Introduction
Lung cancer has top ranking in the malignant tumors
in the world, and there are approximately 1,820,000 incidence cases
and approximately 386,000 deaths (more male deaths than female
deaths) every year (1). Lung cancer
is mainly caused by the surrounding environment, which is related
to air pollution, smoking, heredity, chronic lung diseases and
occupational factors. In the early stage of lung cancer, there is
no obvious specificity, but most patients with lung cancer, at
diagnosis, have lost the optimal opportunity for treatment due to
the rapid progression and high malignant degree of lung cancer
(2). If changes in some tumor
markers can be detected in the early stage of lung cancer, ‘early
detection and early treatment’ can be realized, which is an
important measure to reduce the mortality rate of lung cancer
(3). Therefore, it is extremely
important to search for lung cancer markers with high sensitivity
and specificity.
Lung cancer markers mainly include hormones,
enzymes, alpha-fetoprotein, cell surface membrane antigens and some
cytokines (4). Thioredoxin (Trx) is
a dimeric selenium-enzyme and a member of the pyridine
nucleotide-disulfide oxidoreductase family, which is closely
related to the condition of patients with lung cancer. Previous
studies have demonstrated that Trx plays an important role in the
development of cancer cells, and it is expected to be a new target
for diagnosis, treatment and prognosis of tumor (5). Cytokeratin fragment 21-1 (CYFRA21-1) is
a cytokeratin released into the blood in case of necrosis or lysis
of tumor cells, its sensitivity and concentration increase with the
progression of cancer cells. Therefore, CYFRA21-1 is considered as
one of the best tumor markers for detecting lung cancer (6). Serum squamous cell carcinoma antigen
(SCCA) is a tumor-associated antigen, and its levels in serum and
specificity are higher in lung cancer patients (7). No lung cancer marker with good
specificity and sensitivity has been found yet. Therefore, the
combined detection of multiple tumor markers can complement one
another and make up for deficiencies, thus providing an important
auxiliary basis for the diagnosis and progression of lung cancer
(8). In this study, the diagnostic
value of combined detection of serum Trx, CYFRA21-1 and SCCA in
lung cancer was investigated.
Materials and methods
Clinical data
A total of 65 patients with lung cancer in Weihai
Municipal Hospital (Weihai, China) from January 2014 to June 2017
were selected as the observation group, including 36 males and 29
females with an average age of 55.1±9.6 years. There were 30 cases
of squamous carcinoma, 20 cases of adenocarcinoma and 15 cases of
small cell lung cancer. Another 60 healthy subjects receiving
physical examination were selected as the control group, including
36 males and 24 females with an average age of 52.1±7.2 years.
Inclusion and exclusion criteria
Inclusion criteria: Patients clinically diagnosed
with lung cancer, patients aged ≥18 years, patients who had not
undergone systematic treatment, and patients without other
hereditary diseases. Exclusion criteria: Patients with diseases of
the respiratory system, patients with cardiovascular or
cerebrovascular diseases, handicapped patients, patients infected
with the human immunodeficiency virus (HIV), or patients who had
taken antibiotics in the prior three months. This study was
approved by the Ethics Committee of Weihai Municipal Hospital.
Subjects of the study were informed, agreed to participate in the
clinical study and signed the informed consent.
Methods
After 5 ml fasting venous blood was drawn from
patients and healthy volunteers, it was centrifuged at 2,100 × g
for 15 min at 4°C using a low-temperature high-speed centrifuge to
collect the serum. The levels of serum CYFRA21-1 and SCCA were
detected via chemiluminescence using the full-automatic
chemiluminescence immune analyzer (MKY) and corresponding reagents.
The level of Trx in cells was detected via enzyme-linked
immunosorbent assay (ELISA) with human thioredoxin, Trx ELISA Kit
(Cusabio, Wuhan, China) using a microplate reader (LB942; Berthold,
Bad Wildbad, Germany) and corresponding reagents. The activity of
Trx reductase was analyzed. Samples were collected and instruments
were used strictly according to the manufacturer's
instructions.
