Introduction
Lung cancer develops from the respiratory system.
Incidence and mortality rate of lung cancer ranks first among all
malignancies in the world. With the aggregated environmental
pollution, increased number of smokers and growth of aging
population, incidence of lung cancer shows an increasing trend
(1,2). Lung cancer affects 1.6 million
individuals every year, and this number is still increasing. As the
most common type of lung cancer, non-small cell lung cancer (NSCLC)
accounts for 80–85% of all the cases (3,4).
Radiotherapy and chemotherapy are conventional
treatments for NSCLC. However, chemotherapy has been proved to be
ineffective in improving the survival of patients (5,6). In
recent years, studies on patients with stage II and IIIa NSCLC have
shown that postoperative radiotherapy significantly shortens
patients' survival time. Therefore, identification of novel
therapeutic targets is urgently needed (7,8). miRNAs
are widely expressed in eukaryotic cell organisms and regulate cell
proliferation, differentiation, and apoptosis. Abnormal changes in
miRNA biosynthesis are involved in a variety of pathophysiological
processes (9,10). Chen et al (11) reported that the downregulation of
miR-100 is closely related to the progression and prognosis of
hepatocellular carcinoma. Xu et al (12) found that miR-100 is downregulated in
human bladder epithelial carcinoma and that the elevated miR-100
expression in bladder cancer cells inhibits cell proliferation and
migration.
However, the involvement of miR-100 in lung cancer
still has not been reported. In this study, the expression of
miR-100 in 283 patients with NSCLC was investigated and the
association with patients' clinicopathological features was
investigated to study the effects of miR-100 on patient
prognosis.
Patients and methods
Subjects
A total of 283 patients with NSCLC were selected
from February 2013 to April 2015 in The First Hospital of Jiaxing
(Jiaxing, China). The patients included 128 males and 155 females,
with a mean age of 56.32±11.03 years. All patients were
pathologically diagnosed as NSCLC, including 176 patients with
invasive adenocarcinoma, 70 patients with squamous cell carcinoma
and 37 large cell carcinoma. None of the patients had previous
history of a tumor. Patients with organ dysfunction such as liver
or kidney, abnormal bleeding or abnormal coagulation function were
excluded. All the patients have complete clinical and follow-up
data. Patients who received treatment, patients with large tumors,
patients with other pulmonary or chest wall disease, and patients
who died of other diseases were excluded. This study was approved
by the Ethics Committee of The First Hospital of Jiaxing. All
patients or their families signed an informed consent.
Extraction of total RNA
Total RNA was extracted from cancer and normal
adjacent tissues using TRIzol reagent (Shanghai Mingjing
Biotechnology Co., Ltd.) according to the manufacturers
instructions. A micro-ultraviolet spectrophotometer MD1000
(Thmorgan Biotechnology Co., Ltd.) was used to measure the
concentration and analyze the purity of RNA samples, and 3% agarose
gel electrophoresis (Jingke Chemical Technology Co., Ltd.) was used
to analyze the integrity of RNA.
Total RNA was subjected to reverse transcription
(45°C for 45 min and 95°C for 5 min) to synthesize cDNA, followed
by preparation of PCR reaction system using fluorescence
quantitative SYBR-Green PCR kit (cat. no. 4364344; Thermo Fisher
Scientific, Inc.). PCR reaction conditions were: 95°C for 10 min,
followed by 95°C for 10 sec, 60°C for 20 sec, 72°C for 10 sec, and
72°C for 5 min. U6 was used as an endogenous control, and each
experiment was performed 3 times. Data were analyzed by the
2−ΔΔCq method (13).
Primers were synthesized by Suzhou Yaxun Biotechnology Co., Ltd.
The primer sequences are shown in Table
I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Variables | Forward primers | Reverse primers |
|---|
| miR-100 | 5′-CGACGAGGC | 5′-CCATCGATG |
|
| GTTGCCTGCACC-3′ | GAATCTTTAAC-3 |
| U6 | 5′-CGCTTCGGC | 5′-TTCACGAAT |
|
| AGCACATATAC-3′ | TTGCGTGTCAT-3′ |
Observation indicators
Association between the expression of miR-100 and
clinicopathological features of NSCLC were explored. All patients
were followed up for a maximum of 60 months. The association
between miR-100 expression and survival was analyzed.
