Introduction
Gastric cancer (GC) is the second most common cause
of cancer-associated mortality and the second most common type of
malignancy in China (1). Although
the nationwide incidence and mortality rates of gastric cancer in
China have decreased in recent years, with an incidence rate of
10.79% and a mortality rate of 12.8% in 2014 (2), it remains a major public health
problem. Due to the high rate of recurrence or metastasis, the
5-year survival rate of GC is only 5–20% (3). Chemotherapy before or after a surgical
resection is a crucial adjuvant treatment for advanced GC,
providing a method to expand patients' lifetime (4). In the last several decades, new methods
have been sought to improve the prognosis of advanced GC, whereas
the standard interventions have not been changed. Therefore, novel
pharmaceuticals are urgently needed to improve the outcomes of
patients with advanced GC.
Propyl isothiocyanate (PITC) is one of the natural
isothiocyanates (ITCs) with the characteristic chemical group
-N=C=S (Fig. 1A). Natural ITCs are
generally the hydrolysates of glucosinolates (GLS), which are
innocuous and widely exist in cruciferous plants (5). While GLS are non-toxic, ITCs exert
toxicity by interacting with thiol- and amine-groups in peptides
and proteins (6), exhibiting a wide
spectrum of bioactivities, including anticancer properties
(7). Glutathione (GSH) and several
mitochondrial proteins, such as ATP synthase (Complex I),
cytochrome c oxidase (Complex IV) and thioredoxin-dependent
peroxide reductase have been identified as potential ITC targets,
the inhibition of which may lead to reactive oxygen species (ROS)
accumulation (8–10). Certain ITCs, such as sulforaphane,
allyl ITC (AITC), benzyl ITC and phenethyl ITC have been
demonstrated to effectively inhibit cell proliferation and induce
apoptosis of multiple types of cancer cells (11–14).
However, no detailed studies have investigated the bioactivity of
PITC in vitro and in vivo. In the present study, the
anti-neoplastic activity of PITC in GC cell lines (MGC-803 and
HGC-27) was investigated to provide insight into the molecular
mechanisms involved, which may be the experimental evidence for
using PITC as a new natural anti-cancer medicine for GC.
Materials and methods
Drugs and antibodies
PITC and dimethyl sulfoxide (DMSO) were purchased
from Sigma-Aldrich; Merck KGaA. N-acetyl-L-cysteine (NAC) was
purchased from Beyotime Institute of Biotechnology. PITC was
dissolved in DMSO to prepare stock solutions for in vitro
assays and stored at −20°C. Cells in the control groups were
treated with vehicle at an equal volume. To minimize toxicity, DMSO
concentration was >0.1% for cell culture. Anti-poly-ADP-ribose
polymerase (PARP; catalog no. 9542; 1:1,000), anti-cleaved
caspase-3 (catalog no. 9662; 1:1,000), anti-cleaved caspase-9
(catalog no. 9502; 1:1,000), anti-GAPDH (catalog no. 5174;
1:1,000), anti-cytochrome c (Cyt c; catalog no. 4272; 1:1,000),
anti-p53 (catalog no. 2527; 1:1,000) and anti-phosphorylated p53
(p-p53; catalog no. 2521; 1:1,000) antibodies were purchased from
Cell Signaling Technology, Inc. Anti-cyclin A1 antibody (catalog
no. ab118897; 1:1,000) was obtained from Abcam, Inc. Antibodies
against Bcl-2 (catalog no. A2845; 1:1,000), Bax (catalog no. A0207;
1:1,000) and β-tubulin (catalog no. AC008; 1:1,000 for western blot
analysis and 1:400 for immunofluorescence assay) were purchased
from ABclonal Biotech Co., Ltd.
Cells and cell culture
Human GC MGC-803 and HGC-27 cell lines were
purchased from the Shanghai Institute of Cell Biology, Chinese
Academy of Sciences, and cultured in RPMI-1640 (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) and 100 µg/ml streptomycin
and 100 U/ml penicillin (HyClone; GE Healthcare Life Sciences) in a
37°C incubator with a humidified atmosphere and 5%
CO2.
