Introduction
Hepatocellular carcinoma (HCC) is a common type of
malignant tumor and has been reported as the third leading cause
for cancer-associated mortality (1).
Postoperative recurrence and metastasis remain the leading cause of
liver cancer mortality, however it has been reported that the
progress for HCC diagnosis and treatment has greatly improved
(2). The metastasis of HCC is a
complex process associated with multiple genes and signaling
pathways (3). Epithelial-mesenchymal
transition (EMT) serves an important role in the invasion and
proliferation of tumor cells (4).
It has been reported that drugs used for the
treatment of tumor metastasis may reduce the viscosity of blood
(5), enhance the immune function of
the body (6) and affect the cell
cycle, the proliferation and the apoptosis of tumor cells (7) by interfering with nucleic acid
biosynthesis, destroying DNA structure and function, and affecting
protein synthesis and hormonal balance. However, the aforementioned
drugs have strong side effects and low selectivity (8). Further investigation is required for
the targeting of antitumor drugs so that tumor cells may be
selectively attacked without harming healthy tissues. Myricetin is
a flavonoid widely found in dicotyledonous plants, including
onions, berries and grapes. It has been reported to have an
extensive range of pharmacological activities, including
antithrombotic (9), antioxidant
(10), antitumor (7), anti-inflammatory, including reducing
blood sugar (11) and protecting the
liver and kidney (12). However, it
has been reported that myricetin may have a toxic effect on cells
(13). Shih et al (14) revealed that myricetin affected the
migration and invasion of human lung cancer A549 cells by
inhibiting ERK signaling. It has been reported that myricetin may
inhibit the expression and activity of matrix metalloproteinase
(MMP)-2 in colorectal cancer cells, as well as the migration and
invasion of tumor cells (15). In
addition, Seydi et al (16)
reported that myricetin exhibited cytotoxic responses in HCC cells,
but had no effect on untreated healthy liver cells. However, the
effects of myricetin on migration and invasion of HCC cells remain
unclear.
The adherent human HC epithelial cell line, MHCC97H,
was noted to be highly migratory and invasive by Tian et al
(17). Therefore, MHCC97H cells were
selected to determine the effects of myricetin on migration and
invasion, and to explore the possible mechanisms to support the
utilization of myricetin in the prevention and treatment of
cancer.
Materials and methods
Experimental materials
Myricetin (cat. no. PS1149-0025; Chengdu Pushi
Biotechnology Co., Ltd.; purity, 98%), was maintained at −20°C in
an appropriate storage concentration (3.14×104 µmol/l),
dissolved with dimethylsulfoxide (DMSO). The working solution of
the drug was obtained by adding the corresponding amount of
high-glucose Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Fetal bovine
serum (FBS) was obtained from Shanghai Luoshen Biotechnology Co.,
Ltd. (Shanghai, China). Radioimmunoprecipitation assay (RIPA)
buffer, trypsin, MTT and SDS-PAGE (10% gels) were used (Beyotime
Institute of Biotechnology, Haimen, China). Mouse anti-human GAPDH
(cat. no. 51332), rabbit anti-human epithelial (E)-cadherin (cat.
no. 3195), rabbit anti-human neural (N)-cadherin (cat. no. 13116),
rabbit anti-human vimentin (cat. no. 5741) and the secondary
antibodies, including goat anti-rabbit (cat. no. 6990) and goat
anti-mouse (cat. no. 5946) conjugated to horseradish peroxidase
(HRP) were used (all from Cell Signaling Technology, Inc., Danvers,
MA, USA).
Cell culture
Human HCC MHCC97H cell lines (Nanjing KeyGen Biotech
Co., Ltd., Nanjing, China) were cultured in high-glucose DMEM
containing 10% FBS and maintained at 37°C in a humidified
atmosphere containing 5% CO2.
MTT assay
MHCC97H cells in the logarithmic growth phase were
isolated, digested with 0.25% trypsin cell digestive fluid (cat.
no. C0201; Beyotime Institute of Biotechnology) into a single cell
suspension and seeded onto a 96-well plate (1×104
cells/well), allowed to adhere overnight at 37°C in a humidified
atmosphere containing 5% CO2. Various concentrations (0,
10, 20, 30, 40, 50, 100 and 200 µmol/l) of myricetin were added
into 96-well plates, which were divided into 7 groups with 6 wells
for each group. Subsequent to being cultured for 48 h, 10 µl of MTT
(5 mg/ml) solution was added to each well of the plate and the
cells were cultured for 4 h. A total of 150 µl DMSO was added to
each well. Plates were incubated for 15 min on the table at 100
rev/min in dark. The absorbance was measured with a
spectrophotometer at a wavelength of 490 nm and the cell viability
curve was obtained. All experiments were performed in triplicate
and repeated thrice.