Statistical analysis
SPSS19.0 [AsiaAnalytics (formerly SPSS China)] was
used for all statistical analyses. Enumeration data were expressed
as rate and compared by χ2 test. Measurement data were
expressed as mean ± standard deviation and normal distribution was
tested by K-S test. Normal distribution measurement data were
compared by t-test, and non-normal distribution data were compared
by Chi-square test. Association between the miR-100 expression and
clinicopathological features of patients were analyzed by
cross-tabulation analysis. Univariate analysis was performed using
Kaplan-Meier analysis and the log-rank test. Multivariate analysis
was performed using the Cox model for multivariate analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
General information
Among the 283 NSCLC patients, there were 176
invasive adenocarcinoma, 70 squamous cell carcinoma, and 37 large
cell carcinoma. According to TNM staging, 113 patients were in
stage I, 71 in stage II, 88 in stage III, and 11 in stage IV.
Follow-up time was no more than 60 months, and median was 31
months, and mean follow-up time was 25.32±14.33 months. During
follow-up, 155 patients died and lymph node metastasis occurred in
150 patients (Table II).
 | Table II.General information. |
Table II.
General information.
| Variables | No. (%) |
|---|
| Sex [n (%)] |
| Male | 128 (45.23) |
|
Female | 155 (54.77) |
| Age | 56.32±11.03 |
| Histological type [n
(%)] |
| Invasive
adenocarcinoma | 176 (62.19) |
| Squamous
cell carcinoma | 70
(24.73) |
| Large
cell carcinoma | 37
(13.07) |
| TNM stage [n
(%)] |
| I | 113 (39.93) |
| II | 71
(25.09) |
| III | 88
(31.10) |
| IV | 11 (3.89) |
| Follow-up time
(month) |
| Median
follow-up time | 31 |
| Mean
follow-up time | 25.32±14.33 |
| Follow-up results [n
(%)] |
|
Survival |
|
Metastasis | 15 (5.30) |
|
Non-metastasis | 113 (39.93) |
|
Death |
|
Metastasis | 135 (47.70) |
|
Non-metastasis | 20 (7.07) |
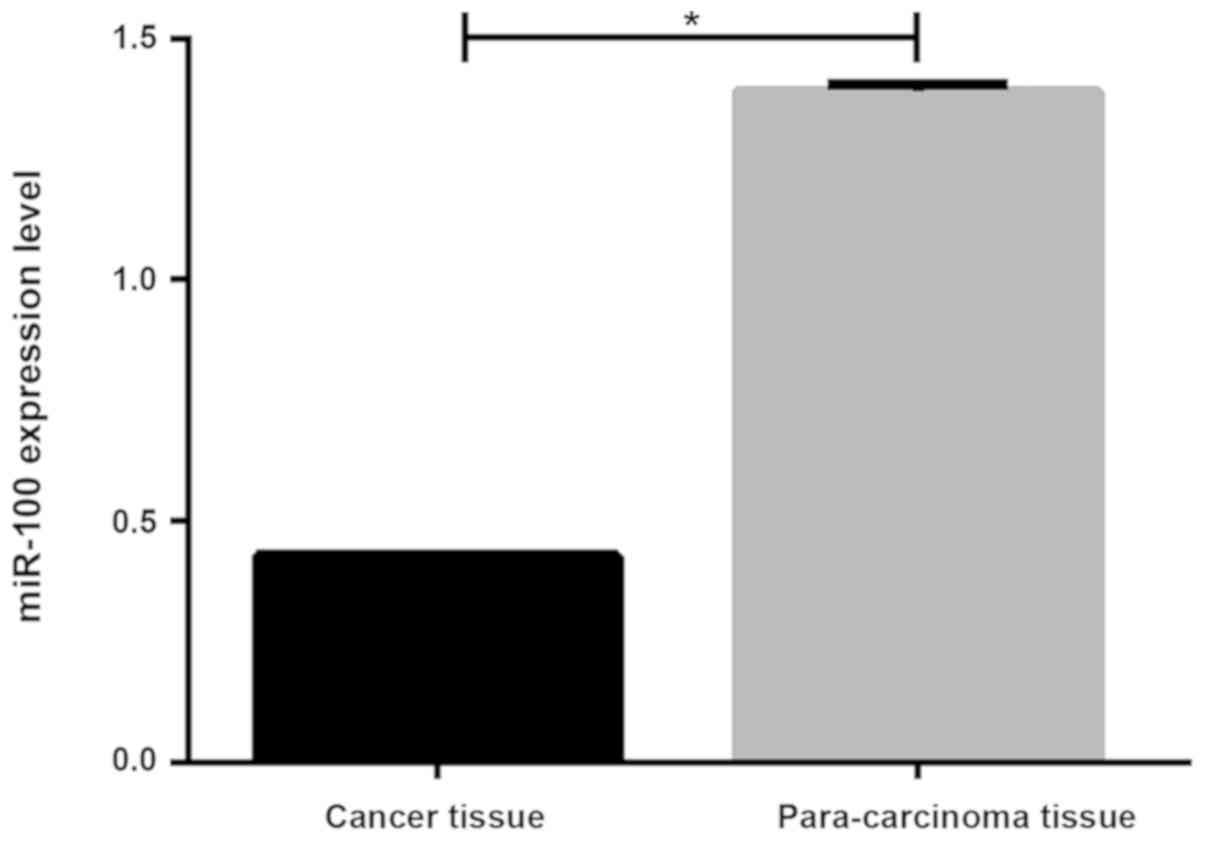
Expression of miR-100 in cancer
tissues and adjacent healthy tissues of NSCLC patients
RT-qPCT results showed that the miR-100 expression
level was significantly lower in tumor tissues than in adjacent
tissues (0.429±0.004 vs. 1.292±0.013, P<0.05, Fig. 1).