Cell viability assay
The effects of PITC on GC cells were evaluated by
Cell Counting Kit-8 [CCK-8; Yeasen Biotechnology (Shanghai) Co.,
Ltd.]. Prior to treatment, MGC-803 and HGC-27 cells were plated in
96-well plates with 1,500 cells per well and incubated at 37°C
overnight. MGC-803 cells were treated with PITC at concentrations
of 0, 50, 100, 150, 200 and 250 µM, whereas HGC-27 cells were
treated with PITC at 0, 20, 30, 40, 50 and 60 µM for 24, 48 and 72
h at 37°C. CCK-8 working solution was prepared with 10 µl CCK-8
solution and 100 µl culture medium; culture medium was replaced
with 100 µl working solution in each well and incubated for 2 h at
37°C. The absorbance at 450 nm was measured by a microplate reader
(Bio-Rad Laboratories, Inc.).
Colony formation assay
A total of 500 cells/well were seeded in 6-well
plates and treated with PITC (0, 50, 100 and 150 µM for MGC-803; 0,
20, 40 and 60 µM for HGC-27) for 48 h at 37°C and allowed to form
colonies in fresh medium for 10 days. During this time, culture
medium was refreshed every three days. Subsequently, the plates
were washed gently with PBS and fixed with 4% paraformaldehyde for
15 min and stained with 0.1% crystal violet (Sigma-Aldrich) for 15
min at room temperature. PBS was used to remove the excess crystal
violet, and images of the plates were captured. Colonies with ≥50
cells were counted.
Apoptosis assay
FITC Annexin V Apoptosis Detection Kit I (BD
Biosciences) was used to detect apoptosis according to the
manufacturer's instructions. MGC-803 and HGC-27 cells were plated
in 6-well plates with 8×104 cells per well and treated
with PITC for 48 h at 37°C following adherence. Floating and
adherent cells were collected and resuspended in 100 µl binding
buffer which containing 5 µl FITC-conjugated annexin-V and 5 µl
propidium iodide (PI), then incubated for 15 min at room
temperature. After which, 400 µl binding buffer was added to the
suspension and then immediately analyzed by flow cytometry (BD
Biosciences).
Cell cycle analysis
MGC-803 and HGC-27 cells were treated with PITC at
different concentrations for 48 h. The cells were harvested, washed
with cold PBS and fixed with cold 70% ethanol at −20°C overnight.
Each sample contained ~2×105 cells was washed with cold
PBS, resuspended in 250 µl staining solution containing 10 mg/ml of
RNase and 1 mg/ml propidium iodide (Sigma-Aldrich; Merck KGaA) and
incubated at 37°C for 30 min in the dark. Cell cycle of all samples
was analyzed by flow cytometry (BD Biosciences).
Intracellular reactive oxygen species
detection
A ROS Assay kit (Beyotime Institute of
Biotechnology) was used to determine the level of intracellular
ROS. Considering that the production of ROS must be earlier than
the presence of cell apoptosis and cell cycle arrest, 24 h was
selected as the treatment time in this assay. MGC-803 and HGC-27
cells were seeded in 6-well plates at a density of 8×104
cells per well and treated with PITC (0, 50 and 150 µM for MGC-803;
0, 20 and 60 µM for HGC-27) for 24 h at 37°C. The following steps
were performed according to the manufacturer's protocol. Briefly,
adherent cells were washed with PBS, and each well was incubated
with 1 ml 10 mM dichloro-dihydro-fluorescein diacetate (DCFH-DA) at
37°C for 25 min. The wells were washed three times with serum-free
RPMI-1640 medium. The ROS in situ was observed under a
fluorescence microscope (Leica Microsystems, Inc).
To evaluate the intracellular ROS with flow
cytometry, the cells were collected by trypsinization prior to
incubation with DCFH-DA. Samples were agitated every 3 min during
incubation and immediately analyzed by flow cytometry.
