Introduction
Lynch syndrome (LS), also called hereditary
nonpolyposis colorectal cancer (HNPCC), is a hereditary cancer
predisposition syndrome that causes an increased risk for many
types of cancers, including colorectal cancer and cancers of the
endometrium, stomach, ovary, small bowel, as well as the urinary
tract (1). The definitive diagnosis
of LS is based on the identification of the germline pathogenic
variants of mismatch repair (MMR) genes, mainly MLH1, PMS2,
MSH2 and MSH6 (1).
Defects in MMR proteins commonly result in the accumulation of
genetic errors during DNA replication and therefore lead to high
microsatellite instability (MSI-H) (1). Pembrolizumab, an anti-programmed cell
death 1 (PD-1) antibody, shows promising efficacy for MSI-H or
deficient MMR (dMMR) tumors (2,3). Through
the pooled analysis of 5 single-arm clinical studies (KEYNOTE 016,
164, 012, 028, 158), the objective response rate (ORR) was 36% in
MSI-H or dMMR patients with colorectal cancer (CRC) and 46% in 14
non-CRC cancers, which promoted the first tissue/site agnostic
approval of pembrolizumab in unresectable or metastatic, MSI-H or
dMMR solid tumors (4). The results
obtained in the current study suggested that different cancers with
MSI-H or dMMR have different responses to anti-PD-1 therapy.
However, the underlying mechanism is unknown.
Synchronous or metachronous multiple primary
carcinomas are found in a subset of patients with LS. It has been
previously reported that >60% of patients with LS with rectal
cancer develop colon cancer within 30 years (5). The 10-year cumulative risk of
endometrial cancer for patients with LS firstly diagnosed with CRC
was ~23% (6). Despite this, to the
best of our knowledge, there is a lack of a well-established
treatment mode, therefore, this case aimed to investigate the
therapeutic choice for the rare concurrent urothelial and colon
cancers. We reported the response of a patient with LS with
metachronous urothelial and colon cancers to pembrolizumab
treatment.
Case report
A 38-year-old male presented to the Cancer Hospital,
Chinese Academy of Medical Sciences (Beijing, China), complaining