Evaluation criteria
The positive evaluation criteria for the three tumor
markers are as follows: SCCA >15 µg/l, CYFRA21-1 >50 µg/l,
and the optical density (OD) value of Trx samples ≥ cut-off value
indicated positive.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
17.0 (supported by Beijing Xinmei Jiahong Technology Co., Ltd.,
Beijing, China) was used for the analysis of all data in this
experiment. Measurement data were expressed as mean ± SD. t-test
was used for the comparison between the two groups, and
multi-factor analysis of variance was used for the comparison among
the groups and the post hoc test was Dunnetts test. Enumeration
data were expressed as rate (%), and Chi-square test was adopted.
The diagnostic values were analyzed using the ROC curve. P<0.05
was considered to indicate a statistically significant
difference.
Results
Analysis of clinical data of
patients
Analysis of sex, age, smoking or not, drinking or
not and body mass index in the observation and control groups
revealed that there were no statistically significant differences
in the groups (P>0.05) (Table
I).
 | Table I.Clinical data of patients [n (%)]. |
Table I.
Clinical data of patients [n (%)].
| Groups | Observation group
(n=65) | Control group
(n=60) | χ2/t
value | P-value |
|---|
| Sex |
|
| 1.346 | 0.285 |
| Male | 36 (55.38) | 27 (45.00) |
|
|
|
Female | 29 (44.62) | 33 (55.00) |
|
|
| Age |
|
| 2.187 | 0.108 |
| ≥55 | 41 (63.08) | 29 (48.33) |
|
|
|
<55 | 24 (36.92) | 31 (51.67) |
|
|
| Smoking |
|
| 0.157 | 0.593 |
| Yes | 38 (58.46) | 32 (53.33) |
|
|
| No | 27 (41.54) | 28 (46.67) |
|
|
| Drinking |
|
| 0.936 | 0.285 |
| Yes | 37 (56.92) | 28 (46.67) |
|
|
| No | 28 (43.08) | 32 (53.33) |
|
|
| Body mass index
(kg/m2) | 21.3±3.9 | 29.6±4.3 | 1.433 | 0.154 |
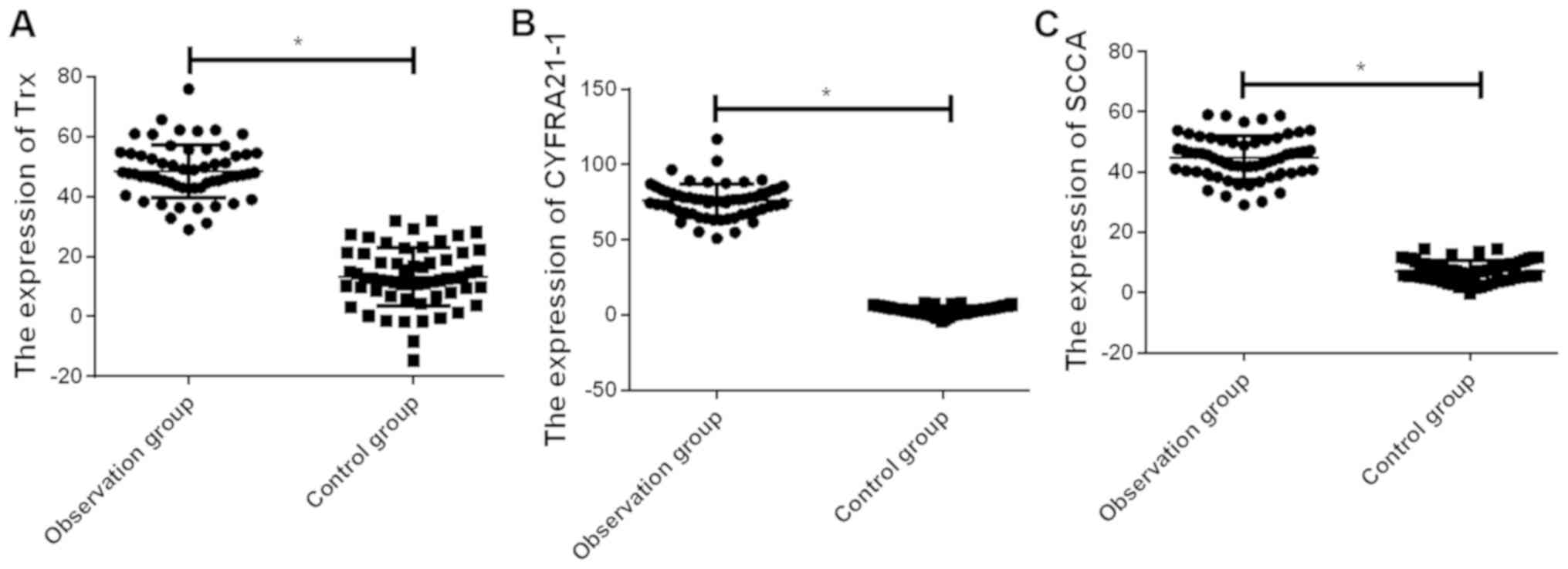
Determination of serum Trx, CYFRA21-1
and SCCA in the observation and control groups
The expression levels of serum Trx, CYFRA21-1 and
SCCA in the observation group were, respectively, 47.6±10.7,
