Introduction
Siblings, particularly twins, are at similar risk of
developing leukemia or non-Hodgkin lymphoma (NHL) (1,2),
presumably due to similarities in both genetic and environmental
factors. In a cohort of child cancer survivors including 211 twin
pairs (1), the standardized incidence
ratio (SIR) for all malignancies in all twins was 8.2 (95%
confidence interval [CI], 3.9–17.1), compared with an SIR of 1.9
(95% CI, 1.7–2.1) in non-twin siblings, with an even higher SIR in
monozygotic twins of 23.3 (9% CI, 11.1–48.9). Notably, the SIR for
NHL was particularly high in monozygotic twins, at 40.5 (95% CI,
5.7–287.5) (1). A few reported cases
of NHL occurring in both members of a pair of twins have been
pathologically classified as similar types. We herein present the
first report of primary conjunctival NHL that developed
metachronously in each of a pair of monozygotic twins. Complete
remission (CR) was achieved with different therapeutic strategies
in each twin.
Case report
Twin 1 (treated at another
hospital)
The present team discovered Twin 1's medical history
while treating Twin 2. Twin 1's attending physician was
subsequently interviewed and the relevant biopsy specimen slides
were sent to our hospital and reviewed by the present team.
A tumor associated with papillary changes was
detected in the right conjunctiva in a 25-year-old woman and
diagnosed as an extranodal marginal zone lymphoma (EMZL) by
examination of a biopsy specimen. No infiltration of any other
organs was detected and the EMZL was staged as Ann Arbor stage
IE. The tumor was excised and the patient (Twin 1) was
closely followed up. At the age of 39 years, an asymptomatic
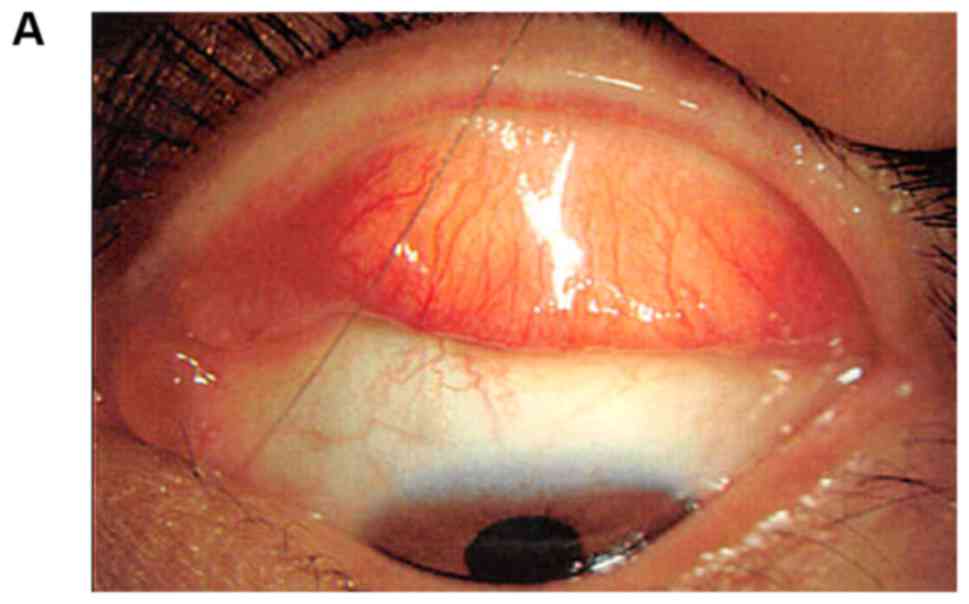
conjunctival tumor was detected in the contralateral upper and
lower conjunctiva (Fig. 1) and
monitored. It did not regress and was therefore completely excised.