of progressive symptoms, including frequent and urgent micturition,
and increased nocturia without apparent causes since February 2015.
His maternal grandmother was diagnosed with carcinoma of the rectum
at 39 years old. His mother was diagnosed with endometrial cancer
at 39 and a metachronous adenocarcinoma of colon at 59 years old.
His three maternal uncles were diagnosed with liver cancer. In
December 2015, computed tomography (CT) imaging and magnetic
resonance imaging (MRI) revealed the left ureteral mass (2.7×2.9
cm) and multiple enlarged lymph nodes. Paraaortic lymph nodes
biopsy and histological examination confirmed the lesions to be
urothelial carcinoma, and the tumor stage was evaluated as T4N2MX
with suspected lung metastasis according to the 2004 World Health
Organization classification (7). The
patient received 4 cycles of gemcitabine and cisplatin chemotherapy
between January 2016 and March 2016. CT scans in July 2016
suggested that the tumor became larger (4.5×3.8 cm). Therefore, the
patient was treated with paclitaxel liposome combined with tegafur,
gimeracil, and oteracil potassium capsules between July 2016 and
August 2016. In September 2016, the patient underwent regular chest
and abdomen/pelvis CT scans, and the results showed local
thickening of sigmoid colon wall (the thickest part was ~1.6 cm,
and ~6.7 cm in length) and multiple enlarged lymph nodes on the
outside of the intestinal wall. Further colonoscopy revealed an
ulcerative tumor on sigmoid colon. The hematoxylin and eosin
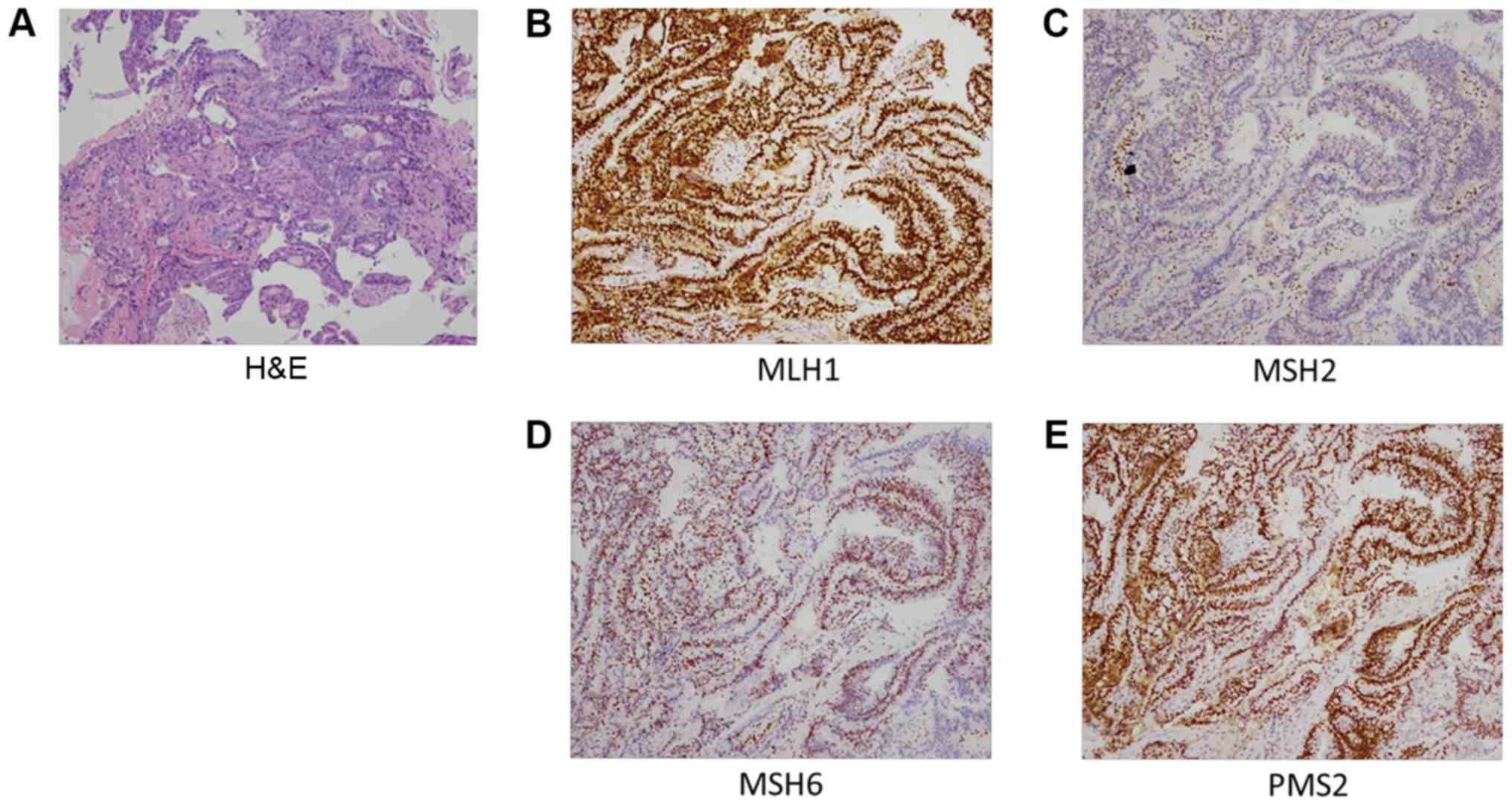
(H&E)-stained slides were reviewed by pathologists to confirm
the diagnosis of moderately differentiated adenocarcinoma (Fig. 1A). Briefly, tissue samples were
placed in 10% formalin for 24 h at room temperature and embedded in
paraffin. Immunohistochemical staining (IHC) was performed using
automated standard procedures (EnVision™ Flex+ Detection System;
cat. no. K8002; Dako; Agilent Technologies, Inc.). The standardized
protocol supplied by the manufacturer was followed. The following
primary antibodies were used: mutL homolog 1 (MLH1; cat. no.