Cell scratch assay
Hepatoma cells in the logarithmic growth phase were
seeded onto a 6-well plate (1×106 cells/well). When the
cells reached 70–90% confluency, they were transferred to the
serum-free DMEM and cultured overnight at 37°C in an atmosphere
containing 5% CO2. On the following day, a scratch wound
was created in the cell monolayer with a 10-µl pipette tip, and the
scratch state at the beginning of cell culture (0 h) was observed
and photographed under a light microscope at ×40 magnification. The
serum-free DMEM containing corresponding concentrations (0, 10, 20,
30, 40, 50, 100 and 200 µmol/l) of myricetin was subsequently added
to the 6-well plates. Cells were photographed after being cultured
for 24 and 48 h at 37°C under an inverted phase contrast
fluorescence microscope at ×40 magnification. Five visual fields
were counted for each experimental group and all the experiments
were repeated thrice.
Transwell migration and invasion
assays
Cell migration experiments were performed with the
HCC MHCC97H cell lines, cultured overnight in serum-free DMEM and
isolated into a single cell suspension. The Transwell chamber was
placed in a 24-well plate and 600 µl of complete medium
supplemented with 10% FBS containing 0, 25, 50 and 100 µM myricetin
was added to the lower chamber of the Transwell. Subsequently, 200
µl of serum-free cell suspension containing 0, 25, 50 and 100 µM
myricetin was added to a total of 2.5×105 cells/ml in
the upper chamber of the Transwell. Cells were incubated at 37°C in
a humidified atmosphere containing 5% CO2 for 24 h. The
Transwell chamber was removed and soaked in Giemsa stain subsequent
to rinsing with PBS (Jiangsu Lvye Biotechnology Co., Ltd.,
Shanghai, China; solution A contained 1% methylene blue, 1% eosin,
1% azure, glycerin and methanol, and was used at 25±1°C for 2 min;
solution B contained 0.2 mol monometallic sodium orthophosphate and
0.2 mol disodium hydrogen phosphate, pH was 6.4±0.2, and was used
at 25±1°C for 8 min). The non-migratory cells in the upper chamber
of the Transwell were removed gently with a cotton bud following
rinsing with PBS. The cells at the bottom of membrane were
observed, photographed and counted under inverted phase contrast
microscope. Six visual fields (magnification, ×100 were randomly
counted for each well. Two wells were set for each group and all
the experiments were performed in triplicate and repeated
thrice.
Invasion assays were performed using a
24-well Transwell chamber coated with Matrigel (1 µg/ml), according
to manufacturer's protocol
The Matrigel was placed in the refrigerator
overnight (8 h) for defrosting and the upper surface of membrane
was covered with 100 µl Matrigel. Cells at a density of
1×106 cells/well were plated in the upper chamber in
serum-free DMEM. Subsequent to incubation for 8 h at 37°C in an
atmosphere containing 5% CO2, invaded cells were stained
with Giemsa stain for 10 min at 25°C and counted under an inverted
phase contrast microscope (×100 magnification) Two wells were set
for each group and of all the experiments were performed in
triplicate and repeated thrice.