76.6±10.4 and 45.3±6.9 µg/l, which were higher than those in the
control group (13.1±8.9, 3.5±2.8 and 7.8±3.2 µg/l)(P<0.01)
(Fig. 1).
Diagnostic value analysis of single
and combined detection of Trx, CYFRA21-1 and SCCA
The area under the receiver operating characteristic
(ROC) curve of combined detection of the three indexes was the
largest, followed by CYFRA21-1, SCCA and Trx. The value of combined
detection of the three indexes was higher than that of single
detection, and the sensitivity of combined detection of the three
indexes was obviously higher than that of single detection,
displaying statistically significant differences (P<0.05). The
combined detection of the three indexes in lung cancer was the best
with the sensitivity of 86.2% and specificity of 75.0% (Table II and Fig. 2).
 | Table II.ROC curve analysis of single and
combined detection of Trx, CYFRA21-1 and SCCA. |
Table II.
ROC curve analysis of single and
combined detection of Trx, CYFRA21-1 and SCCA.
| Index | Area under curve | OR | 95%CI | P-value | Sensitivity (%) | Specificity (%) |
|---|
| Trx | 0.674 | 0.048 | 0.580–0.768 | 0.001 | 58.5 | 73.3 |
| CYFRA21-1 | 0.829 | 0.037 | 0.757–0.901 | 0.001 | 70.8 | 85.0 |
| SCCA | 0.684 | 0.048 | 0.590–0.779 | 0.001 | 64.6 | 76.0 |
| Combined
detection | 0.851 | 0.035 | 0.782–0.919 | 0.001 | 86.2 | 75.0 |
Comparison of serum Trx, CYFRA21-1 and
SCCA levels in different pathological types of lung cancer
The levels of CYFRA21-1 and SCCA were the highest in
squamous carcinoma, and there were statistically significant
differences compared with those in adenocarcinoma and small cell
lung cancer (P<0.05). The level of Trx was the highest in small
cell lung cancer, and it had statistically significant differences
compared with those in squamous carcinoma and adenocarcinoma
(P<0.05) (Table III).
 | Table III.Comparison of serum Trx, CYFRA21-1 and
SCCA levels in different pathological types of lung cancer. |
Table III.