MAB-0642), mutS homolog 2 (MSH2; cat. no. MAB-0291), mutS homolog 6
(MSH6; cat. no. MAB-0643), PMS1 homolog 2, mismatch repair system
component (PMS2; cat. no. MAB-0656), PD-1 (cat. no. MAB-0734),
cytokeratin 20 (CK20; cat. no. Kit-0025), CD7 (cat. no. Kit-0021)
and IL-12 P40 monomer (P40; cat. no. RMA-0815; all from Fuzhou
Maixin Biotech Co., Ltd.), HER-2 (cat. no. 790-4493), BRAF-V600E
(cat. no. 790-4855) and PD-L1 (cat. no. 741-4905; all from Roche
Diagnostics) and caudal type homeobox 2 (CDX2; cat. no. ZA-0520;
OriGene Technologies, Inc.). All antibodies were ready-to-use and
were used without dilution. The IHC staining demonstrated that the
tumor was positive for HER-2 (+), CDX2) (3+) and CK20 (3+), and was
negative for CD7 and P40, which is significantly different from
that of metastatic lymph nodes of urothelial carcinoma (HER-2: ++;
fluorescence in situ hybridization revealed no
amplification; CDX2: -; CK20: ++; CD7: +++; P40: +++). In addition,
BRAF-V600E, PD-1 and PD-L1 for colon were not expressed. For MMR
proteins, MSH2 was negative, however this was not the case for
MLH1, MSH6 and PMS2 (Fig. 1). A
diagnosis of primary moderately differentiated sigmoid
adenocarcinoma was made.
Taking into consideration the clinical diagnosis,
the MSH2-deficiency and the overwhelming family history, the
patient was suspected to harbor a germline mutation in MMR genes,
especially in MSH2. A genetic test was performed using DNA
from the patient's peripheral blood by next-generation sequencing
(Geneplus-Beijing Institute). As a result, 43 somatic mutations
were identified and a germline variant was detected on MSH2
(Table SI; Fig. 2A). The MSH2 c.1759+1G>T
germline variant is a novel splice site mutation that may affect
splicing process and result in a defective MSH2 protein (8,9).
According to the American College of Medical Genetics and Genomics
guidelines (10), the variant was
judged as a likely pathogenic mutation. Sanger sequencing was
performed to confirm the presence of MSH2 c.1759+1G>T
variant as previously described (11) (Fig.
2B). Therefore, the patient was finally diagnosed as
MSH2-associated LS with urothelial and colon cancer. As the sigmoid
colon lesions were observed 6 months after the diagnosis of
urothelial carcinoma, it should be defined as metachronous
carcinoma.
After 3 cycles of chemotherapy (irinotecan and
pemetrexed) combined with bevacizumab treatment, the tumors
progressed. The patient was subsequently administered pembrolizumab
treatment (200 mg every three weeks) in December 2016. Compared
with the lesions before pembrolizumab treatment (Fig. 3A and D), the imaging examinations in
March 2017 suggested that the sigmoid colon lesion slightly
increased and the ureteral lesion had progressed (Fig. 3B and E). In view of the notable level
reduction of tumor markers detected by electrogenerated
chemiluminescence (Table I), the
patient was suspected to have pseudoprogression and therefore
continued pembrolizumab treatment for another 4 months. The PET-CT
performed in July 2017 showed the shrinkage of the left ureteral
tumor. By contrast, progression was present on the sigmoid colon
wall (Fig. 3C and F). According to
the Response Evaluation Criteria In Solid Tumors v1.1 (12), the sigmoid colon lesion was evaluated
as stable disease (SD) at the first evaluation, and progressive
disease (PD) at the second evaluation, while the ureteral lesion
was assessed as PD at the first evaluation and partial response
(PR) at the second evaluation. Taking into consideration the
unsatisfactory efficacy, the patient then returned to his local
hospital to receive symptomatic and supportive treatment.
 | Table I.Tumor markers of the patient between
November 2016 and July 2017 as detected by electrogenerated
chemiluminescence. |
Table I.
