Angiocentric glioma (AG) is a rare central nervous
system (CNS) neoplasm that was first reported by Lellouch-Tubiana
et al (1) and Wang et
al (2) in 2005. AG was
recognized as a distinct clinicopathologic entity by the World
Health Organization (WHO) classification of CNS tumors in 2007 and
was defined as ‘an epilepsy-associated, stable or slow-growing
cerebral tumor primarily affecting children and young adults,
histologically characterized by an angiocentric pattern of growth,
monomorphous bipolar cells and features of ependymal
differentiation (3,4). Since its initial description, an
increasing number of cases of AG have been reported in the
literature. In the 2016 WHO classification of CNS tumors (5), AG was considered as a WHO grade I tumor
and was classified as ‘other gliomas’. The majority of studies on
AG focus on the cytological features of the disease (6,7), while
there is a lack of clinical and imaging data, as well as
descriptions of the surgical treatment. The present study describes
two patients with AG that received surgical treatment and provides
a review of all previously reported cases to date.
The first case was an 8-year-old male who presented
with a 3-month history of seizures, headaches and vomiting. The
patient was admitted to the Chinese PLA General Hospital (Beijing,
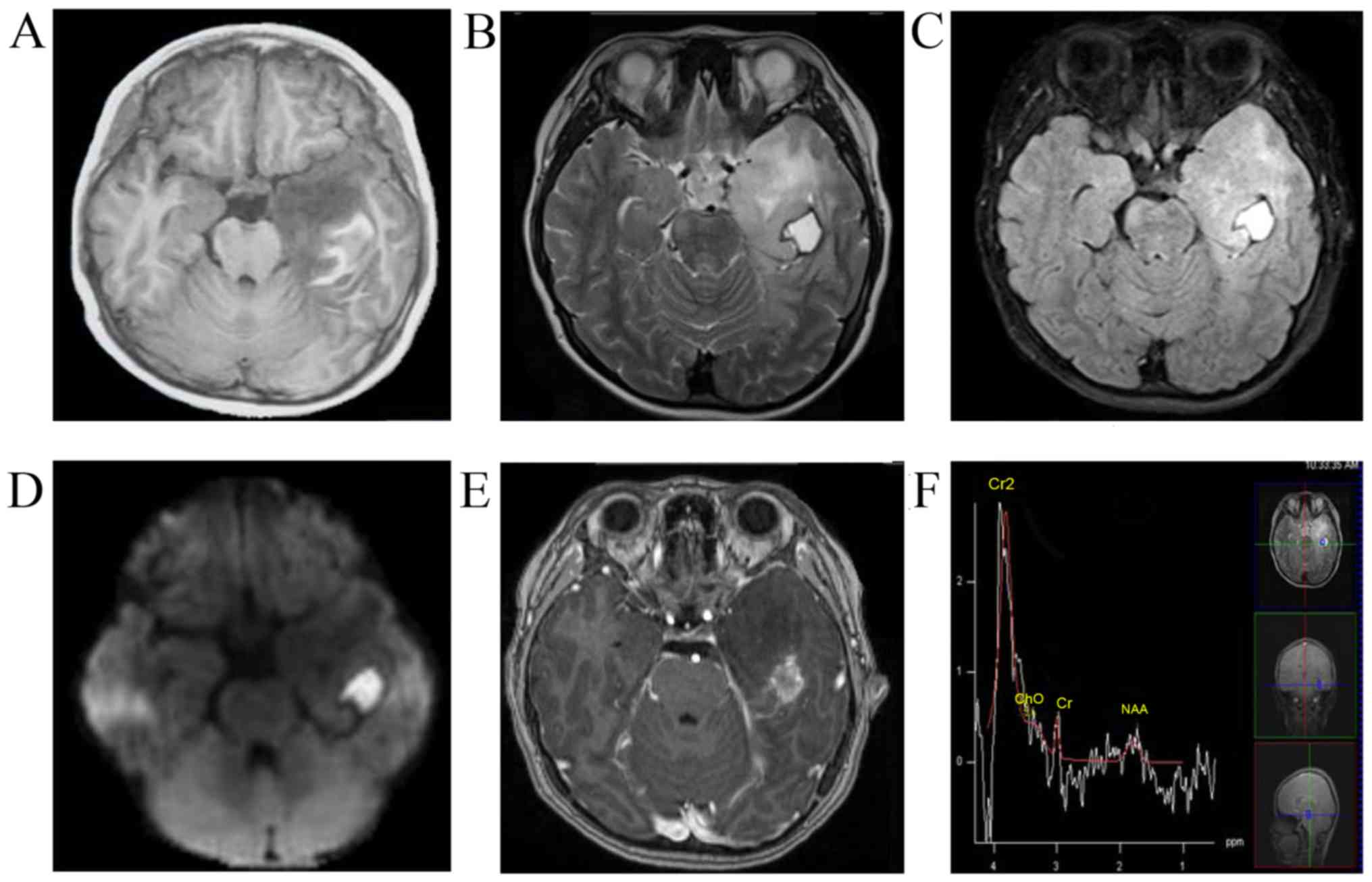
China) in June 2016. MRI revealed a left temporal non-enhancing
lesion [T1 hypointense, T2 hyperintense, diffusion-weighted imaging
hyperintense, fluid-attenuated inversion recovery (FLAIR)
hyperintense], measuring 2×2×1.5 cm, with a peripherally enhanced
1×1 mm cystic lesion and obvious brain edema around the lesion. The
patient underwent magnetic resonance spectroscopy, which revealed a
decrease in the N-acetylaspartate peak and no significant increase
in the choline peak (Fig. 1). The
patient was then subjected to a left craniotomy and underwent gross
total resection (GTR). The tumor was located in the inferior
temporal lobe and had a relatively clear boundary. Part of the
tumor tissue was fish flesh-like in appearance and the patient had
recurrent hemorrhage without vascular changes. Intra-operative
frozen histological analysis suggested low-grade glioma. The final
pathological assessment (Fig. 2)
revealed that tumor cells surrounded the blood vessels and neurons
in the cortex. The infiltrating tumor cells were glial fibrillary
acidic protein (GFAP)-positive and epithelial membrane antigen
(EMA) staining was observed in a distinct dot-like pattern in the
cytoplasm. The Ki-67 proliferative rate was 5% and the cells were
S-100- and neurospecific nucleoprotein (NeuN)-positive, and protein
53 (p53)-, synaptophysin (Syn)-, oligodendrocyte transcription
factor-2 (Olig-2)- and creatine kinase (CK)-negative. According the
2016 WHO classification of CNS tumors (3,4), tumors
with an angiocentric pattern of growth, GFAP-positive, NeuN-
positive and low Ki-67 proliferative rate were diagnosed as AG (WHO
grade I). At the 3.5-year follow-up, the patient continued to be
seizure-free and did not exhibit any neurological deficits.