Comet assay
The comet assay, also known as single cell gel
electrophoresis (SCGE), was performed according to the protocol
described by Speit et al (15) with minor modifications. Briefly,
following 24 h treatment with PITC, MGC-803 and HGC-27 cells were
harvested and washed with PBS, and cell viability was determined by
trypan blue. After incubation with trypan blue for 5 min at room
temperature, the cells were counted under light microscope and
living cells were those without being stained by trypan blue. A
sample with <5% dead cells was considered acceptable. Normal
melting agarose (1%) was prepared to form the bottom layer of the
gel. The following steps were performed in the dark. A total of 10
µl cell suspensions containing 1×104 cells were mixed
with 90 µl 0.5% low melting point agarose and distributed on
slides. Following solidification, cells were lysed in cold, freshly
made lysing solution (2.5 M NaCl, 0.1 M EDTA, 0.01 M Tris, 1%
Triton X-100, 10% DMSO, pH=10) at 4°C for 1.5 h. The slides were
placed in an electrophoresis tank filled with electrophoresis
buffer (0.001 M Na2EDTA, 0.3 M NaOH, pH=13) for 20 min,
and electrophoresis was performed for 25 min at 25 V in an ice
bath. The slides were set in a neutralization buffer for 15 min at
room temperature, which was replaced for every 5 min.
4′,6-diamidino-2-phenylindole (Beyotime Institute of Biotechnology)
was used as a nucleic acid stain to identify the DNA tracks. The
comets were magnified 100 times and detected with a fluorescence
microscope and Leica Application Suite software (version 4.2, Leica
Microsystems, Inc.). For each slide, five fields were counted and
each experiment was performed in triplicate.
Intracellular GSH/glutathione
disulfide (GSSG) level detection
GSH and GSSG Assay kit (catalog no. S0053; Beyotime
Institute of Biotechnology) was used to detect the level of
GSH/GSSG in MGC-803 and HGC-27 cells. Following treatment with PITC
(0, 50 and 150 µM for MGC-803; 0, 20 and 60 µM for HGC-27) for 12 h
at 37°C, the cells were harvested and counted. Cells were
transferred to new tubes to ensure equal the cell numbers in each
group and washed with PBS. The protein removal solution was used to
resuspend cells. The tubes were placed in liquid nitrogen and 37°C
water twice for fast freezing and thawing to split the cell
membrane and subsequently placed in 4°C for 5 min and centrifuged
at 10,000 × g at 4°C for 10 min. The supernatants were collected to
detect the amount of total (t)GSH. GSH and GSSG Assay kit (Beyotime
Institute of Biotechnology) was used to detect tGSH following the
manufacturer's instructions. Briefly, GSH assay working solution
and samples were added to 96-well plates and incubated at room
temperature for 5 min; reduced nicotinamide adenine dinucleotide
phosphate was added into the reactive system and incubated for 25
min at room temperature. Absorbance at 412 nm was measured by a
microplate reader.
Cellular immunofluorescence
staining
Cellular immunofluorescence staining was conducted
to detect the expression of β-Tubulin. A total of 4×104
of MGC-803 and HGC-27 cells were seeded in 12-well plates, which
were previously laid with sterile cover glasses and cultured for 24
h at 37°C until the cells adhered to the glass slides. The cells
were then treated with PITC (60 µM), DMSO or PITC (60 µM) and NAC
(5 µM) respectively for 12 h at 37°C. Cold 4% paraformaldehyde was
used to fix cells at 4°C for 20 min, 0.2% Triton-X was used to
perforate cell membrane for 10 min, and cells were blocked with 1%
bovine serum albumin (Gibco; Thermo Fisher Scientific, Inc.) for 1
h at room temperature. Subsequently, cells were incubated with
antibody against β-tubulin (1:200) at 4°C overnight. Following
incubation with the primary antibodies, cells were then incubated
with donkey anti-rabbit IgG HRP-conjugated fluorescent secondary
antibody (1:200; catalog no. 34206ES60; Yeasen Biotechnology, Co.,
Ltd.) for 30 min and the nuclei were stained with DAPI for 5 min at
room temperature. Finally, the expression and morphology of
β-tubulin were detected by fluorescence microscopy at ×400
magnification.