Tumor markers of the patient between
November 2016 and July 2017 as detected by electrogenerated
chemiluminescence.
| Markers (unit) | 11/28/2016 | 03/20/2017 | 07/05/2017 | Upper limit of the
normal range |
|---|
| CA19-9 (U/ml) | 307.9 | 133.3 | 116.7 | 37 |
| CA72-4 (U/ml) | 82.04 | 41.84 | 56.48 | 9.8 |
| CEA (ng/ml) | 216.2 | 159.5 | 327 | 5 |
Discussion
LS is an autosomal-dominant disease caused by a
pathogenic germline mutation in a DNA MMR, including MLH1, MSH2,
MSH6, PMS2, or EPCAM gene (13,14).
Individuals with LS may have increased risk for many types of
cancer. Particularly, the lifetime risk of colorectal cancer and
endometrial cancer are 42 and 35%, respectively (13,14). LS
is also associated with an increased risk of gastric cancer,
ovarian cancer, hepatobiliary tract cancer, urinary tract cancer,
small bowel cancer, brain cancer, pancreatic cancer, as well as
sebaceous neoplasms (13,14). MSH2, being the most frequently
mutated gene, has a cumulative incidence of ureter and kidney
cancers for patients with MSH2-associated LS of 17.8% by 75 years
old (15). LS-associated
hepatocellular carcinoma, although not common, was reported in a
number of cases (16,17). In this case, a germline MSH2
mutation was identified in a patient with metachronous urothelial
and colon cancer. The cancerous manifestations of the patient and
his family members were all considered as part of the tumor
spectrum of LS, though this case was unable to get access to other
family members' DNA samples.
The traditional two-hit model is widely used to
describe the genesis of LS. A germline loss-of-function mutation
accompanied with somatic inactivation of the other allele promotes
tumorigenesis (18). In this case,
the patient carried a germline MSH2 mutation, while no
MSH2 somatic mutation was detected. Biallelic inactivation
of MSH2 may be caused by other mechanisms, such as loss of
heterozygosity (19). IHC revealed
the loss of expression of MSH2 protein and made the conclusion of
dMMR.
Given that MMR deficiency is a validated biomarker
for immunotherapy (2,20), the patient switched to pembrolizumab
treatment after failure of chemotherapy. The lesion on the sigmoid
colon appeared to have no response to pembrolizumab with continuous
aggravation, whereas the ureteral lesion was evaluated as PD at the
first response evaluation, and as PR at the second evaluation,
which was considered as pseudoprogression.
Pseudoprogression is an immunotherapy-associated
phenomenon. Increased tumor size, revealed by imaging examination,
may reflect tumor cells that are infiltrated with lymphocytes and
macrophages (21). These patients
may benefit from immunotherapy, however may switch to other
treatment regimens based on the imaging evaluation. These
inflammatory-based processes have prompted the development of
immune-related response criteria (irRC), which can identify 10% of
patients with pseudoprogression (22). However, recent studies demonstrated
that circulating tumor DNA monitoring can accurately distinguish
pseudoprogression from true progression in patients with lung
adenocarcinoma and melanoma treated with immunotherapy (23,24).
The response to PD-1 inhibition varies among
different tumor types (25). The
efficacy of pembrolizumab in patients with previously treated
urothelial carcinoma was evaluated in study KEYNOTE-045. For
patients treated with pembrolizumab, ORR was 21% and overall
survival time was 10.3 months (26).
A phase 2 clinical study evaluated the efficacy of pembrolizumab in
metastatic carcinomas with or without dMMR (19). ORR for dMMR colorectal cancers was
40%. For MMR proficient colorectal cancers, ORR was 0 (19). The objective response rate of
pembrolizumab in MSI-H or dMMR non-CRC cancers (36%) is higher than
in patients with CRC (46%) (4,25). In
this case, no response was observed on sigmoid colon lesion, while
the ureteral lesion achieved PR after pseudoprogression
Immunotherapy-associated biomarkers may contribute to the different
responses. IHC analysis of colon lesion showed negative expression
of MSH2 and PD-L1. However, the expression of these proteins in
ureteral lesion was unknown and no tumor tissue was left to allow
the performance of PD-1/PD-L1 IHC staining. One research
investigated the states of MSI, PD-L1 and TMB in 11,348 patients
with cancer (27). High MSI, PD-L1
and TMB were identified in 5.7, 6.7 and 7.2% of colorectal
adenocarcinoma (1,395 patients), and in 0.0, 16.8 and 42.7% of
bladder cancer (143 patients) (27).