Association between miR-100 expression
and clinicopathological features
According to the median expression level of miR-100
in cancer tissue (0.413), patients were divided into the high
expression and low expression groups. Results of cross-tabulation
analysis showed that the low expression level of miR-100 was
associated with the patients' age, TNM stage, metastasis,
histological type (P<0.05), but not sex (P>0.05). The
proportion of patients with low miR-100 expression was higher in
patients who died than in those who survived (P<0.05; Table III).
 | Table III.Association between miR-100 expression
and clinicopathological features. |
Table III.
Association between miR-100 expression
and clinicopathological features.
| Variables | Low expression | High expression | χ2
value | P-value |
|---|
| Cases | 170 | 113 |
|
|
| Sex |
|
| 1.134 | 0.233 |
| Male | 75 | 53 |
|
|
|
Female | 95 | 60 |
|
|
| Age |
|
| 2.050 | 0.042 |
| <56.32
years | 71 | 59 |
|
|
| ≥56.32
years | 99 | 54 |
|
|
| TNM stage |
|
| 2.039 | 0.041 |
| I+II | 101 | 83 |
|
|
|
III+IV | 69 | 30 |
|
|
| Metastasis |
|
| 8.138 | 0.001 |
| Yes | 145 | 5 |
|
|
| No | 25 | 108 |
|
|
| Histological
type |
|
| 2.012 | 0.045 |
| Invasive
adenocarcinoma (n=176) | 113 | 63 |
|
|
| Squamous
cell carcinoma (n=70) | 36 | 34 |
|
|
| Large
cell carcinoma (n=37) | 21 | 16 |
|
|
| Follow-up
results |
|
| 2.349 | 0.019 |
|
Survival | 61 | 67 |
|
|
|
Death | 109 | 46 |
|
|
Univariate and multivariate analysis
of association between miR-100 expression and prognosis using
Kaplan-Meier analysis and the log-rank test
Cox single factor regression analysis was performed
on 283 patients. Univariate prognostic analysis showed that the
miR-100 expression level, age, TNM staging, and metastasis may be
risk factors for poor prognosis in patients with NSCLC. Cox
multivariate regression analysis showed that the low miR-100
expression levels, advanced TNM stages and tumor metastasis are
independent risk factors for poor prognosis of NSCLC (Tables IV and V).
 | Table IV.Univariate and multivariate analysis
of association between miR-100 expression and prognosis. |
Table IV.
Univariate and multivariate analysis
of association between miR-100 expression and prognosis.
|
| Univariate
analysis |
|---|
|
|
|
|---|
| Variables | HR (95% CI) | P-value |
|---|
| miRNA-100 (low vs.
high) | 1.648
(1.122–2.896) | 0.010 |
| Sex (male vs.
female) | 1.012
(0.797–1.620) | 0.559 |
| Age (<56.32 vs.
≥56.32 years) | 1.132
(1.028–1.715) | 0.014 |
| TNM stages (I and
II vs. III and IV) | 2.821
(1.346–2.857) | 0.013 |
| Metastasis (yes vs.
no) | 3.053
(1.282–7.323) | 0.011 |
| Histological type
(ACC vs. SCC) | 0.921
(0.831–1.525) | 0.325 |
 | Table V.Multivariate analysis of association
between miR-100 expression and prognosis. |
Table V.