Western blot analysis
Cells were treated with PITC at various
concentrations for 24 h (0, 50, 100 and 150 µM for MGC-803; 0, 20,
40 and 60 µM for HGC-27) and washed twice with cold PBS prior to
protein extraction. Radio Immunoprecipitation Assay (RIPA) buffer
(Beyotime Institute of Biotechnology) containing 1% protease
inhibitor cocktail (Beyotime Institute of Biotechnology) was used
to lyse cells, which were then removed from the dishes and
continued to be lysed on ice for 30 min. The extractions were
centrifuged at 14,000 × g at 4°C for 15 min, and the concentration
was determined by a bicinchoninic acid (BCA) assay kit (Beyotime
Institute of Biotechnology) according to the manufacturer's
instructions. Western blot analysis was conducted to detect the
expression of proteins as previously described (16). Briefly, an equal amount (10 µg) of
proteins was separated by SDS-PAGE (10% gel) and transferred to
PVDF membranes. Subsequently, the PVDF membranes were blocked with
5% skimmed milk and probed with primary antibodies against PARP,
β-tubulin, p-53, p-p53, cleaved caspase-9, cleaved caspase-3,
Bcl-2, Bax, cyclin A1 and Cyt c (1:1,000) at 4°C overnight and
further incubated with HRP-conjugated secondary antibodies at room
temperature for 1 h. The bands were visualized by chemiluminescence
(EMD Millipore) and Image J software (version 1.48; National
Institues of Health) was used to quantify the densitometric values
of the detected bands. GAPDH was used as an endogenous control.
Statistical analysis
All values are presented as the mean ± SD and were
analyzed using GraphPad Prism 5 (GraphPad Software, Inc.) and IBM
SPSS Statistics 24 (IBM Corp.). Student's t-test was used to
compare the differences between two groups, and multiple
comparisons of means were conducted using one-way analysis of
variance with Tukey's Honestly Significant Difference. P<0.05
was considered to indicate a statistically significant
difference.
Results
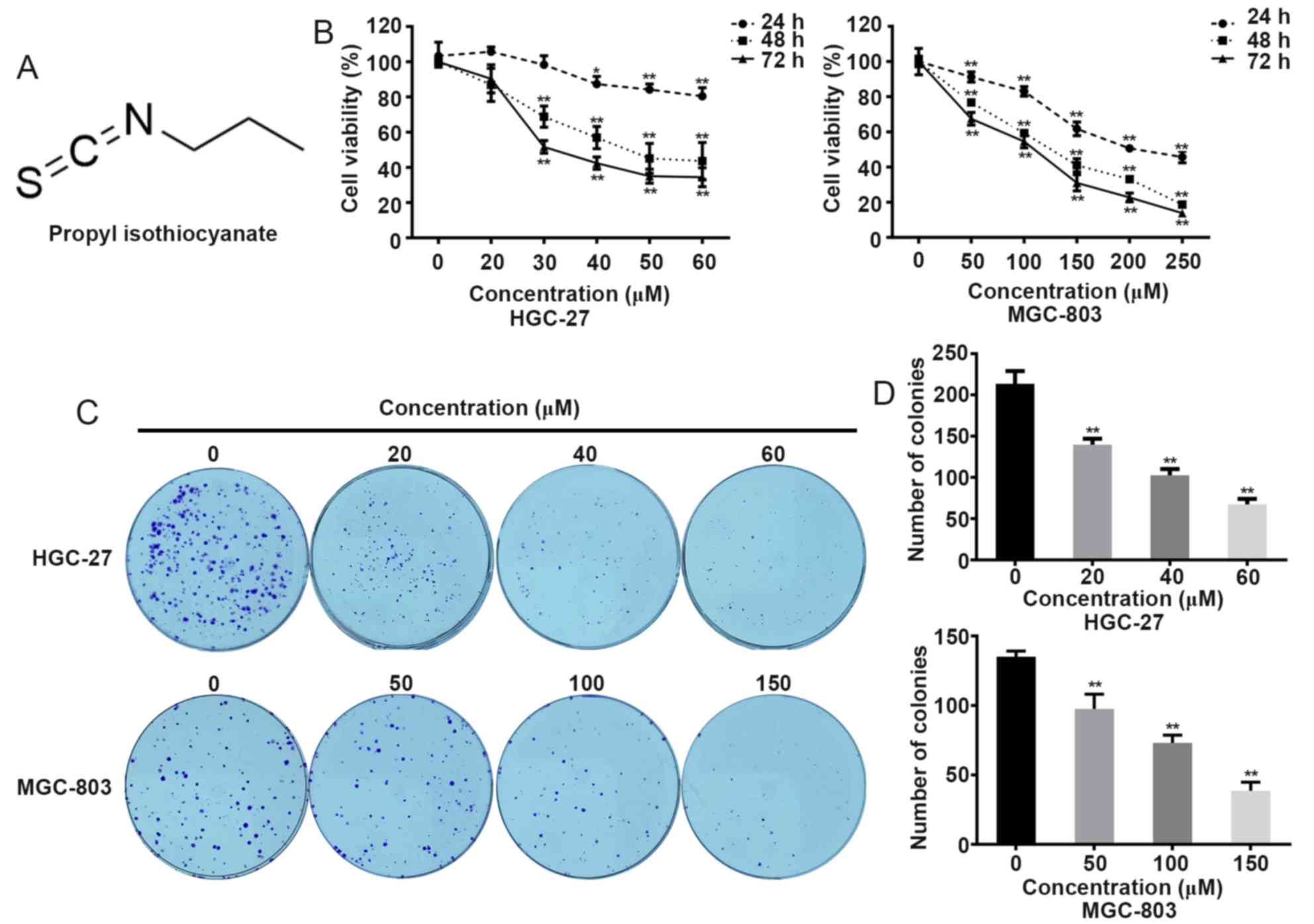
PITC inhibits GC cell proliferation
and viability
Cell proliferation and viability were analyzed using
CCK-8 and colony formation assays. Treatment with PITC inhibited
the viability of MGC-803 and HGC-27 cells; the inhibitory effect
grew as the concentration and treatment time of PITC increased
(Fig. 1B). The half maximal
inhibitory concentration (IC50) values of MGC-803 and
HGC-27 cells at 48 h were ~100 and ~40 µM, respectively. According
to the curves, 50, 100 and 150 µM were selected as the treatment
concentrations for the MGC-803 cell line, whereas 20, 40 and 60 µM