Histopathological examination revealed lympho-epithelial lesions
with monotonous infiltration of small atypical lymphocytes
extending into the epithelium. Reactive follicle formation at the
center of the lesion was accompanied by follicular colonization. On
immunostaining, the lesion was positive for cluster of
differentiation (CD) 20 and negative for CD10. On the basis of
these findings, an EMZL was again diagnosed (Fig. 2). Detailed protocols of the
experimental procedures for Twin 1 are unavailable, but staining
was performed and the magnifications are shown. No infiltration of
any other organs was detected by either fluorodeoxyglucose-positron
emission tomography/computed tomography (FDG-PET/CT) or bone marrow
biopsy and the EMZL was staged as Ann Arbor stage IE. At
the time of writing, Twin 1 had been closely followed up for 1.5
years since the most recent surgery, with no recurrence.
Twin 2 (treated at our hospital)
Twin 1's monozygotic twin sister, Twin 2, presented
with the sensation of a foreign body in the right lower conjunctiva
at age 38 years. Three months later, at age 39 years, a tumor with
papillary changes was detected in the right lower conjunctiva.
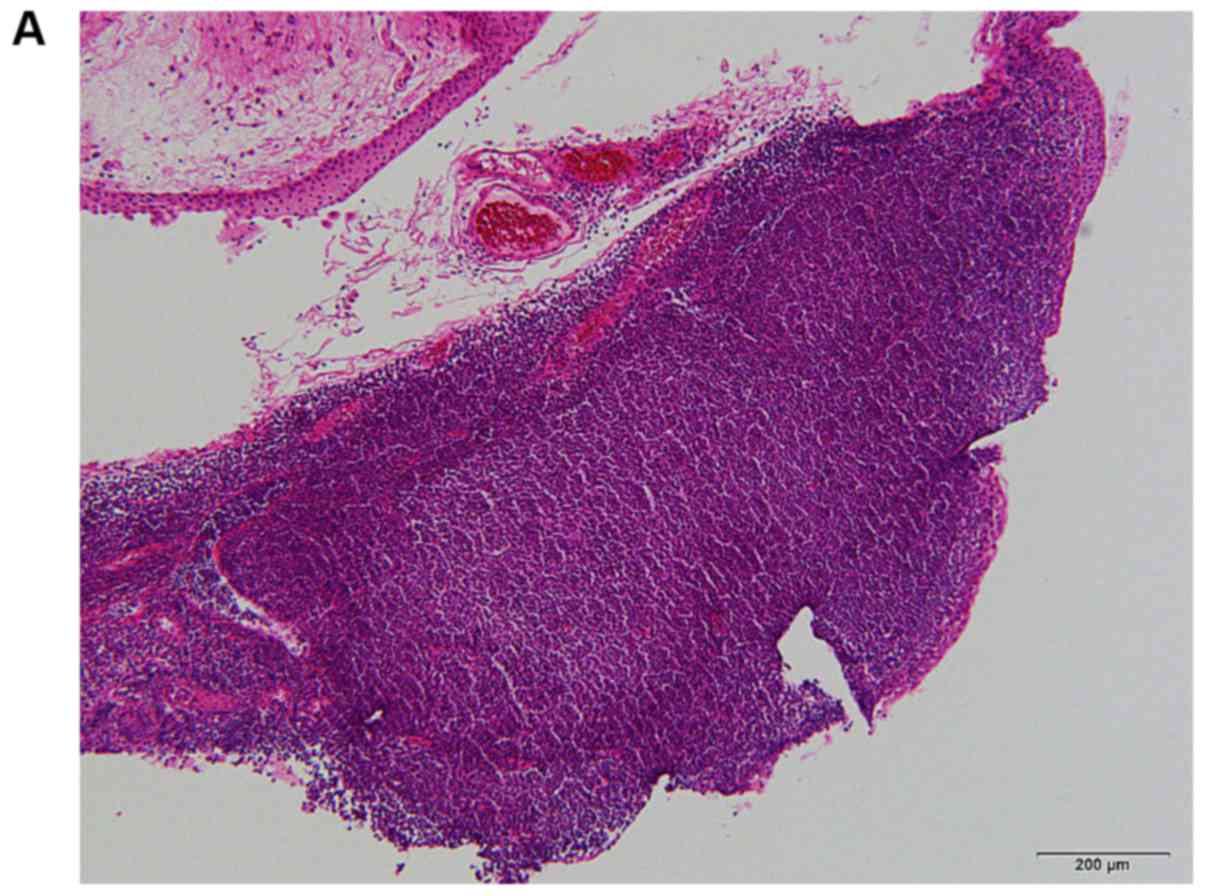
Although examination of a biopsy of the same lesion revealed
lymphocytic infiltration, no evidence of lymphoma was detected.