Multivariate analysis of association
between miR-100 expression and prognosis.
|
| Multivariate
analysis |
|---|
|
|
|
|---|
| Variables | HR (95% CI) | P-value |
|---|
| miRNA-100 (low vs.
high) | 1.328
(1.022–2.416) | 0.006 |
| TNM stages (I and
II vs. III and IV) | 2.231
(1.357–2.547) | 0.005 |
| Metastasis (yes vs.
no) | 3.053
(1.282–7.323) | 0.011 |
Discussion
Occurrence and development of NSCLC, including
adenocarcinoma, squamous cell carcinoma, and large cell carcinoma,
are closely related to internal gene expression and external
environment (14). miR-100 is
located on human chromosome 11q24.1 position, and its nucleic acid
sequence is highly conserved, and may be closely related to the
growth and development of human body (15). Studies have shown that miR-100 is
closely associated with the occurrence, development, invasion and
metastasis of colorectal, endometrial and breast cancers (16–18). In
this study, we explored the expression and clinical significance of
miR-100 in patients with NSCLC with an expectation of providing a
new target for clinical treatment and prognosis assessment of this
disease.
Results of this study showed that the expression
level of miR-100 was significantly lower in cancer tissues of NSCLC
patients than in normal adjacent tissues. Therefore, we
hypothesized that miR-100 may act as a tumor suppressor gene in
this disease. Huang et al (19) found that the miR-100 expression was
low in cancerous tissues of patients with pancreatic cancer. Leite
et al (20) also showed that
miR-100 functions as a tumor suppressor gene in patients with
prostate cancer. In this study, we analyzed the association between
the expression level of miR-100 and clinicopathological features of
patients with NSCLC. We found that the low expression level of
miR-100 was associated to the patients' age, TNM stage, metastasis,
and histological type. The involvement of miR-100 in NSCLC still
has not been well studied. Chen et al (11) found that the low expression of
miR-100 was closely associated with clinical grade, lymph node
metastasis, and TNM stages of hepatocellular carcinoma. Consistent
with this study, we also found that advanced tumor stages and tumor
metastasis were associated with the low expression level of
miR-100. However, Chen et al (11) reported no significant association
between the low expression of miR-100 and the age of the patients,
which may be explained by the number of subjects included in this
study. We also analyzed the association between miR-100 expression
and patients' 5-year follow-up results. We found that the
proportion of patients with low miR-100 expression was higher in
patients who died than in those who survived. Therefore, we
hypothesize that the low expression level of miR-100 is closely
related to the patients' age, TNM staging, metastasis, histological
type, and affects the prognosis of patients.
We analyzed the expression level of miR-100 and
prognosis of patients. Analysis of the results showed that the low
expression of miR-100, advanced TNM stage and metastasis are
independent risk factors for NSCLC. A meta-analysis carried out by
Chen et al (21) showed that
expression of miR-100 is associated with the survival of cancer
patients and may be a clinical prognostic factor for cancer. Cao
et al (22) also found that
the low expression of miR-100 may be a prognostic risk factor for
bladder cancer. Similar results were found in this study,
indicating that expression level of miR-100 may serve as a
predictor of prognosis of NSCLC. Our study provided new insights
for diagnosis and treatment of NSCLC. However, only cancer tissues
and adjacent healthy tissues were used in this study and the
clinical value of serum miR-100 was not investigated. In addition,
the sample size of this study is small. Therefore, further studies
with a larger number of samples are needed to confirm the
conclusions in this study.
In summary, the expression level of miR-100 is
relatively lower in cancer tissues than in normal adjacent tissues
in NSCLC patients. The low expression level of miR-100 is closely
associated with poor prognosis of patients. Therefore, miR-100
shows potential as a prognostic marker for NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XM drafted the manuscript and performed PCR. JZ and
HM extracted total RNA. YY was responsible for statistical
analysis. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The First Hospital of Jiaxing (Jiaxing, China). Signed informed
consents were obtained from the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rizvi NA, Hellmann MD, Snyder A, Kvistborg
P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al: Cancer
immunology. Mutational landscape determines sensitivity to PD-1
blockade in non-small cell lung cancer. Science. 348:124–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lortet-Tieulent J, Soerjomataram I, Ferlay
J, Rutherford M, Weiderpass E and Bray F: International trends in
lung cancer incidence by histological subtype: adenocarcinoma
stabilizing in men but still increasing in women. Lung Cancer.
