotherapy for CRPC indicated improved PSA response rates for
dexamethasone over prednisolone (9).
PSA response rates and median time to PSA progression were 41 vs.
22% and 9.7 vs. 5.1 months for dexamethasone and prednisolone,
respectively. However, this trend did not reach statistical
significance (9).
Furthermore, a recent retrospective study examined
patients with mCRPC who had undergone a ‘steroid switch’ from
prednisolone to dexamethasone during abiraterone treatment. In this
trial, 11 of 30 patients (39%) had confirmed decline (≥30%) in PSA
after the ‘steroid switch’ with a median time to PSA progression of
11.7 weeks (10). ‘Steroid switch’ is
also able to occasionally induce a long-lasting biochemical
response accompanied by a clinical and radiological improvement
(10).
Potential mechanisms of responses induced by
‘steroid switch’ include: i) Pharmacokinetic differences between
different corticosteroids, ii) activation of the glucocorticoid
receptor (GR) and/or mineralocorticoid receptor (MR), iii)
resistance occurs due to androgen receptor (AR) mutations and iv)
responses derived from various cytokines and cellular growth
factors, including interleukin (IL)6, IL8 and vascular endothelial
growth factor (Fig. 4).
 | Figure 4.Suggested mechanisms of responses that
are induced by the ‘steroid switch’: i) Activation of the GR and/or
MR, ii) resistance occurs due to AR mutations and iii) responses
derived from various cytokines and cellular growth factors,
including IL-6, IL-8, HGF, TGF and VEGF. GR, glucocorticoid
receptor; MR, mineralocorticoid receptor; HGF, hepatocyte growth
factor; TGF, transforming growth factor; VEGF, vascular endothelial
growth factor; IL, interleukin; AR, androgen receptor. |
Pharmacokinetic differences between dexamethasone
and prednisolone might explain the mechanisms. The half-life of
dexamethasone is longer, which may produce a more effective
suppression of ACTH and higher antitumoral activity than
prednisolone (16). Dexamethasone is
thought to be a more potent agent than prednisolone due to its
stronger glucocorticoid activity and lower mineralocorticoid
activity (17). A 10 mg of
prednisolone recommended in the EAU guidelines, represents a higher
glucocorticoid activity compared with commonly used low doses of
dexamethasone (0.5–1.5 mg daily) (16).
Differences in the activation of the GR between
dexamethasone and prednisolone might also be responsible for this
phenomenon. GR expression was significantly increased in patients
with prostate cancer who were exposed to androgen deprivation
(18). The AR and GR, which both are
members of the nuclear steroid receptors, share common structures,
mechanisms of action and several transcriptional targets (18,19).
Cross-talk between AR and GR has been recently speculated. As a
result of AR inhibition, AR signaling is bypassed via activated GR,
which binds to nuclear androgen response elements and regulate AR
target genes (18,19). As patients progress to CRPC,
prednisolone is able to activate GR more, which may reverse with a
‘steroid switch’ to dexamethasone, which has a lower affinity for
GR.
Although a limited number of studies have examined
the role of MRs in CRPC, differences between dexamethasone and
prednisolone in the activation of the MR may also cause alterations
in efficacy (20). Resistances that
occurs secondary to MR activation may be reversed with a ‘steroid
switch’ to dexamethasone, which has a lower affinity for MR
(17,20). MR is expressed in prostate cancer
cells regardless of AR status and appears to be regulated by
inflammatory cytokines, which are highly involved in the
progression of prostate cancer (21).
Subsequent changes in MR expression from inflammatory cytokines is
also speculated to be involved in prostate cancer carcinogenesis
(22).
It might also be possible that resistance occurs due
to corticosteroid-responsive AR mutations that are activated by
prednisolone but not by dexamethasone. AR mutations in the
ligand-binding domain and/or hinge region are hypothesized to be
responsible for stimulating effects of alternative ligands other
than testosterone (23). AR mutations
that are activated by corticosteroids, including prednisolone and
dexamethasone, have been identified (24,25).
Glucocorticoids have an anti-angiogenic and
anti-inflammatory effect on prostate cancer by modulating
transcription factors, cellular growth factors and cytokines, which
may also contribute to the differences in the antitumor activity
(26,27). Dexamethasone has an anti-angiogenic
effect on prostate cancer, which mediated by the activation of
GR-mediated signaling, leading to a reduction in IL-6, IL-8 and
vascular endothelial growth factor expression (28,29). IL-6
is known to stimulate the growth of prostate cancer cells through
GRs in an androgen-independent manner and has been demonstrated to
activate the AR through a STAT3-dependent pathway (17,30). In
addition, changes in serum levels of IL-6 were significantly
associated with the response to dexamethasone in patients with CRPC
(8).