Immunofluorescence assay
MHCC97H cells were seeded in a 6-well plate and
cultured at 37°C in a humidified atmosphere containing 5%
CO2. The complete medium containing 0, 25, 50 and 100 µM
myricetin was added into the well. The cells were cultured for 48
h, when the cell reached 70% confluency. The cover slip was removed
and the plate was rinsed twice with Tris-buffered saline containing
0.05% Tween-20 (PBST). The cells were subsequently immobilized for
30 min, fixed with 4% paraformaldehyde at 25°C for 30 min and
rinsed thrice with PBST. PBS containing 0.2% Triton X-100 was added
to the plate for 10 min for permeabilization and the plate was
subsequently rinsed thrice with PBST. The cells were incubated for
1 h at 25°C away from light with rhodamine phalloidin (PHDR1; 1:40
dilution; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and rinsed
thrice with PBST. Cells were subsequently incubated for 15 min away
from light with 5 µg/ml DAPI and rinsed thrice with PBST. Finally,
the cells were observed and photographed under fluorescence
microscope at ×1,000 magnification following the addition of
anti-fade mounting medium (cat. no. P0126; Beyotime Institute of
Biotechnology).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Cells in the logarithmic growth phase were grown to
~70% confluence in 6-well plates and treated with 0, 25, 50 and 100
µM myricetin for 48 h. Total RNA extracted using Trizol from each
sample was used to determine concentration and purity and the rest
of the RNA samples were subjected to reverse transcription using
Prime Script RT Master Mix kit (TaKaRa Biotechnology Co., Ltd.,
Dalian, China). RNA was reverse-transcribed into cDNA using AceQ
qPCR SYBR Green Master Mix (Vazyme, Piscataway, NJ, USA). A total
of 3 wells were used for cDNA samples of each concentration and the
experiments were repeated thrice. Primers used for real-time PCR
analysis are presented in Table I.
GAPDH was used as a reference gene. The thermocycling conditions
were as follows: Initial denaturation at 95°C for 5 min prior to 40
cycles at 95°C for 10 sec and at 60°C for 34 sec. Relative mRNA
levels were evaluated by 2−ΔΔCq method (18).
 | Table I.Primers and sequences used in reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers and sequences used in reverse
transcription-quantitative polymerase chain reaction.
| Name | Base sequence
(5′-3′) |
|---|
| E-cadherin |
|
|
Forward |
5′-AGGCCAAGCAGCAGTACATT-3′ |
|
Reverse | 5′-
ATTCACATCCAGCACATCCA-3′ |
| N-cadherin |
|
|
Forward |
5′-CCATCAAGCCTGTGGGAATC-3′ |
|
Reverse |
5′-GCAGATCGGACCGGATACTG-3′ |
| GAPDH |
|
|
Forward |
5′-GCACCGTCAAGGCTGAGAAC-3′ |
|
Reverse |
5′-TGGTGAAGACGCCAGTGGA-3′ |
Western blotting
Western blot analysis was performed to determine the
expression of E-cadherin, N-cadherin and vimentin proteins. The
cells were grown to ~50% in 6-well plates and treated with 0, 25,
50 and 100 µM myricetin for 48 h. Cells were lysed by adding RIPA
buffer (cat. no. P0013B; Biyun Biotechnology Co., Ltd.) subsequent
to being harvested and the supernatant was collected. SDS-PAGE
protein sample buffer (cat. no. P0015L; Biyun Biotechnology Co.,
Ltd) was added into the supernatant prior to protein sample
preparation by boiling the aforementioned mixed liquid for 5–10 min
in a water bath at 100°C. Simultaneously, the supernatant was
collected to determine the concentration of protein samples by
bicinchoninic acid protein assay. Protein samples (40 µg) were
loaded onto SDS-PAGE (10% gels) and transferred to a polyvinylidene
difluoride membrane. Membranes were blocked in 5% non-fat dried
milk and incubated overnight at 4°C subsequent to adding diluted
monoclonal antibody, including E-cadherin (1:1,000), N-cadherin
(1:1,000), vimentin (1:1,000) and GAPDH (1:1,000). Subsequently,
the corresponding horseradish peroxidase (HRP)-conjugated secondary
antibody [HRP-conjugated anti-mouse and anti-rabbit IgG (1:2,000)]
was added to incubated for 2 h at 25°C. The chemiluminescence was
determined using a gel imaging system and Pierce Enhanced
Chemiluminescence Plus (Thermo Fisher Scientific, Inc.). The
membranes were incubated with GAPDH antibody as a control and the
intensity of the bands was quantified using ImageJ 1.42q software
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
The experimental data were analyzed using SPSS 16.0
(SPSS, Inc., Chicago, IL, USA) and Excel 2003 (Microsoft
Corporation, Redmond, WA, USA) through one-way analysis of variance
followed by Tukey's post hoc test for multiple comparisons and
Student's t-test. All the experimental data were expressed as mean
± standard deviation The variance analysis was used to compare the
differences between groups of measurement data. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of myricetin on activity of
MHCC97H cells
Compared with the control group (0 µM), the
viability of MHCC97H cells did not significantly change when cells
were treated with 10–100 µM of myricetin. However, there was a
significant decrease in viability at 200 µM (P<0.01; Fig. 1). This suggests that the cytotoxicity
of myricetin at 0–100 µM decreased compared with that at 200 µM.