84:13–22. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Markou A, Sourvinou I, Vorkas PA, Yousef
GM and Lianidou E: Clinical evaluation of microRNA expression
profiling in non small cell lung cancer. Lung Cancer. 81:388–396.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang JJ, Chen HJ, Yan HH, Zhang XC, Zhou
Q, Su J, Wang Z, Xu CR, Huang YS, Wang BC, et al: Clinical modes of
EGFR tyrosine kinase inhibitor failure and subsequent management in
advanced non-small cell lung cancer. Lung Cancer. 79:33–39. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
a randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó
L, Ahn MJ, De Pas T, Besse B, Solomon BJ, Blackhall F, et al:
Crizotinib versus chemotherapy in advanced ALK-positive lung
cancer. N Engl J Med. 368:2385–2394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Molina R, Filella X, Augé JM, Fuentes R,
Bover I, Rifa J, Moreno V, Canals E, Viñolas N, Marquez A, et al:
Tumor markers (CEA, CA 125, CYFRA 21-1, SCC and NSE) in patients
with non-small cell lung cancer as an aid in histological diagnosis
and prognosis. Comparison with the main clinical and pathological
prognostic factors. Tumour Biol. 24:209–218. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okamura K, Takayama K, Izumi M, Harada T,
Furuyama K and Nakanishi Y: Diagnostic value of CEA and CYFRA 21-1
tumor markers in primary lung cancer. Lung Cancer. 80:45–49. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Köttgen A, Albrecht E, Teumer A, Vitart V,
Krumsiek J, Hundertmark C, Pistis G, Ruggiero D, O'Seaghdha CM,
Haller T, et al LifeLines Cohort Study; CARDIoGRAM Consortium;
DIAGRAM Consortium; ICBP Consortium; MAGIC Consortium, :
Genome-wide association analyses identify 18 new loci associated
with serum urate concentrations. Nat Genet. 45:145–154. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen P, Zhao X and Ma L: Downregulation of
microRNA-100 correlates with tumor progression and poor prognosis
in hepatocellular carcinoma. Mol Cell Biochem. 383:49–58. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu C, Zeng Q, Xu W, Jiao L, Chen Y, Zhang
Z, Wu C, Jin T, Pan A, Wei R, et al: miRNA-100 inhibits human
bladder urothelial carcinogenesis by directly targeting mTOR. Mol
Cancer Ther. 12:207–219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ashworth A, Rodrigues G, Boldt G and Palma
D: Is there an oligometastatic state in non-small cell lung cancer?
A systematic review of the literature. Lung Cancer. 82:197–203.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fu HL, Wu DP, Wang XF, Wang JG, Jiao F,
Song LL, Xie H, Wen XY, Shan HS, Du YX, et al: Altered miRNA
expression is associated with differentiation, invasion, and
metastasis of esophageal squamous cell carcinoma (ESCC) in patients
from Huaian, China. Cell Biochem Biophys. 67:657–668. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang XD, Xu XH, Zhang SY, Wu Y, Xing CG,
Ru G, Xu HT and Cao JP: Role of miR-100 in the radioresistance of
colorectal cancer cells. Am J Cancer Res. 5:545–559.
2015.PubMed/NCBI
|
|
17
|
Qin C, Huang RY and Wang ZX: Potential
role of miR-100 in cancer diagnosis, prognosis, and therapy. Tumour
Biol. 36:1403–1409. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gong Y, He T, Yang L, Yang G, Chen Y and
Zhang X: The role of miR-100 in regulating apoptosis of breast
cancer cells. Sci Rep. 5:116502015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang JS, Egger ME, Grizzle WE and McNally
LR: MicroRNA-100 regulates IGF1-receptor expression in metastatic
pancreatic cancer cells. Biotech Histochem. 88:397–402. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leite KR, Morais DR, Reis ST, Viana N,
Moura C, Florez MG, Silva IA, Dip N and Srougi M: MicroRNA 100: a
context dependent miRNA in prostate cancer. Clinics (São Paulo).
68:797–802. 2013. View Article : Google Scholar
|
|
21
|
Chen J, Zheng B, Wang C, Chen Y, Du C,
Zhao G, Zhou Y and Shi Y: Prognostic role of microRNA-100 in
various carcinomas: evidence from six studies. Tumour Biol.
35:3067–3071. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cao YH, Zhang HH, Xu HF, Duan YJ, Li Q and
Huang B: Prognostic role of microRNA-100 in patients with bladder
cancer. Genet Mol Res. 14:15948–15954. 2015. View Article : Google Scholar : PubMed/NCBI
|