were selected for the HGC-27 cell line in subsequent experiments.
The results of the colony formation assay indicated that PITC
repressed the ability of GC cells to form colonies, which was
associated with the drug concentrations (Fig. 1C and D). These results suggested that
PITC may significantly affect gastric cancer cell viability and
proliferation.
PITC induces apoptosis and cell cycle
arrest in GC cells
To further demonstrate whether PITC induces cell
cycle arrest and apoptosis, the effects of PITC on MGC-803 and
HGC-27 cells were evaluated by flow cytometry. The number of
apoptotic cells, including early and late apoptosis, were
apparently increased in a dose-dependent manner following PITC
treatment (Fig. 2A and B). The
results presented in Fig. 2C-E
demonstrated that PITC increased the percentage of cells in the
S-phase compared with the control groups and decreased the
expression of Cyclin A1. These results suggested that apoptosis and
cell cycle arrest induced by PITC may serve a vital role in the
inhibition of cell viability and proliferation.
PITC reduces tGSH and increases ROS
production in GC cells and causes DNA damage
The results of the present study demonstrated that
the levels of tGSH were decreased in PITC-treated groups compared
with the control groups (Fig. 3A).
Simultaneously, an apparent increase in ROS levels was detected by
fluorescence microscopy (Fig. 3B).
SCGE was performed to examine the effects of PITC on DNA; the
results suggested that PITC may induce DNA damage in the two GC
cell lines (Fig. 3C), and higher
doses of PITC could lead to longer tails of comets which means more
severe damage of DNA. Therefore, treatment with PITC may lead to
the accumulation of ROS in the two GC cell lines and subsequently
induce DNA damage.
PITC affects the signaling pathway of
caspase and Bcl-2 family members
Apoptosis is a physiological process triggered by
several pathways. Mitochondria-dependent apoptosis is one of these
pathways which is initiated by Cyt c (17). To confirm the involvement of
mitochondria in PITC-induced apoptosis, a number of
apoptosis-related proteins were analyzed by western blotting. As
demonstrated in Fig. 3D, PITC
significantly increased the expression of Cyt c, cleaved caspase-9,
cleaved caspase-3 and cleaved PARP in a dose-dependent manner. The
expression of p-p53, which is activated by DNA damage, was
upregulated and total p53 was downregulated; in addition, Bax
expression was upregulated and Bcl-2 expression was downregulated.