Therefore, the concentrations of 25, 50 and 100 µM were selected to
treat MHCC97H cells.
Effects of myricetin on migration and
invasion of MHCC97H cells
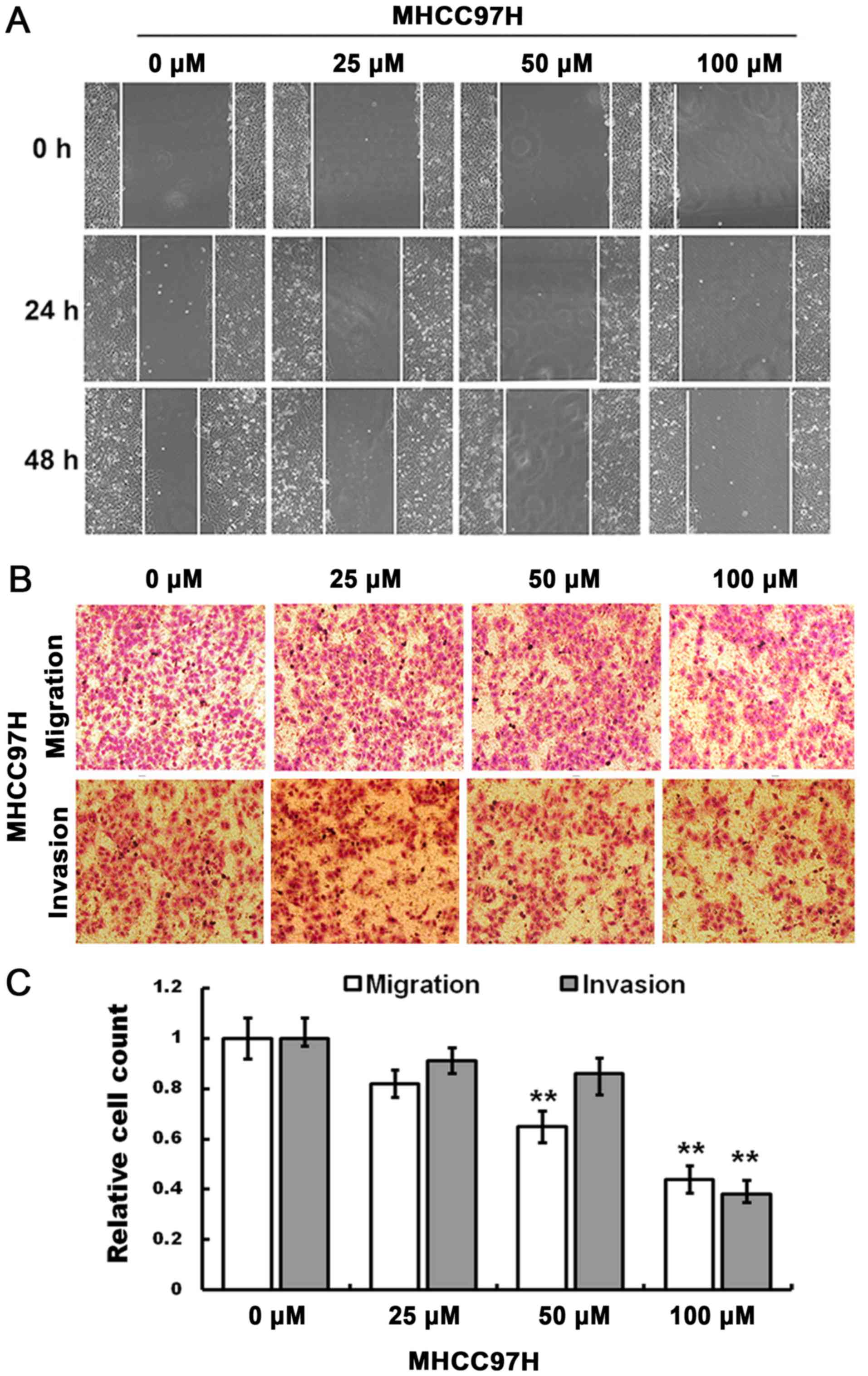
The cell scratch assay indicated that compared with
the control group, the migration of MHCC97H cells was inhibited by
100 µM myricetin when the cells were treated for 24 and 48 h
(Fig. 2A).