Twin 2 was closely followed up and a second biopsy of the tumor in
the right lower conjunctiva taken at the age of 40 years because
the lesion had grown (Fig. 3).
Histopathological examination revealed massive infiltration of
small to medium-sized lymphocytes with atypical centrocytes under
the epithelium. The lesion contained closely packed follicles and
no tangible body macrophages were identified in the germinal
centers of the reactive follicles. The follicular cells were
positive for CD20 and almost negative for CD10. A pattern of CD21
staining was detected in the follicular dendritic cells, which had
created well-formed meshworks. Bcl-2 protein was expressed not in
the germinal center but in the peripheral zone of follicles.
Bcl-6-positive cells in the interfollicular areas were
Bcl-2-negative and these Bcl-6-positive cells were the remaining
reactive follicular center cells. Ki-67-positive cells had lost
polarity and there were a few large centroblastic cells. Thus Twin
2 was diagnosed as EMZL with intense follicular colonization
(Fig. 4). No infiltration of any
other organs was detected by FDG-PET/CT and the EMZL was staged as
Ann Arbor stage IE. Because this tumor widely involved
the conjunctiva and it was believed that the completely excision
was difficult, external beam radiation therapy (EBRT) was
administered. Because the lesion involved the conjunctival surface
in a way that was undetectable by magnetic resonance imaging, the
entire conjunctiva was irradiated with a 5-MeV electron beam. A
lead shield was placed on the cornea to prevent development of a
radiation-induced cataract. CR was achieved by irradiation at a
dose of 2 Gy/fraction to a total dose of 30 Gy. There were no
adverse effects of radiation therapy. Twin 2 has been closely
followed up for 6 months after this therapy to date, with no
recurrence.
Hematoxylin and eosin staining (Twin
2)
Sections (4 µm) were cut from each
paraffin-embedded, formalin-fixed (FFPE) tissue blocks and stained
with Mayer's hematoxylin (Sakura Finetek Japan Co., Ltd., Tokyo,
Japan) and eosin-floxine (Sakura Finetek Japan Co., Ltd.) using by
Tissue-Tek ® prisma (Sakura Finetek Japan Co., Ltd.).
Mayer's hematoxylin was reacted for 3 min at room temperature, and
eosin-floxine was reacted for 3 min at room temperature.
Immunohistochemistry (Twin 2)
Sections (4 µm) were deparaffinized and treated with
Envision Flex High pH visualisation system (Dako; Agilent
Technologies Inc., Santa Clara, CA, USA), as antigen retrieval, for
20 min at 97°C in a microwave and blocked using Peroxidase-Blocking
Solution (Dako; Agilent Technologies, Inc.) at room temperature for
5 min. The immunohistochemical stainings were performed using by
Autostainer Link 48 (Dako; Agilent Technologies, Inc.). Primary
antibodies were used in the present study as follows: B-cell
lymphoma 2 (Bcl2; clone SC-509; host, mouse; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA, cat no. M0887; 1:50), Bcl6
(clone, PG-B6p; host, mouse; Dako; Agilent Technologies, Inc.; cat
no. IR625), CD10 (clone, 56C6; host, mouse; Novocastra, Leica
Microsystems GmbH, Wetzlar, Germany.; cat no. NCL-CD10-270; 1:10),
CD20 (clone, L26; host, mouse; Dako; Agilent Technologies, Inc.;
cat no. IR604; 1:1,600), CD21 (clone, 1F8; host, mouse; Dako;
Agilent Technologies, Inc.; cat no. IR608). Counterstaining was
performed with hematoxylin. All of the assays were validated using
proper positive controls tonsil FFPE tissue sections. Results were
evaluated using a light microscope (BX-41, Olympus Co., Tokyo,
Japan). The positive cut-off for all antibodies was considered to
be 30% (3).