Therefore, the dysfunction of mitochondria and DNA damage may be
associated with PITC-induced apoptosis.
NAC rescues the effects of PITC on
apoptosis of GC cells
To investigate whether the effect of PITC on the
intracellular redox balance is the cause of apoptosis, reversion
tests with NAC were performed. Following co-treatment with PITC and
NAC, the levels of ROS were analyzed by flow cytometry, which
demonstrated that the increase of ROS in PITC-treated groups were
downregulated by NAC (Fig. 4A). In
addition, adding NAC reversed the viability inhibition by PITC and
decreased the percentage of apoptotic cells compared with the
groups treated with PITC only (Fig.
4B-D). The results of the western blotting also suggested that
NAC may rescue the changes of several apoptosis-related proteins
induced by PITC (Fig. 4E). In
summary, these results indicated that the accumulation of ROS
induced by PITC triggers apoptosis of MGC-803 and HGC-27 cells.
PITC induces β-tubulin degradation in
gastric cancer cells
In order to confirm the effect of PITC on β-tubulin
of MGC-803 and HGC-27 cells, the present study performed cellular
immunofluorescence staining. As a result, the treatment of PITC
decreases the expression level of β-tubulin both in MGC-803 and
HGC-27 cells (Fig. S1A), and the
consequence was supported by the results of the western blot
analysis in Fig. S1B. Furthermore,
the effect of PITC on β-tubulin was reversed by NAC (Fig. S1C). These findings indicated that
PITC induces the degradation of β-tubulin in gastric cancer
cells.
Discussion
ITCs are a category of compounds extracted from
cruciferous plants that exhibit versatile biological activities.
The anticancer activities of PITC have not been reported to date.
In the present study, the effects of PITC on the biological
behavior of GC cells were investigated, and the relevant molecular
mechanisms were explored. The results demonstrated that PITC
inhibited the viability of MGC-803 and HGC-27 cells. HGC-27 cells
exhibited higher sensitivity to PITC compared with that of MGC-803
cells, the reason of which is unknown and requires further study.
The underlying mechanisms were preliminarily studied by flow
cytometry; the results demonstrated significant cell cycle arrest
at the S-phase and apoptosis induction, which suggested the
potential of PITC to suppress cancer cell proliferation. The
mechanisms of PITC-induced apoptosis were subsequently
explored.
GSH is a metabolite involved in the maintenance of
redox homeostasis in cells; the balance of ROS is disturbed when
GSH is dysregulated (18). According
to previous studies, the anticancer activities of ITCs may be
associated with their direct and indirect interactions with
cellular components. Conjugation with GSH is the first step in ITCs
metabolism, which consumes the natural anti-oxidant and possibly
influences the intracellular redox status (8,19). After
binding with GSH, ITCs will then be excreted from the cytosol,
which may cause the depletion of cytosolic GSH (5,20,21). In
the present study, the intracellular tGSH levels were measured, and
a concentration-dependent decrease of tGSH following PITC treatment
was revealed, consistent with the increase in ROS. DNA damage in
MGC-803 and HGC-27 cells, which is one of the consequences of
increased ROS levels (22,23), was also observed in the present
study. The results suggested that the accumulation of ROS, which
may be induced by GSH depletion, resulted in PITC-induced apoptosis
in MGC-803 and HGC-27 cells.
To further investigate the mechanisms of
PITC-induced apoptosis, the damage induced by excessive ROS was
considered. Proteins, lipids, DNA and other intracellular
components are targets of ROS (24).
Mitochondria can be damaged by excessive ROS through direct
oxidation of proteins and lipids, leading to changes in their
structure and function (25). As the
permeability of the mitochondrial membrane increases, Cyt c is
released into the cytosol and triggers mitochondria-dependent
apoptosis by activating caspase (25,26).