The Transwell migration assay indicated that,
compared with the control group, an increase in drug concentration
(50 and 100 µM) significantly decreased the number of migrated
cells, and the invasive ability of MHCC97H cells was reduced in
response to 100 µM myricetin (Fig.
2B). The relative cell count at the bottom of the membrane is
indicated in Fig. 2C (P<0.01).
Therefore, migration and invasion of MHCC97H cells may be inhibited
in response to myricetin treatment.
RT-qPCR analysis indicated that the relative mRNA
expression of E-cadherin in MHCC97H cells significantly increased
at 25 µM myricetin (P<0.01; Fig.
3A) and the relative mRNA expression level of N-cadherin
significantly decreased at 100 µM, along with that of vimentin
(P<0.01; Fig. 3A). The
aforementioned results suggest that myricetin may affect the mRNA
expression level of genes associated with migration and
invasion.
 | Figure 3.Effects of myricetin on protein
expression. (A) The relative mRNA expression level of E-cadherin,
N-cadherin and vimentin in MHCC97H cells treated with 0, 25, 50 and
100 µM of myricetin. (B) Expression of E-cadherin, N-cadherin and
vimentin in MHCC97H cells treated with 0, 25, 50 and 100 µM of
myricetin with GAPDH used as a reference. (C) Relative expression
level of E-cadherin, N-cadherin and vimentin in MHCC97H cells
treated with 0, 25, 50 and 100 µM of myricetin. *P<0.05,
**P<0.01, compared with 0 µM myricetin. |
Western blotting indicated that the relative
expression of E-cadherin in MHCC97H cells significantly increased
(P<0.01; Fig. 3B and C) and the
expression level of N-cadherin and vimentin protein significantly
decreased (P<0.01; Fig. 3B and
C). RT-qPCR and western blotting indicated that the migration
and invasion of MHCC97H cells may be inhibited by myricetin
treatment through the upregulation of E-cadherin and the
downregulation of N-cadherin and vimentin.
Effects of myricetin on assembly of
actin cytoskeleton in two MHCC97H cell lines
The filamentous actin (F-actin) cytoskeleton serves
an important role in cell migration and invasion (19). Compared with the control group, the
number of fibers decreased in MHCC97H cells, and the filopodia and
lamellipodia at the cell edge were also weakened with increased
myricetin concentration (Fig. 4).
The effects of myricetin on the migration and invasion of MHCC97H
cells may be mediated by the rearrangement of F-actin cytoskeleton
fibers.
Discussion
It has been reported that primary liver cancer is
the second most common type of malignant tumor (1). HCC has been reported to account for
70–85% of primary liver cancer, with the main mortality cause among
patients with HCC being the invasion of HCC cells (2). In EMT, the epithelial cells have been
demonstrated to lose their polarity and their adhesion
characteristics, changing into mesenchymal cell groups and
resulting in strong migratory and invasive ability of the cells
(20). Therefore, tumor cells have
been indicated to have a greater probability to be exacerbated
in situ infiltration or transfer to other parts of the body
through the blood or the lymphatic system (21). It has been reported that cancer cell
migration may even cause a healthy tissue or organ to become
cancerous. Myricetin has been evaluated in numerous studies as an
antitumor drug. Ko et al (22) confirmed that myricetin may inhibit
the expression and activity of MMP-2 in human colorectal cancer
cells, as myricetin may prevent the degradation of extracellular
matrix and decrease cell activity.
In the present study, the effects of myricetin on
EMT were examined by evaluating cell viability and focusing on the
changes in the migration and invasion of tumor cells. It was
demonstrated that with increased myricetin concentration, the
migratory and invasive ability of HCC cells weakened. The
underlying mechanisms may involve cytoskeletal remodeling, as the
number of fibers in cells and filopodia and lamellipodia at the
cell edge significantly decreased in response to myricetin
treatment. Therefore, the present study suggests that the main
reasons behind the migration and invasion of tumor cells are the
regulated proteins, including E-cadherin, N-cadherin and
vimentin.