Compared with bladder cancer, the percentage of MSI was higher in
colorectal carcinoma. On the contrary, TMB-H and PD-L1 positivity
were present more often in bladder cancer, which may explain the
different responses of the two primary sites (27).
This case presents the treatment course in a
challenging case of a patient with MSH2-associated LS manifested
with metachronous ureteral urothelial cancer and colon
adenocarcinoma. Taking into consideration dMMR confirmed by IHC
staining, the patient was treated with immune checkpoint inhibitor
pembrolizumab, after disease progression on chemotherapy. The
response patterns of the two primary lesions to pembrolizumab
differed. This case report discussed the potential explanations
underlying this phenomenon; however, further clinical
investigations are required.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YF, YC and XH were major contributors in writing the
manuscript. YF and XH were responsible for the analysis of clinical
information. MY and RC were responsible for genetic analysis and
literature review. MY edited the manuscript. YC and XJ conducted
imaging examinations and analysis. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the clinical data and any accompanying
images.
Competing interests
The authors declare no conflicts of interest.
References
|
1
|
Lynch HT, Snyder CL, Shaw TG, Heinen CD
and Hitchins MP: Milestones of Lynch syndrome: 1895–2015. Nat Rev
Cancer. 15:181–194. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Le DT, Durham JN, Smith KN, Wang H,
Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et
al: Mismatch repair deficiency predicts response of solid tumors to
PD-1 blockade. Science. 357:409–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marcus L, Lemery SJ, Keegan P and Pazdur
R: FDA approval summary: Pembrolizumab for the treatment of
microsatellite instability-high solid tumors. Clin Cancer Res.
25:3753–3758. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Food and Drug Administration (FDA), . FDA
grants accelerated approval to pembrolizumab for first tissue/site
agnostic indicationFDA; Silver Spring, MD: 2018, https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm560040.htmJuly.
30, 2018
|
|
5
|
Win AK, Parry S, Parry B, Kalady MF,
Macrae FA, Ahnen DJ, Young GP, Lipton L, Winship I, Boussioutas A,
et al: Risk of metachronous colon cancer following surgery for
rectal cancer in mismatch repair gene mutation carriers. Ann Surg
Oncol. 20:1829–1836. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Obermair A, Youlden DR, Young JP, Lindor
NM, Baron JA, Newcomb P, Parry S, Hopper JL, Haile R, Jenkins MA,
et al: Risk of endometrial cancer for women diagnosed with
HNPCC-related colorectal carcinoma. Int J Cancer. 127:2678–2684.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eble JL, Sauter G and Epstein JI:
Pathology and genetics of tumours of the urinary system and male
genital organs: WHO classification of tumours. World Health Organ.
2004.
|
|
8
|
Bianchi F, Rosati S, Belvederesi L,
Loretelli C, Catalani R, Mandolesi A, Bracci R, Bearzi I, Porfiri E
and Cellerino R: MSH2 splice site mutation and endometrial cancer.