Comparison of serum Trx, CYFRA21-1 and
SCCA levels in different pathological types of lung cancer.
| Pathological
types | n | Trx (µg/l) | CYFRA21-1 (µg/l) | SCCA (µg/l) |
|---|
| Squamous
carcinoma | 30 | 22.6±21.1 |
95.9±23.9b |
101.8±29.8b |
| Adenocarcinoma | 20 | 18.6±10.8 | 39.9±10.6 | 17.6±10.2 |
| Small cell lung
cancer | 15 | 56.9±8.7a | 30.5±10.6 | 17.5±8.9 |
| F value |
| 12.77 | 10.32 | 14.64 |
| P-value |
| 0.001 | 0.001 | 0.001 |
Comparison of serum Trx, CYFRA21-1 and
SCCA levels in different clinical stages of lung cancer
The levels of serum Trx, CYFRA21-1 and SCCA in lung
cancer patients in clinical stage III–IV were obviously higher than
those in patients in clinical stage I–II, displaying statistically
significant differences (P<0.001). There were no statistically
significant differences in levels of Trx, CYFRA21-1 and SCCA in
patients with lymph node metastasis and distant metastasis
(P>0.05) (Table IV).
 | Table IV.Comparison of serum Trx, CYFRA21-1 and
SCCA levels in different clinical stages of lung cancer. |
Table IV.
Comparison of serum Trx, CYFRA21-1 and
SCCA levels in different clinical stages of lung cancer.
| Groups | n | Trx | CYFRA21-1 | SCCA |
|---|
| Stage I–II | 20 | 17.9±5.7 | 16.5±4.9 | 14.3±5.6 |
| Stage III–IV | 45 | 30.9±6.1 | 28.6±8.4 | 25.5±6.3 |
| t value |
| 8.086 | 5.989 | 6.835 |
| P-value |
| 0.001 | 0.001 | 0.001 |
| Lymph node
metastasis | 28 | 26.3±5.3 | 21.6±2.5 | 24.8±4.3 |
| Distant
metastasis | 25 | 24.5±4.2 | 20.4±5.6 | 25.6±5.9 |
| t value |
| 1.359 | 1.026 | 0.568 |
| P-value |
| 0.180 | 0.310 | 0.572 |
Comparison of positive rates of Trx,
CYFRA21-1 and SCCA in different pathological types of lung
cancer
The positive rate of Trx was the highest in small
cell lung cancer, and the positive rates of CYFRA21-1 and SCCA were
the highest in squamous carcinoma, showing statistically
significant differences compared with other cancers (P<0.05)
(Table V).
 | Table V.Comparison of positive rates of Trx,
CYFRA21-1 and SCCA in different pathological types of lung cancer
[n (%)]. |
Table V.
Comparison of positive rates of Trx,
CYFRA21-1 and SCCA in different pathological types of lung cancer
[n (%)].
| Pathological
types | n | Trx | CYFRA21-1 | SCCA |
|---|
| Squamous
carcinoma | 30 | 16 (53.33) | 24
(80.00)b | 26
(86.67)c |
| Adenocarcinoma | 25 | 13 (52.00) | 9 (36.00) | 10 (40.00) |
| Small cell lung
cancer | 10 | 9
(90.00)a | 4 (40.00) | 6
(60.00) |
| χ2
value |
| 4.851 | 12.147 | 13.099 |
| P-value |
| 0.050 | 0.002 | 0.001 |
Discussion
The incidence and mortality rates of lung cancer
have shown increasing trends in recent years, seriously threatening
people's health. However, it is noteworthy that there are generally
no obvious symptoms in the early stage of lung cancer, and it is
hard for patients to notice. At the same time, rich blood supply in
lung tissues provides favourable conditions for the metastasis of
cancer cells, so that patients are often in the late stage once
there are obvious clinical symptoms, leading to difficult
treatment, which is also an important cause of the high mortality
rate of lung cancer (9). Therefore,
the early diagnosis and treatment of lung cancer are of great
importance. In the diagnosis of lung cancer, tissue biopsy serves
as a golden standard in clinic, but it is only applied when
symptoms develop and patients need to be diagnosed, and cannot be
used in the early screening of lung cancer due to trauma (10). Therefore, the value of tumor markers
in the diagnosis of early lung cancer has gained extensive
attention.