Detection of immunoglobulin gene
rearrangements by BIOMED-2 study protocols
To analyze immunoglobulin gene clonal rearrangements
by multiplex PCR, we used the BIOMED-2 primer system (4). In the BIOMED-2 guidelines, three sets of
VH FR regions primers (FR1:6 primers, FR2:7 primers, and FR3: 7
primers) and a JH primer were designed. Furthermore, immunoglobulin
kappa chain (IGK) VH primers (FR: 6 primers) and two sets of JH
primers (JH1: 2 primers and JH2: 1 primer), and immunoglobulin
lambda chain (IGL) VH primer and JH primer were prepared as
previously reported by Lu et al (5). RNase P was used as the internal control.
The PCR products were analyzed by agarose gel electrophoreses.
Clonal immunoglobulin gene rearrangements were found by multiplex
PCR, but the patterns of these rearrangement were different between
both Twin 1 and Twin 2. In Twin 1, clonal rearrangement was
detected by IGH FR2 primer set, and by IGH FR1 primer set in Twin
2. In both Twin 1 and Twin 2, IGK (kappa chain) were detected but
IGL (lambda chain) were absent. Multiplex PCR analysis showed that
immunoglobulin kappa light chain was dominant in both twins (data
not shown).
Fluorescence in situ hybridization for
immunoglobulin heavy chain (IGH)-mucosa associated lymphoid tissue
lymphoma translocation gene 1 (MALT1) rearrangement detection.
Four-micrometer sections were cut and deparaffinized with xylene.
Glass slides were heated at 70°C for 10 min. After the hydrophilic
procedure and washing, the samples were placed in citric buffer at
98°C for 15 min. After washing, proteinaseK solution was added to
the samples and incubated at room temperature for 15 min. Next,
Cytocell aquarius t(14;18)(32.33:21.31–21.32)/IGH-MALT1 dual fusion
probe (Cytocell Ltd., Cambridge, UK) was added to the individual
samples and the samples were covered with coverslips. After sealing
the coverslips, the samples were denatured at 75°C for 10 min and
the slides were transferred to a hybridization oven overnight at
37°C. Further processing included washing and counterstaining with
DAPI. Signals were detected using an Axio Imager Z2 with a
fluorescent microscope (Carl Zeiss Microscopy, Jena, Germany) with
appropriate filters (Chroma Technology Corporation, Bellows Falls,
VT, USA). The images were analyzed with the ZEN2 Pro software (Carl
Zeiss Microscopy). These results showed the not rearranged
IGH-MALT1 signals in both twins' samples (data not shown).
Species-specific real-time PCR for the
diagnosis of Chlamydia psittaci (C. psittaci) infection
DNA samples were extract from formalin fixed and
paraffin embedded (FFPE) sections using by the commercial kit
(GeneRead DNA FFPE kit, Qiagen GmbH, Hilden, Germany). The first
PCR reaction was carried out with each DNA by using the AmpliTaq
Gold® 360 Master Mix (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and the species-specific primers that have been
reported by Opota et al (6).
Samples were incubated at 95°C for 10 min before being subjected to
40 cycles of denaturation at 95°C for 30 sec, annealing at 60°C for
1 min, and polymerization at 72°C for 1 min. The first reaction was
performed on a conventional PCR machine (PC808, ASTEC Co. Ltd.,
Fukuoka, Japan). Two microliters of each resulting product were
used as the template in the second semi-nested (snq) PCR (7,8)
amplification performed by QuantStudio 3 (Thermo Fisher Scientific,
Inc.) with the species-specific primers, as same as the first PCR
reaction, and TaqMan®probes that have been reported by
Opota et al (6). TaqMan Copy
Number Reference Assay, RNase P, Human (Thermo Fisher
Scientific, Inc.) were used as an internal control gene. Although
internal control gene RNase P was detected from both Twin 1
and Twin 2 sample DNA, neither C. psittaci nor C.
abortus were detected from both twins (data not shown).