The exact starting point for turning to ‘steroid
switch’ is not determined yet. ‘Steroid switch’ is generally
performed at progression during abiraterone treatment (10,11). It is
reasonable to consider switching concomitant steroids when the
level of PSA increases during abiraterone treatment or cytotoxic
chemotherapy. Drug resistances for concomitant corticosteroids
might be possible. In addition, steroids used in palliative care
for a long time period (5) should be
reconsidered, which may conversely accelerate the growth of
prostate cancer.
The ‘steroid switch’ from prednisolone to
dexamethasone at PSA progression might be feasible options, which
may delay the development of the disease. The underlying mechanisms
require further study. Although it is unclear whether the ‘steroid
switch’ would contribute to the prognosis of the patient, the time
to abiraterone failure was elongated through ‘steroid switch’ in
our case. When the conditions of the patient allow, it would be
acceptable to switch steroids for a brief period before starting
further therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TK designed the study, contributed to analysis and
interpretation of data, and wrote the initial draft of the
manuscript. SK, AK and YN contributed to analysis and assisted in
the preparation of the manuscript. KY contributed to pathological
diagnosis. All authors, including AF, KO, TS, KH, KA and HM, have
contributed to clinical management of the reported case. All
authors critically reviewed the manuscript and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of the present case report and any
accompanying images.
Competing interests
None declared under financial, general, and
institutional competing interests.
Glossary
Abbreviations
Abbreviations:
|
PSA
|
prostate specific antigen
|
|
ACTH
|
adrenocorticotropic hormone
|
|
CRPC
|
castration-resistant prostate
cancer
|
|
mCRPC
|
metastatic castration resistant
prostate cancer
|
|
AR
|
androgen receptor
|
|
GR
|
glucocorticoid receptor
|
|
MR
|
mineralocorticoid receptor
|
|
IL
|
interleukin
|
References
|
1
|
de Bono JS, Logothetis CJ, Molina A,
Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F,
et al: Abiraterone and increased survival in metastatic prostate
cancer. N Engl J Med. 364:1995–2005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tannock IF, de Wit R, Berry WR, Horti J,
Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et
al: Docetaxel plus prednisone or mitoxantrone plus prednisone for
advanced prostate cancer. N Engl J Med. 351:1502–1512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
James ND, Sydes MR, Clarke NW, Mason MD,
Dearnaley DP, Spears MR, Ritchie AW, Parker CC, Russell JM, Attard
G, et al: Addition of docetaxel, zoledronic acid, or both to
first-line long-term hormone therapy in prostate cancer (STAMPEDE):
Survival results from an adaptive, multiarm, multistage, platform
randomized controlled trial. Lancet. 387:1163–1177. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fizazi K, Tran N, Fein L, Matsubara N,
Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S,
Protheroe A, et al: Abiraterone plus prednisone in metastatic,
castration-sensitive prostate cancer. N Engl J Med. 377:352–360.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leppert W and Buss T: The role of
corticosteroids in the treatment of pain in cancer patients. Curr
Pain Headache Rep. 16:307–313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khandwala HM, Vassilopoulou-Sellin R,
Logethetis CJ and Friend KE: Corticosteroid-induced inhibition of
adrenal androgen production in selected patients with prostate
cancer. Endocr Pract. 7:11–15. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eichholz A, Ferraldeschi R, Attard G and
de Bono JS: Putting the brakes on continued androgen receptor
signaling in castration resistant prostate cancer. Mol Cell
Endocrinol. 360:68–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Komiya A, Shimbo M, Suzuki H, Imamoto T,
Kato T, Fukasawa S, Kamiya N, Naya Y, Mori I and Ichikawa T: Oral
low-dose dexamethasone for androgen-independent prostate cancer
patients. Oncol Lett. 1:73–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Venkitaraman R, Lorente D, Murthy V,
Thomas K, Parker L, Ahiabor R, Dearnaley D, Huddart R, De Bono J
and Parker C: A randomised phase 2 trial of dexamethasone versus
prednisolone in castration-resistant prostate cancer. Eur Urol.
67:673–679. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lorente D, Omlin A, Ferraldeschi R, Pezaro
C, Perez R, Mateo J, Altavilla A, Zafeirou Z, Tunariu N, Parker C,
et al: Tumour responses following a steroid switch from prednisone
to dexamethasone in castration resistant prostate cancer patients
progressing on abiraterone. Br J Cancer. 111:2248–2253. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmid S, Fornaro J, Rothermundt C, Omlin
A, Brändle M, Rupp NJ and Gillessen S: Abiraterone-what is wrong
with the adrenal glands? Clin Genitourin Cancer. 12:e133–e137.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tannock I, Gospodarowicz M, Meakin W,
Panzarella T, Stewart L and Rider W: Treatment of metastatic
prostatic cancer with low-dose prednisone: Evaluation of pain and
quality of life as pragmatic indices of response. J Clin Oncol.