Int J Gynecol Cancer. 16:1419–1423. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rakobradović J, Krivokuća A, Jovandić S,
Kesić V and Branković-Magić M: Confirmation of damaging effect of
MSH2 c. 2634+ 1G>C mutation on splicing, its classification and
implications for counseling. Cancer Genet. 239:1–7. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Richards S, Aziz N, Bale S, Bick D, Das S,
Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al:
Standards and guidelines for the interpretation of sequence
variants: A joint consensus recommendation of the American College
of Medical Genetics and Genomics and the Association for Molecular
Pathology. Genet Med. 17:405–424. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Q, Wang LA, Su J, Tong D, Lan W, Wang
L, Liu G, Zhang J, Zhang VW, Zhang D, et al: Giant bilateral
adrenal myelolipomas in two Chinese families with congenital
adrenal hyperplasia. Endocr Connect. Sep 1–2018.(Epub ahead of
print). View Article : Google Scholar
|
|
12
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bonadona V, Bonaiti B, Olschwang S,
Grandjouan S, Huiart L, Longy M, Guimbaud R, Buecher B, Bignon YJ,
Caron O, et al: Cancer risks associated with germline mutations in
MLH1, MSH2 and MSH6 genes in Lynch syndrome. JAMA. 305:2304–2310.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Møller P, Seppälä T, Bernstein I,
Holinski-Feder E, Sala P, Evans DG, Lindblom A, Macrae F, Blanco I,
Sijmons R, et al: Cancer incidence and survival in Lynch syndrome
patients receiving colonoscopic and gynaecological surveillance:
First report from the prospective Lynch syndrome database. Gut.
66:464–472. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Møller P, Seppälä TT, Bernstein I,
Holinski-Feder E, Sala P, Gareth Evans D, Lindblom A, Macrae F,
Blanco I, Sijmons RH, et al: Cancer risk and survival in path_MMR
carriers by gene and gender up to 75 years of age: A report from
the Prospective Lynch Syndrome Database. Gut. 67:1306–1316. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Casper M, Weber SN, Kloor M, Müllenbach R,
Grobholz R, Lammert F and Zimmer V: Hepatocellular carcinoma as
extracolonic manifestation of Lynch syndrome indicates SEC63 as
potential target gene in hepatocarcinogenesis. Scand J
Gastroenterol. 48:344–351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vernez M, Hutter P, Monnerat C, Halkic N,
Gugerli O and Bouzourene H: A case of Muir-Torre syndrome
associated with mucinous hepatic cholangiocarcinoma and a novel
germline mutation of the MSH2 gene. Fam Cancer. 6:141–145. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan H, Jin H, Xue G, Mei Q, Ding F, Hao L
and Sun SH: Germline hMSH2 promoter mutation in a Chinese HNPCC
kindred: Evidence for dual role of LOH. Clin Genet. 72:556–561.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hemminki A, Peltomäki P, Mecklin JP,
Järvinen H, Salovaara R, Nyström-Lahti M, de la Chapelle A and
Aaltonen LA: Loss of the wild type MLH1 gene is a feature of
hereditary nonpolyposis colorectal cancer. Nat Genet. 8:405–410.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Q, Gao J and Wu X: Pseudoprogression
and hyperprogression after checkpoint blockade. Int
Immunopharmacol. 58:125–135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Agarwala SS: Practical approaches to
immunotherapy in the clinic. Semin Oncol. 42 (Suppl 3):S20–S27.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guibert N, Mazieres J, Delaunay M,
Casanova A, Farella M, Keller L, Favre G and Pradines A: Monitoring
of KRAS-mutated ctDNA to discriminate pseudo-progression from true
progression during anti-PD-1 treatment of lung adenocarcinoma.
Oncotarget. 8:38056–38060. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee JH, Long GV, Menzies AM, Lo S,
Guminski A, Whitbourne K, Peranec M, Scolyer R, Kefford RF, Rizos H
and Carlino MS: Association between circulating tumor DNA and
pseudoprogression in patients with metastatic melanoma treated with
anti-programmed cell death 1 antibodies. JAMA Oncol. 4:717–721.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yarchoan M, Hopkins A and Jaffee EM: Tumor
mutational burden and response rate to PD-1 inhibition. N Engl J
Med. 377:2500–2501. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y,
Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK,
et al: Pembrolizumab as second-line therapy for advanced Urothelial
Carcinoma. N Engl J Med. 376:1015–1026. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vanderwalde A, Spetzler D, Xiao N,
Gatalica Z and Marshall J: Microsatellite instability status
determined by next-generation sequencing and compared with PD-L1
and tumor mutational burden in 11,348 patients. Cancer Med.
7:746–756. 2018. View Article : Google Scholar : PubMed/NCBI
|