Discussion
When one member of a twin pair develops leukemia or
NHL, the other member is at increased risk of developing the same
disease. A cohort study on familial risk of NHL showed that the SIR
for lifetime cumulative risk of NHL in siblings of an individual
with NHL is 1.6 (95% CI, 1.2–1.9), compared with a cumulative
lifetime risk for twins, depending on sex and age at diagnosis, of
3.1–12.9% (2). Possible reasons for
both members of a pair of twins developing NHL include genetic
factors, such as chromosomal translocation, and exposure to similar
environmental factors as a result of living in the same region.
Only eight instances of both members of a pair of twins developing
NHL have been reported (Table I)
(9–16). In all these cases, both twins had
similar primary sites, the commonest being the skin, which was
affected in three pairs. Moreover, both members of each of the
eight twin pairs had similar pathological subtypes. No cases of NHL
of the conjunctiva in twins have been reported to date. The current
case report is the first to document the development of
conjunctival NHL in monozygotic twins.
 | Table I.Overview of reported twin pairs with
non-Hodgkin lymphoma. |
Table I.
Overview of reported twin pairs with
non-Hodgkin lymphoma.
|
|
|
|
| Twin 1 | Twin 2 |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Author, year | Pair | Twin type | Sex | Histology, invaded
organ | Age, years | Histology, invaded
organ | Age, years | (Refs.) |
|---|
| Granet, 1949 | 1 | MZ | M/M | ML, rectum | 31 | ML, rectum | 38 | (9) |
| Schneider et
al, 1995 | 2 | MZ | F/F | T cell lymphoma,
skin | 46 | T celllymphoma,
skin | 47 | (10) |
| Jensen et al,
1997 | 3 | MZ | F/F | EBV positive NHL,
CNS | 18 | EBV positive NHL,
CNS | 19 | (11) |
| Salawu et al,
1997 | 4 | MZ | M/M | Burkitt's lymphoma,
nodal | 8 | Burkitt's lymphoma,
nodal | 11 | (12) |
| Marco et al,
1999 | 5 | MZ | M/M | Follicular
lymphoma, nodal | 48 | Follicular
lymphoma, nodal | 48 | (13) |
| Chakravarti et
al, 2005 | 6 | DZ | M/F | Follicular
lymphoma, nodal | 52 | Follicular
lymphoma, salivary gland | 53 | (14) |
| Dickinson et
al, 2011 | 7 | MZ | M/M | Marginal zone
lymphoma, skin | 45 | Marginal zone
lymphoma, skin | 45 | (15) |
| Bahig et al,
2016 | 8 | MZ | F/F | Marginal zone
lymphoma, skin | 18 | Marginal zone
lymphoma, skin | 25 | (16) |
|
| The present
case | MZ | F/F | Marginal zone
lymphoma, conjunctiva | 25 and 39 | Marginal zone
lymphoma, conjunctiva | 40 |
|
|---|
NHL is reportedly associated with several
chromosomal translocations and genetic factors may be considered to
be responsible in our pair of twins. In recent years, to diagnose
B-cell lymphoma, the immunoglobulin gene rearrangement detection
system is used as a valuable tool (5). Our both twins have clonal B-cell
immunoglobulin gene rearrangements, but the patterns of these
rearrangements were different between Twin 1 and Twin 2. Recently,
t(14;18)(q32;q21) involving the IgH/MALT1 has been reported in
ocular adnexal mucosa-associated lymphoid tissue (MALT) lymphoma
(17). However, our both twins were
negative for t(14;18)(q32;q21).