It has been revealed that numerous factors induce
EMT and may be defined by several regulatory networks (23). EMT has been reported to be influenced
by various genes and their translation, cellular signaling, as well
as the regulation of various proteins (20,21). It
has been demonstrated that high CDH1L expression induces EMT
through Cdc42 guanine nucleotide exchange factor 9-mediated Cdc42
activation (20). Chemokine ligand
18 promotes EMT through expression of PITPNM3 family member 3 and
the activation of the nuclear factor-κB subunit signaling pathway
(21). ECM proteins, including
collagen-I, fibronectin and hyaluronan, and ECM remodeling through
extracellular lysyl oxidase have been reported to be involved in
EMT regulation (24). In addition,
Ricciardi et al (4) indicated
that inflammation may induce EMT, which involves various
immunoregulatory processes. Jung et al (24) concluded that tumor
micro-environmental factors, including pro-inflammatory cytokines
secreted by locally activated stromal cells, hypoxia conditions,
ECM components and mechanical properties may also affect EMT and
therefore cancer cell invasion.
It has been revealed that intercellular connections
containing E-cadherin are often adjacent to cytoskeletal
microfilaments containing actin (20). The increase in microfilaments has
been indicated to enhance the adhesion strength among cells and
reduce the activity of cells, subsequently inhibiting the invasion
of cancer cells to surrounding tissues through the basement
membrane (21). It has been reported
that adhesion proteins on the cancerous animal cell significantly
reduce or become eliminated, enhancing the migratory ability of the
cancer cells (4). Therefore,
cytoskeleton remodeling, decreased expression of the epithelial
marker E-cadherin and increased expression of the interstitial
marker N-cadherin have been reported as marked changes during EMT
(21). Zhao et al (19) indicated that deguelin may inhibit the
migration and invasion of lung cancer cells lines A549 and H460 by
regulating actin cytoskeleton rearrangement. In the present study,
the aforementioned factors were reversed in liver cancer cells
treated with various concentrations of myricetin, as revealed by
immunofluorescence assay, RT-qPCR and western blotting.
Myricetin has been demonstrated to be not only an
effective antitumor medicine, but also a common ingredient of
various foods and beverages (13).
Jose et al (12) indicated
that myricetin did not produce any toxicity in mice and may protect
the cellular architecture of the liver through histopathology
studies. Further studies on the effects of myricetin on EMT are
required.
The results of the present study provide an
experimental and theoretical basis for further research on the
treatment of tumors and the development of antitumor metastasis
drugs. They also provide a novel target for the application of the
drugs. The underlying molecular mechanism of myricetin requires
further study, although it was indicated in the present study that
the MHCC97H cells exhibited transformation of EMT to MET in
response to myricetin treatment, which was concentration and
time-dependent. Further research in order to determine the genes,
expression, activity of enzymes, glycoproteins and signaling
pathways associated with EMT should be conducted so that a more
comprehensive experimental basis is provided for the application of
myricetin as a drug against HCC migration and invasion.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 31071171) and the
State Key Laboratory of Genetic Engineering program (grant no.
SKLGE-1405).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZX made substantial contributions to conception and
design. WS performed and analyzed cellular and molecular
experiments. HM examined the molecular-level indicators and wrote
the manuscript. LZ cultivated cells, examined molecular-level
indicators and participated in writing the manuscript. JR and YK
cultivated and detected cells. BR and MS examined cell and
molecular levels. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures were approved by the Animal Care and
Use Committee of the Faculty of Veterinary Medicine of Yangzhou
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen JG and Zhang SW: Liver cancer
epidemic in China: Past, present and future. Semin Cancer Biol.
21:59–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang H and Chen L: Tumor microenviroment
and hepatocellular carcinoma metastasis. J Gastroenterol Hepatol.
28 (Suppl 1):S43–S48. 2013. View Article : Google Scholar
|
|
3
|
Liu T, Zhang S, Chen J, Jiang K, Zhang Q,
Guo K and Liu Y: The transcriptional profiling of glycogenes
associated with hepatocellular carcinoma metastasis. PLoS One.
9:e1079412014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ricciardi M, Zanotto M, Malpeli G, Bassi
G, Perbellini O, Chilosi M, Bifari F and Krampera M:
Epithelial-to-mesenchymal transition (EMT) induced by inflammatory
priming elicits mesenchymal stromal cell-like immune-modulatory
properties in cancer cells. Br J Cancer. 112:1067–1075. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pawitan Y, Yin L, Setiawan A, Auer G,
Smedby KE and Czene K: Distinct effects of anti-inflammatory and
anti-thrombotic drugs on cancer characteristics at diagnosis. Eur J
Cancer. 51:751–757. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Briso EM, Guinea-Viniegra J, Bakiri L,
Rogon Z, Petzelbauer P, Eils R, Wolf R, Rincón M, Angel P and
Wagner EF: Inflammation-mediated skin tumorigenesis induced by
epidermal c-Fos. Genes Dev. 27:1959–1973. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feng J, Chen X, Wang Y, Du Y, Sun Q, Zang
W and Zhao G: Myricetin inhibits proliferation and induces
apoptosis and cell cycle arrest in gastric cancer cells. Mol Cell
Biochem. 408:163–170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baldo BA and Pham NH: Adverse reactions to
targeted and non-targeted chemotherapeutic drugs with emphasis on
hypersensitivity responses and the invasive metastatic switch.