The oxidative active amino acid composition of Trx
is Cys-Gly-Pys-Cys, and Trx can catalyze the redox reaction and
also maintain intracellular homeostasis, regulate apoptosis and
resist oxidative stress response under biological functions
(11). A number of studies have
demonstrated that the expression of Trx is increased in a variety
of malignant tumors, such as gastric, breast, liver, lung, rectal
and cervical cancers (7,12), indicating that Trx is closely related
to malignant tumors. It was found in related studies that Trx can
inhibit apoptosis of tumor cells, promote cell proliferation and
disturb normal cell cycle (13).
CYFRA21-1 is a cytokeratin existing in cells of epithelium-derived
malignant tumors. A large amount of soluble CYFRA21-1 enters the
blood circulation in case of lysis or death of tumor cells
(14). Moreover, studies have
revealed that the expression level of serum CYFRA21-1 in patients
with lung cancer is remarkably higher than that in healthy
individuals, so it can be used in the identification of malignant
lesions in the lung (15). SCCA is a
tissue antigen, which is mainly used in the diagnosis of squamous
carcinoma, including squamous carcinoma in the head and neck, lung
and trachea, esophagus, cervix, anal canal, genitourinary tract and
brain, monitoring of condition and prognosis and therapeutic
evaluation (16).
In this study, it was found that the expression
levels of serum Trx, CYFRA21-1 and SCCA in the observation group
were higher than those in the control group (P<0.01), and there
were significant differences between the two groups, indicating
that the tumor markers (Trx, CYFRA21-1 and SCCA) have important
value in the clinical diagnosis of lung cancer. The above results
are basically consistent with those in related results, and the
more severe the disease is, the higher the levels of tumor markers
are (17), suggesting that the
levels of serum Trx, CYFRA21-1 and SCCA can provide a certain
reference basis for the diagnosis of lung cancer. In this study,
the levels of CYFRA21-1 and SCCA were the highest in squamous
carcinoma, and there were statistically significant differences
compared with those in adenocarcinoma and small cell lung cancer
(P<0.05). The level of Trx was the highest in small cell lung
cancer, and it had statistically significant differences compared
with those in squamous carcinoma and adenocarcinoma (P<0.05),
suggesting that CYFRA21-1 and SCCA are superior in detecting
squamous carcinoma to adenocarcinoma and small cell lung cancer,
and Trx is more valuable in detecting small cell lung cancer. SCCA
is a good marker for the diagnosis of squamous carcinoma, which can
monitor the disease development and evaluate the prognosis. Trx,
CYFRA21-1 and SCCA are of important significance in guiding the
diagnosis and treatment of lung squamous carcinoma. The three
indexes can improve the diagnostic accuracy rate of lung cancer and
help provide diagnostic information, so as to perform the targeted
treatment, improve the therapeutic efficiency and prolong the life
of patients, which are basically consistent with results in related
studies (18). In this study, the
levels of serum Trx, CYFRA21-1 and SCCA in lung cancer patients in
clinical stage III–IV were obviously higher than those in patients
in clinical stage I–II, indicating that Trx, CYFRA21-1 and SCCA are
related to the progression of lung cancer, and they can be used to
monitor metastasis and recurrence of malignant tumor in clinic. The
concentration of serum tumor markers is positively correlated with
the tumor size, which is also consistent with related literature
reports (19). Results of this study
manifested that despite high specificity of single detection of
Trx, CYFRA21-1 and SCCA, the sensitivity is unsatisfactory, failing
to meet the clinical requirements. Therefore, lung cancer should
not be diagnosed using only one single index at present, but
multiple indexes should be combined for joint diagnosis, thus
improving the accuracy. The sensitivity of combined detection of
the three indexes is improved, and the sensitivity, specificity and
accuracy are 86.8, 80.4 and 89.6%, respectively. It can be seen
that both sensitivity and specificity are significantly improved
compared with single detection, thus benefiting the early screening
of lung cancer patients, performing the treatment as early as
possible improves the prognosis, which is consistent with the
report of Jiang et al (20).