Studies of monozygotic twins, who share a common
genome, provide an effective way of assessing the potential
contribution of environmental factors to NHL development. According
to a questionnaire survey on medical history (e.g., infections and
atopic disease) and microbial exposure history (e.g., sucking on a
pacifier) in 162 twin pairs with discordant development of NHL, the
risk of NHL was higher in the twin who had been more frequently
exposed to microbes than their co-twin (18). Conjunctival lymphoma is believed to be
caused by prolonged antigen stimulation resulting from chronic
conjunctival infection with Chlamydia psittaci and other
organisms, causing loss of B-lymphocyte control and 80% of ocular
adnexal lymphoma samples carried C. psittaci DNA (19). Therefore to detect C. psittaci
infection, we performed species-specific real-time PCR by using the
primers that have been reported by Opota et al recently
(6). However, our both twins were
negative for C. psittaci in real-time PCR. Helicobacter
pylori is associated with gastric MALT lymphoma but has not
been identified in any reported cases of conjunctival MALT lymphoma
(20). Because the twins in this case
report lived together until Twin 1 developed her first conjunctival
lymphoma at age 25 years, Twin 2 might have the another chronic
infection at living alone. Autoimmune disorders such as Sjögren
syndrome are also reportedly associated with conjunctival lymphoma;
however, our twins had no history of such diseases. The commonest
subtype of conjunctival lymphoma is EMZL, accounting for 81% of all
cases, whereas the second most common is follicular lymphoma (FL),
accounting for 8% (21). The
incidence of FL is lower in Asian countries, including Japan, than
in the West, and only four cases of conjunctival FL in Japanese
individuals have been reported to date (22–25). In
the current twins, both NHL in the conjunctiva was EMZL.
EBRT is a highly effective and commonly administered
therapeutic strategy for conjunctival lymphoma; nonetheless,
complete surgical excision followed by monitoring is also sometimes
performed. However, lymphoma cells may infiltrate the surrounding
tissues at a microscopic level, resulting in a high recurrence rate
in the absence of additional treatment, approximately one in three
patients reportedly developing progression or recurrence during a
3-year follow-up (21,26). The total radiation dose of 30 Gy is
generally considered sufficient to successfully and safely control
conjunctival EMZL (27,28). In the current Twin 1, NHL developed in
the contralateral conjunctiva 13.5 years after complete excision of
the first NHL deposit; however, no further recurrence has been
identified in either conjunctiva in the 1.5 years since the second
surgery. The current Twin 2 was treated with EBRT alone, receiving
a total dose of 30 Gy, and has since shown no recurrence in either
conjunctiva. Ocular adnexal EMZL may develop in the contralateral
orbit, the reported incidence being 6.4% even after radiation
therapy (28). However, the prognosis
of ocular adnexal EMZL is favorable, with a 10-year overall
survival rate of approximately 95.3% (28). CR was achieved in both twins in the
current case report with different therapeutic strategies being
used. Further follow-up of both twins is essential.
We here provide the first reported cases of primary
conjunctival NHL occurring metachronously in the conjunctiva of
each of a pair of monozygotic twins. When NHL occurs in one of a
twin pair, the other twin is at increased risk of developing NHL.
When conjunctival lymphoma, which causes few symptoms, develops in
a twin, it is therefore important to examine the other, apparently
healthy twin.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NI treated Twin 2 and analyzed the twins' data, as
well as being a major contributor to writing the manuscript. SS
biopsied the conjunctiva of Twin 2. HT examined Twin 2. HN and YN
performed the histological examinations of the twins' samples. YC
examined Twin 1. TM treated Twin 2. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The patients provided written informed consent.
Patient consent for publication
The patients provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EBRT
|
external beam radiation therapy
|
|
EMZL
|
extranodal marginal zone lymphoma
|
|
FDG-PET/CT
|
fluorode-oxyglucose-positron emission
tomography/computed tomography
|
|
NHL
|
non-Hodgkin lymphoma
|
References
|
1
|
Kadan-Lottick NS, Kawashima T, Tomlinson
G, Friedman DL, Yasui Y, Mertens AC, Robison LL and Strong LC: The
risk of cancer in twins: A report from the childhood cancer
survivor study. Pediatr Blood Cancer. 46:476–481. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fallah M, Kharazmi E, Pukkala E, Tretli S,
Olsen JH, Tryggvadottir L, Sundquist K and Hemminki K: Familial
risk of non-Hodgkin lymphoma by sex, relationship, age at diagnosis
and histology: A joint study from five Nordic countries. Leukemia.