Therefore, it may be speculated that PITC-induced apoptosis of
MGC-803 and HGC-27 cells may be mediated by the mitochondria. In
the present study, the exposure to PITC increased the expression of
Cyt c, induced the upregulation of active forms of caspase-3 and
−9, and led to the activation of PARP. In addition, other targets
of ROS contribute to apoptosis. ROS-induced DNA damage promotes the
phosphorylation of p53 by activating ataxia telangiectasia-mutated
kinase and subsequently regulates the expression of downstream
proteins, including the Bcl-2 family (27). In the present study, PITC increased
the ratio of p-p53/total p53 and the expression of Bax, as well as
induced the downregulation of Bcl-2 in MGC-803 and HGC-27 cells.
These results suggested that ROS-induced mitochondrial dysfunction
and DNA damage may mediate PITC-induced apoptosis.
The effects of PITC were rescued by the ROS
scavenger NAC, although they were not fully reversed, which
suggests that there may be other ways for PITC to induce apoptosis.
β-tubulin is a potential target of ITCs due to being rich in
cysteine residues (28). The results
of the present study demonstrated that the degradation of β-tubulin
was promoted following treatment with PITC. This effect was
partially attenuated by NAC, which may serve as a competitor of
β-tubulin to bind PITC.
In summary, the results of the present study
indicate that PITC may exhibit potent anticancer activities in GC
cell lines by promoting apoptosis and inducing cell cycle arrest.
PITC may trigger apoptosis by inducing GSH depletion, leading to
the accumulation of ROS, mitochondrial dysfunction and DNA damage.
Mitochondria-associated apoptosis and DNA damage are the possible
mechanisms mediating these effects in the two studied GC cell
lines. To explore the efficacy and safety of PITC in vivo,
further research is required using xenograft tumor models.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81572819) and the Shanghai
Key Laboratory of Biliary Tract Disease Research Foundation (grant
no. 17DZ2260200).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Author contributions
JG and GFH conceived the study. LH, CC and WD
collected and analyzed the data and finalized the manuscript. LH
and CC performed the experiments. JHL assessed the data and revised
the manuscript. JG and GFH supervised and managed the project. All
authors have approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang L, Zheng R, Wang N, Yuan Y, Liu S, Li
H, Zhang S, Zeng H and Chen W: Incidence and mortality of stomach
cancer in China, 2014. Chin J Cancer Res. 30:291–298. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van Cutsem E, Sagaert X, Topal B,
Haustermans K and Prenen H: Gastric cancer. Lancet. 388:2654–2664.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beran F, Sporer T, Paetz C, Ahn SJ, Betzin
F, Kunert G, Shekhov A, Vassão DG, Bartram S, Lorenz S and Reichelt
M: One pathway is not enough: The cabbage stem flea beetle
psylliodes chrysocephala uses multiple strategies to overcome the
glucosinolate-myrosinase defense in its host plants. Front Plant
Sci. 9:17542018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brown KK and Hampton MB: Biological
targets of isothiocyanates. Biochim Biophys Acta. 1810:888–894.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Paul S, Geng CA, Yang TH, Yang YP and Chen
JJ: Phytochemical and health-beneficial progress of turnip
(Brassica rapa). J Food Sci. 84:19–30. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mi L, Di Pasqua AJ and Chung FL: Proteins
as binding targets of isothiocyanates in cancer prevention.
Carcinogenesis. 32:1405–1413. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang C, Wu H, Zhao Y, Ma Z and Zhang X:
Comparative studies on mitochondrial electron transport chain
complexes of Sitophilus zeamais treated with allyl isothiocyanate
and calcium phosphide. Pestic Biochem Physiol. 126:70–75. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang T and Chen W: The Candida albicans
inhibitory activity of the extract from papaya (Carica papaya L.)