Combined detection plays an important role in the diagnosis of lung
cancer, which can be used in the early screening of patients with
lung cancer. The ROC curve can reflect the specificity, sensitivity
and accuracy of detection indexes objectively and accurately. The
closer the area under the curve is to 0.5, the lower the diagnostic
accuracy will be, and the closer it is to 1.0, the higher the
diagnostic accuracy will be. In this study, the area under the ROC
curve of combined detection of the three indexes was the largest,
followed by CYFRA21-1, SCCA and Trx. The area under the curve was
<0.9 in single detection of the three indexes and 0.906 in
combined detection, and the combined detection of the three indexes
in lung cancer was optimal with the sensitivity of 86.2% and
specificity of 75.0%, proving that both accuracy and efficiency of
combined detection are superior to those of single detection of any
index, which are consistent with results of Zhang et al
(21). The above results suggest
that the combined detection is able to improve the diagnostic
rate.
The combined detection of the three indexes can
improve the accuracy and can also be used in the early screening
and early treatment of lung cancer and the improvement of
prognosis. However, there are still some false-negative and
false-positive cases in the combined detection of the three indexes
in the diagnosis of lung cancer, indicating that lung cancer still
cannot be diagnosed only using one means, but imaging and
pathological methods should be combined for comprehensive
evaluation, thereby avoiding misdiagnosis and improving diagnostic
accuracy. The sample size in this study was small, and there was a
certain selection bias, so further study is still necessary for
verification.
In conclusion, the detection of serum Trx, CYFRA21-1
and SCCA is of great significance in the diagnosis, progression and
pathological type of lung cancer, and combined detection can
improve both specificity and sensitivity, which is more conducive
to the positive rate of diagnosis of lung cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TQ, JZ, NX and BL collected and analyzed the general
data of patients. ML and ALiu performed ELISA. ALi and HT evaluated
the tumor markers. All the authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Weihai Municipal Hospital (Weihai, China). Signed informed consents
were obtained from the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
D'Addario G, Früh M, Reck M, Baumann P,
Klepetko W and Felip E; ESMO Guidelines Working Group, : Metastatic
non-small-cell lung cancer: ESMO Clinical Practice Guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 21 (Suppl
5):v116–v119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vansteenkiste J, Crinò L, Dooms C,
Douillard JY, Faivre-Finn C, Lim E, Rocco G, Senan S, Van Schil P,
Veronesi G, et al Panel Members, : 2nd ESMO Consensus Conference on
Lung Cancer: Early-stage non-small-cell lung cancer consensus on
diagnosis, treatment and follow-up. Ann Oncol. 25:1462–1474. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boonstra MC, de Geus SW, Prevoo HA,
Hawinkels LJ, van de Velde CJ, Kuppen PJ, Vahrmeijer AL and Sier
CF: Selecting targets for tumor imaging: An overview of
cancer-associated membrane proteins. Biomark Cancer. 8:119–133.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang B, Shao W, Zhu C, Wen G, Yue X, Wang
R, Quan J, Du J and Bu X: Mitochondria-targeted approach:
remarkably enhanced cellular bioactivities of TPP2a as selective
inhibitor and probe toward TrxR. ACS Chem Biol. 11:425–434. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Negrin LL, Halat G, Kettner S, Gregori M,
Ristl R, Hajdu S and Heinz T: Club cell protein 16 and cytokeratin
fragment 21-1 as early predictors of pulmonary complications in
polytraumatized patients with severe chest trauma. PLoS One.
12:e01753032017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holdenrieder S, Molina R, Qiu L, Zhi X,
Rutz S, Engel C, Kasper-Sauer P, Dayyani F and Korse CM: Technical
and clinical performance of a new assay to detect squamous cell
carcinoma antigen levels for the differential diagnosis of
cervical, lung, and head and neck cancer. Tumour Biol.