30:373–378. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choi WW, Weisenburger DD, Greiner TC,
Piris MA, Banham AH, Delabie J, Braziel RM, Geng H, Iqbal J, Lenz
G, et al: A new immunostain algorithm classifies diffuse large
B-cell lymphoma into molecular subtypes with high accuracy. Clin
Cancer Res. 15:5494–5502. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Dongen JJ, Langerak AW, Brüggemann M,
Evans PA, Hummel M, Lavender FL, Delabesse E, Davi F, Schuuring E,
García-Sanz R, et al: Design and standardization of PCR primers and
protocols for detection of clonal immunoglobulin and T-cell
receptor gene recombinations in suspect lymphoproliferations:
Report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia.
17:2257–2317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu C, He Q, Zhu W, Fu C, Zhou J, Tao Y,
Liu S and Xiao D: The value of detecting immunoglobulin gene
rearrangements in the diagnosis of B-cell lymphoma. Oncotarget.
8:77009–77019. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Opota O, Jaton K, Branley J, Vanrompay D,
Erard V, Borel N, Longbottom D and Greub G: Improving the molecular
diagnosis of Chlamydia psittaci and Chlamydia abortus infection
with a species-specific duplex real-time PCR. J Med Microbiol.
64:1174–1185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakanishi Y, Shimizu T, Tsujino I, Obana
Y, Seki T, Fuchinoue F, Ohni S, Oinuma T, Kusumi Y, Yamada T, et
al: Semi-nested real-time reverse transcription polymerase chain
reaction methods for the successful quantitation of cytokeratin
mRNA expression levels for the subtyping of non-small-cell lung
carcinoma using paraffin-embedded and microdissected lung biopsy
specimens. Acta Histochem Cytochem. 46:85–96. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iida Y, Masuda S, Nakanishi Y, Shimizu T,
Nishimaki H, Takahashi M, Hikichi M, Maruoka S, Gon Y, Takahashi N
and Hashimoto S: Clinicopathological characteristics of thyroid
transcription factor 1-negative small cell lung cancers. Hum
Pathol. 79:127–134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Granet E: Simple lymphoma of the
sphincteric rectum in identical twins. J Am Med Assoc.
141:990illust. 1949. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schneider BF, Christian M, Hess CE and
Williams ME: Familial occurrence of cutaneous T cell lymphoma: A
case report of monozygotic twin sisters. Leukemia. 9:1979–1981.
1995.PubMed/NCBI
|
|
11
|
Jensen MK, Koch-Henriksen N, Johansen P,
Varming K, Christiansen CB and Knudsen F: EBV-positive primary
central nervous system lymphomas in monozygote twins with common
variable immunodeficiency and suspected multiple sclerosis. Leuk
Lymphoma. 28:187–193. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Salawu L, Fatusi OA, Kemi-Rotimi F, Adeodu
OO and Durosinmi MA: Familial Burkitt's lymphoma in Nigerians. Ann
Trop Paediatr. 17:375–379. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marco F, Manjón R, Richard C, Mazorra F,
García-Valtuille A, Delgado MD, Loyo ML, Cuadrado MA and
Zubizarreta A: Simultaneous occurrence of follicular lymphoma in
two monozygotic twins. Br J Haematol. 107:461–462. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chakravarti A, Leslie WT and Venugopal P:
Relapsed non-Hodgkin lymphoma in fraternal twins managed
successfully with rituximab maintenance therapy. Clin Adv Hematol
Oncol. 3:136–140. 2005.PubMed/NCBI
|
|
15
|
Dickinson PD, Patel A, Wootton C, Bessell
EM and O'Connor S: Primary cutaneous marginal zone B cell lymphoma
in monozygotic twins. BMJ Case Rep. 3:pii: bcr1120103515. 2011.