seed relates to mitochondria dysfunction. Int J Mol Sci. 18(pii):
E18582017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sita G, Hrelia P, Graziosi A and Morroni
F: Sulforaphane from cruciferous vegetables: Recent advances to
improve glioblastoma treatment. Nutrients. 10(pii): E17552018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bo P, Lien JC, Chen YY, Yu FS, Lu HF, Yu
CS, Chou YC, Yu CC and Chung JG: Allyl isothiocyanate induces cell
toxicity by multiple pathways in human breast cancer cells. Am J
Chin Med. 44:415–437. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang YP, Jiang YW, Chen HY, Hsiao YT,
Peng SF, Chou YC, Yang JL, Hsia TC and Chung JG: Benzyl
isothiocyanate induces apoptotic cell death through
mitochondria-dependent pathway in gefitinib-resistant NCI-H460
human lung cancer cells in vitro. Anticancer Res. 38:5165–5176.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chou YC, Chang MY, Lee HT, Shen CC, Harnod
T, Liang YJ, Wu RS, Lai KC, Hsu FT and Chung JG: Phenethyl
isothiocyanate inhibits in vivo growth of xenograft tumors of human
glioblastoma cells. Molecules. 23(pii): E23052018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Speit G and Rothfuss A: The comet assay: A
sensitive genotoxicity test for the detection of DNA damage and
repair. Methods Mol Biol. 920:79–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiang SS, Wang XA, Li HF, Shu YJ, Bao RF,
Zhang F, Cao Y, Ye YY, Weng H, Wu WG, et al: Schisandrin B induces
apoptosis and cell cycle arrest of gallbladder cancer cells.
Molecules. 19:13235–13250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang H, Kou Y, Li J, Chen L, Mao Z, Han
XX, Zhao B and Ozaki Y: Nickel nanowires combined with
surface-enhanced raman spectroscopy: Application in label-free
detection of cytochrome c-mediated apoptosis. Anal Chem.
91:1213–1216. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang JY, Farooqi AA, Ou-Yang F, Hou MF,
Huang HW, Wang HR, Li KT, Fayyaz S, Shu CW and Chang HW: Oxidative
stress-modulating drugs have preferential anticancer
effects-involving the regulation of apoptosis, DNA damage,
endoplasmic reticulum stress, autophagy, metabolism, and migration.
Semin Cancer Biol. Aug 24–2018.(Epub ahead of print).
|
|
19
|
Øverby A, Stokland RA, Åsberg SE,
Sporsheim B and Bones AM: Allyl isothiocyanate depletes glutathione
and upregulates expression of glutathione S-transferases in
Arabidopsis thaliana. Front Plant Sci. 6:2772015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim YJ, Lee DH, Ahn J, Chung WJ, Jang YJ,
Seong KS, Moon JH, Ha TY and Jung CH: Pharmacokinetics, tissue
distribution, and anti-lipogenic/adipogenic effects of
allyl-isothiocyanate metabolites. PLoS One. 10:e01321512015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Yao S and Li J: Vegetable-derived
isothiocyanates: Anti-proliferative activity and mechanism of
action. Proc Nutr Soc. 65:68–75. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yun M, Choi AJ, Lee YC, Kong M, Sung JY,
Kim SS and Eun YG: Carbonyl reductase 1 is a new target to improve
the effect of radiotherapy on head and neck squamous cell
carcinoma. J Exp Clin Cancer Res. 37:2642018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kivisaar M: The effect of cellular redox
status on the evolvability of new catabolic pathways. MBio. 9(pii):
e01981–18. 2018.PubMed/NCBI
|
|
24
|
Radi R: Oxygen radicals, nitric oxide, and
peroxynitrite: Redox pathways in molecular medicine. Proc Natl Acad
Sci USA. 115:5839–5848. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Piantadosi CA and Suliman HB: Redox
regulation of mitochondrial biogenesis. Free Radic Biol Med.
53:2043–2053. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sinha K, Das J, Pal PB and Sil PC:
Oxidative stress: The mitochondria-dependent and
mitochondria-independent pathways of apoptosis. Arch Toxicol.
87:1157–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang DG, Wang S, Huang B and Liu F: Roles
of cellular heterogeneity, intrinsic and extrinsic noise in
variability of p53 oscillation. Sci Rep. 9:58832019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mi L, Xiao Z, Hood BL, Dakshanamurthy S,
Wang X, Govind S, Conrads TP, Veenstra TD and Chung FL: Covalent
binding to tubulin by isothiocyanates. A mechanism of cell growth
arrest and apoptosis. J Biol Chem. 283:22136–22146. 2008.
View Article : Google Scholar : PubMed/NCBI
|