40:10104283187722022018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakamura H and Nishimura T: History,
molecular features, and clinical importance of conventional serum
biomarkers in lung cancer. Surg Today. 47:1037–1059. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen YW, Zhang XM, Li ST, Lv M, Yang J,
Wang F, Chen ZL, Wang BY, Li P, Chen L, et al: Efficacy and safety
of icotinib as first-line therapy in patients with advanced
non-small-cell lung cancer. OncoTargets Ther. 9:929–935. 2016.
View Article : Google Scholar
|
|
10
|
He WJ, Li WH, Jiang B, Wang YF, Xia YX and
Wang L: MicroRNAs level as an initial screening method for
early-stage lung cancer: A bivariate diagnostic random-effects
meta-analysis. Int J Clin Exp Med. 8:12317–12326. 2015.PubMed/NCBI
|
|
11
|
Arjune S, Schwarz G and Belaidi AA:
Involvement of the Cys-Tyr cofactor on iron binding in the active
site of human cysteine dioxygenase. Amino Acids. 47:55–63. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng X, Xu W, Sun R, Yin H, Dong C and
Zeng H: Synergism between thioredoxin reductase inhibitor ethaselen
and sodium selenite in inhibiting proliferation and inducing death
of human non-small cell lung cancer cells. Chem Biol Interact.
275:74–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
You BR, Kim SH and Park WH: Reactive
oxygen species, glutathione, and thioredoxin influence suberoyl
bishydroxamic acid-induced apoptosis in A549 lung cancer cells.
Tumour Biol. 36:3429–3439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dohmoto K, Hojo S, Fujita J, Yang Y, Ueda
Y, Bandoh S, Yamaji Y, Ohtsuki Y, Dobashi N, Ishida T, et al: The
role of caspase 3 in producing cytokeratin 19 fragment (CYFRA21-1)
in human lung cancer cell lines. Int J Cancer. 91:468–473. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu L, Teng J, Zhang L, Cong P, Yao Y, Sun
G, Liu Z, Yu T and Liu M: The combination of the tumor markers
suggests the histological diagnosis of lung cancer. BioMed Res Int.
2017:20139892017.PubMed/NCBI
|
|
16
|
Zhao W, Yu H, Han Z, Gao N, Xue J and Wang
Y: Clinical significance of joint detection of serum CEA, SCCA, and
bFGF in the diagnosis of lung cancer. Int J Clin Exp Pathol.
8:9506–9511. 2015.PubMed/NCBI
|
|
17
|
Chen F, Wang XY, Han XH, Wang H and Qi J:
Diagnostic value of Cyfra21-1, SCC and CEA for differentiation of
early-stage NSCLC from benign lung disease. Int J Clin Exp Med.
8:11295–11300. 2015.PubMed/NCBI
|
|
18
|
Chen MS, Xu Y, Ma J, Wu CG, Hao XK, Lu BB
and Liu T: Relation between the level of TPS, NSE, CEA and beta2-mG
in the serum and the biological behavior of small cell lung cancer.
Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 23:751–753. 2007.(In Chinese).
PubMed/NCBI
|
|
19
|
Schmidt B, Beyer J, Dietrich D, Bork I,
Liebenberg V and Fleischhacker M: Quantification of cell-free
mSHOX2 Plasma DNA for therapy monitoring in advanced stage
non-small cell (NSCLC) and small-cell lung cancer (SCLC) patients.
PLoS One. 10:e01181952015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang ZF, Wang M and Xu JL: Thymidine
kinase 1 combined with CEA, CYFRA21-1 and NSE improved its
diagnostic value for lung cancer. Life Sci. 194:1–6. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang L, Liu D, Li L, Pu D, Zhou P, Jing
Y, Yu H, Wang Y, Zhu Y, He Y, et al: The important role of
circulating CYFRA21-1 in metastasis diagnosis and prognostic value
compared with carcinoembryonic antigen and neuron-specific enolase
in lung cancer patients. BMC Cancer. 17:962017. View Article : Google Scholar : PubMed/NCBI
|