7:590–597. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Venkitaraman R, Thomas K, Huddart RA,
Horwich A, Dearnaley DP and Parker CC: Efficacy of low-dose
dexamethasone in castration-refractory prostate cancer. BJU Int.
101:440–443. 2008.PubMed/NCBI
|
|
14
|
De Santis M and Saad F: Practical guidance
on the role of corticosteroids in the treatment of metastatic
castration resistant prostate cancer. Urology. 96:156–164. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weitzman AL, Shelton G, Zuech N, Owen CE,
Judge T, Benson M, Sawczuk I, Katz A, Olsson CA, Bagiella E, et al:
Dexamethasone does not significantly contribute to the response
rate of docetaxel and estramustine in androgen independent prostate
cancer. J Urol. 163:834–837. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Diederich S, Scholz T, Eigendorff E,
Bumke-Vogt Ch, Quinkler M, Exner P, Pfeiffer AF, Oelkers W and Bähr
V: Pharmacodynamics and pharmacokinetics of synthetic
mineralocorticoids and glucocorticoids: Receptor transactivation
and prereceptor metabolism by 11beta-hydroxysteroid-dehydrogenases.
Horm Metab Res. 36:423–429. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishimura K, Nonomura N, Yasunaga Y,
Takaha N, Inoue H, Sugao H, Yamaguchi S, Ukimura O, Miki T and
Okuyama A: Low doses of oral dexamethasone for hormone-refractory
prostate carcinoma. Cancer. 89:2570–2576. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Crona DJ and Whang YE: Androgen
receptor-dependent and -independent mechanisms involved in prostate
cancer therapy resistance. Cancers (Basel). 9(pii): E672017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arora VK, Schenkein E, Murali R, Subudhi
SK, Wongvipat J, Balbas MD, Shah N, Cai L, Efstathiou E, Logothetis
C, et al: Glucocorticoid receptor confers resistance to
antiandrogens by bypassing androgen receptor blockade. Cell.
155:1309–1322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lan NC, Graham B, Bartter FC and Baxter
JD: Binding of steroids to mineralocorticoid receptors:
Implications for in vivo occupancy by glucocorticoids. J Clin
Endocrinol Metab. 54:332–342. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dovio A, Sartori ML, De Francia S, Mussino
S, Perotti P, Saba L, Abbadessa G, Racca S and Angeli A:
Differential expression of determinants of glucocorticoid
sensitivity in androgen-dependent and androgen-independent human
prostate cancer cell lines. J Steroid Biochem Mol Biol. 116:29–36.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leach DA, Powell SM and Bevan CL: Women in
Cancer Thematic Review: New roles for nuclear receptors in prostate
cancer. Endocr Relat Cancer. 23:T85–T108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Taplin ME, Bubley GJ, Shuster TD, Frantz
ME, Spooner AE, Ogata GK, Keer HN and Balk SP: Mutation of the
androgen-receptor gene in metastatic androgen-independent prostate
cancer. N Engl J Med. 332:1393–1398. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao XY, Malloy PJ, Krishnan AV, Swami S,
Navone NM, Peehl DM and Feldman D: Glucocorticoids can promote
androgen-independent growth of prostate cancer cells through a
mutated androgen receptor. Nat Med. 6:703–706. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Richards J, Lim AC, Hay CW, Taylor AE,
Wingate A, Nowakowska K, Pezaro C, Carreira S, Goodall J, Arlt W,
et al: Interactions of abiraterone, eplerenone, and prednisolone
with wild-type and mutant androgen receptor: A rationale for
increasing abiraterone exposure or combining with MDV3100. Cancer
Res. 72:2176–2182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nishimura K, Nonomura N, Satoh E, Harada
Y, Nakayama M, Tokizane T, Fukui T, Ono Y, Inoue H, Shin M, et al:
Potential mechanism for the effects of dexamethasone on growth of
androgen-independent prostate cancer. J Natl Cancer Inst.
93:1739–1746. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lam JS, Leppert JT, Vemulapalli SN,
Shvarts O and Belldegrun AS: Secondary hormonal therapy for
advanced prostate cancer. J Urol. 175:27–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yano A, Fujii Y, Iwai A, Kageyama Y and
Kihara K: Glucocorticoids suppress tumor angiogenesis and in vivo
growth of prostate cancer cells. Clin Cancer Res. 12:3003–3009.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Akakura K, Suzuki H, Ueda T, Komiya A,
Ichikawa T, Igarashi T and Ito H: Possible mechanism of
dexamethasone therapy for prostate cancer: Suppression of
circulating level of interleukin-6. Prostate. 56:106–109. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ueda T, Bruchovsky N and Sadar MD:
Activation of the androgen receptor N-terminal domain by
interleukin-6 via MAPK and STAT3 signal transduction pathways. J
Biol Chem. 277:7076–7085. 2002. View Article : Google Scholar : PubMed/NCBI
|