|
|
16
|
Bahig H, Petrogiannis-Haliotis T, Pehr KL
and Roberge D: Primary cutaneous B-cell lymphoma in young
monozygotic twins: A case report. J Cutan Med Surg. 20:582–585.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Streubel B, Lamprecht A, Dierlamm J,
Cerroni L, Stolte M, Ott G, Raderer M and Chott A:
T(14;18)(q32;q21) involving IGH and MALT1 is a frequent chromosomal
aberration in MALT lymphoma. Blood. 101:2335–2339. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Mack TM, Hamilton AS, Hwang AE,
Nathwani BN, Masood K, Buchanan LH, Bernstein L, Deapen DM,
Martínez-Maza O and Cozen W: Common immune-related
exposures/conditions and risk of non-Hodgkin lymphoma: A
case-control study of disease-discordant twin pairs. Am J
Epidemiol. 182:417–425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ferreri AJ, Guidoboni M, Ponzoni M, De
Conciliis C, Dell'Oro S, Fleischhauer K, Caggiari L, Lettini AA,
Dal Cin E, Ieri R, et al: Evidence for an association between
Chlamydia psittaci and ocular adnexal lymphomas. J Natl Cancer
Inst. 96:586–594. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sjö NC, Foegh P, Juhl BR, Nilsson HO,
Prause JU, Ralfkiaer E, Wadström T and Heegaard S: Role of
Helicobacter pylori in conjunctival mucosa-associated
lymphoid tissue lymphoma. Ophthalmology. 114:182–186. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kirkegaard MM, Coupland SE, Prause JU and
Heegaard S: Malignant lymphoma of the conjunctiva. Surv Ophthalmol.
60:444–458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Azumi A, Hirai K, Tamura Y, Saito K,
Yamamoto H, Negi A and Obayashi C: A case of follicular lymphoma
derived from the conjunctiva. Nippon Ganka Gakkai Zasshi.
106:420–425. 2002.PubMed/NCBI
|
|
23
|
Takahira M, Okumura H, Minato H,
Urushisaki N, Sakurai M and Sugiyama K: Primary conjunctival
follicular lymphoma treated with the anti-CD20 antibody rituximab
and low-dose involved-field radiotherapy. Jpn J Ophthalmol.
51:149–151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Otomo T, Fuse N, Ishizawa K, Seimiya M,
Shimura M and Ichinohasama R: Japanese case of follicular lymphoma
of ocular adnexa diagnosed by clinicopathologic,
immunohistochemical, and molecular genetic techniques. Clin
Ophthalmol. 4:1397–1402. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Al-Kader Abd L, Sato Y, Takata K, Ohshima
K, Sogabe Y, Fujii K, Iwaki N and Yoshino T: A case of conjunctival
follicular lymphoma mimicking mucosa-associated lymphoid tissue
lymphoma. J Clin Exp Hematop. 53:49–52. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baldini L, Blini M, Guffanti A, Fossati V,
Colombi M, La Targia ML, Bertoni F, Alietti A, Neri A and Bertoni
G: Treatment and prognosis in a series of primary extranodal
lymphomas of the ocular adnexa. Ann Oncol. 9:779–781. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hata M, Omura M, Koike I, Tomita N, Iijima
Y, Tayama Y, Odagiri K, Minagawa Y, Ogino I and Inoue T: Treatment
effects and sequelae of radiation therapy for orbital
mucosa-associated lymphoid tissue lymphoma. Int J Radiat Oncol Biol
Phys. 81:1387–1393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hashimoto N, Sasaki R, Nishimura H,
Yoshida K, Miyawaki D, Nakayama M, Uehara K, Okamoto Y, Ejima Y,
Azumi A, et al: Long-term outcome and patterns of failure in
primary ocular adnexal mucosa-associated lymphoid tissue lymphoma
treated with radiotherapy. Int J Radiat Oncol Biol Phys.
82:1509–1514. 2012. View Article : Google Scholar : PubMed/NCBI
|