Cancer Metastasis Rev. 32:723–761. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tong Y, Zhou XM, Wang SJ, Yang Y and Cao
YL: Analgesic activity of myricetin isolated from Myrica rubra
Sieb. et Zucc. leaves. Arch Pharm Res. 32:527–533. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan X, Liu Y, Hua X, Deng X, Sun P, Yu C,
Chen L, Yu S, Liu S and Pang H: Myricetin ameliorates the symptoms
of collagen-induced arthritis in mice by inhibiting cathepsin K
activity. Immunopharmacol Immunotoxicol. 37:513–519. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kandasamy N and Ashokkumar N: Protective
effect of bioflavonoid myricetin enhances carbohydrate metabolic
enzymes and insulin signaling molecules in streptozotocin-cadmium
induced diabetic nephrotoxic rats. Toxicol Appl Pharmacol.
279:173–185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jose J, Dhanya AT, Haridas KR, Sumesh
Kumar TM, Jayaraman S, Variyar EJ and Sudhakaran S: Structural
characterization of a novel derivative of myricetin from Mimosa
pudica as an anti-proliferative agent for the treatment of cancer.
Biomed Pharmacother. 84:1067–1077. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Semwal DK, Semwal RB, Combrinck S and
Viljoen A: Myricetin: A dietary molecule with diverse biological
activities. Nutrients. 8:902016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shih YW, Wu PF, Lee YC, Shi MD and Chiang
TA: Myricetin suppresses invasion and migration of human lung
adenocarcinoma A549 cells: Possible mediation by blocking the ERK
signaling pathway. J Agric Food Chem. 57:3490–3499. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumamoto T, Fujii M and Hou DX: Myricetin
directly targets JAK1 to inhibit cell transformation. Cancer Lett.
275:17–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seydi E, Rasekh HR, Salimi A, Mohsenifar Z
and Pourahmad J: Myricetin selectively induces apoptosis on
cancerous hepatocytes by directly targeting their mitochondria.
Basic Clin Pharmacol Toxicol. 119:249–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tian J, Tang ZY, Ye SL, Liu YK, Lin ZY,
Chen J and Xue Q: New human hepatocellular carcinoma (HCC) cell
line with highly metastatic potential (MHCC97) and its expressions
of the factors associated with metastasis. Br J Cancer. 81:814–821.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Meth. 25:402–408. 2001. View Article : Google Scholar
|
|
19
|
Zhao H, Jiao Y and Zhang Z: Deguelin
inhibits the migration and invasion of lung cancer A549 and H460
cells via regulating actin cytoskeleton rearrangement. Int J Clin
Exp Pathol. 8:15582–15590. 2015.PubMed/NCBI
|
|
20
|
Chen L, Chan TH, Yuan YF, Hu L, Huang J,
Ma S, Wang J, Dong SS, Tang KH, Xie D, et al: CHD1L promotes
hepatocellular carcinoma progression and metastasis in mice and is
associated with these processes in human patients. J Clin Invest.
120:1178–1191. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin Z, Li W, Zhang H, Wu W, Peng Y, Zeng
Y, Wan Y, Wang J and Ouyang N: CCL18/PITPNM3 enhances migration,
invasion, and EMT through the NF-κB signaling pathway in
hepatocellular carcinoma. Tumour Biol. 37:3461–3468. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ko CH, Shen SC, Lee TJ and Chen YC:
Myricetin inhibits matrix metalloproteinase 2 protein expression
and enzyme activity in colorectal carcinoma cells. Mol Cancer Ther.
4:281–290. 2005.PubMed/NCBI
|
|
23
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jung HY, Fattet L and Yang J: Molecular
pathways: Linking tumor microenvironment to epithelial-mesenchymal
transition in metastasis. Clin Cancer Res. 21:962–968. 2015.
View Article : Google Scholar : PubMed/NCBI
|