Post-operative MRI also revealed no recurrence of the tumor.
The second case was a 16-year-old male who presented
with a 23-day history of recurrent seizures. The patient was
admitted to the Chinese PLA General Hospital (Beijing, China) in
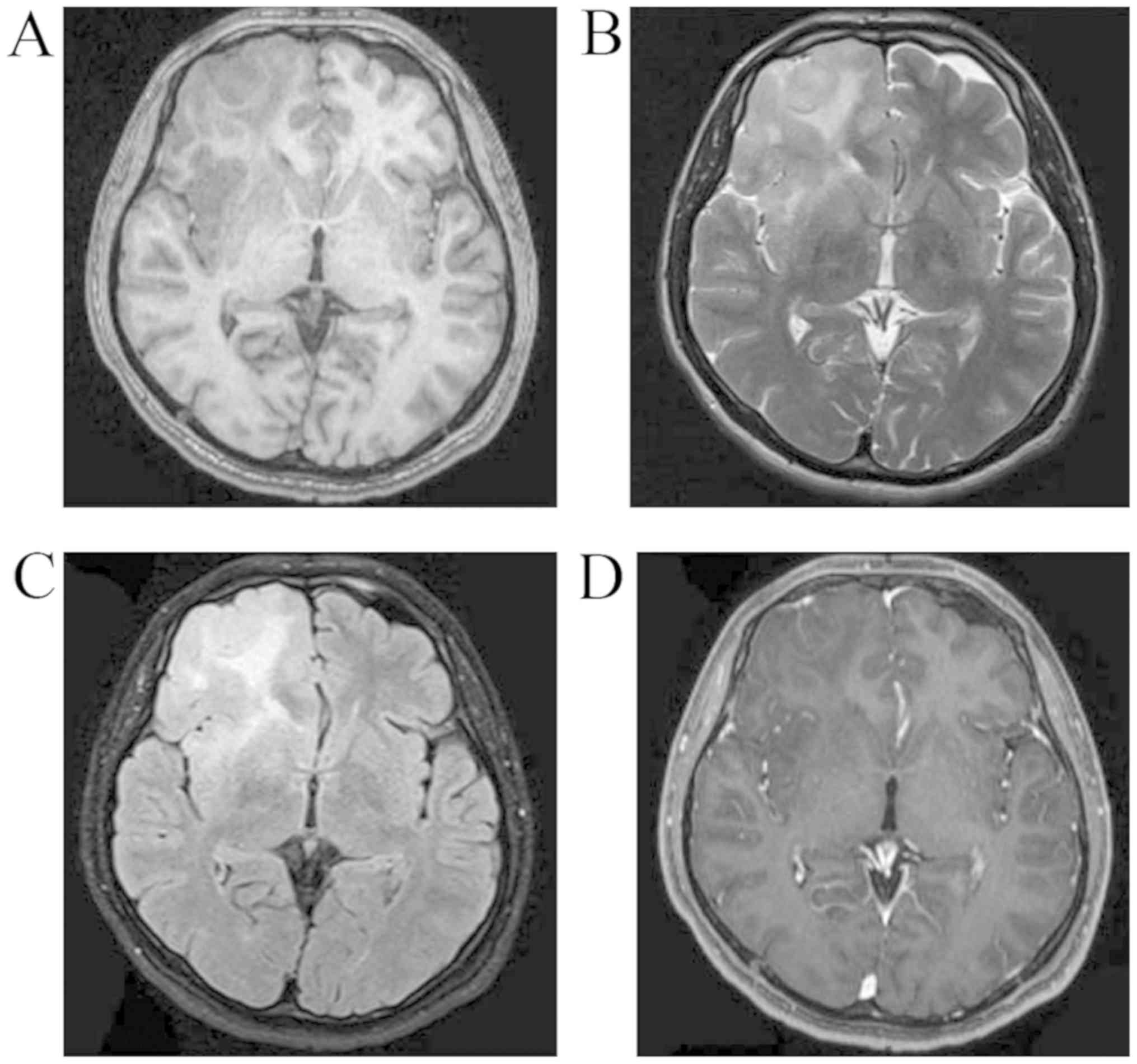
July 2017. A CT scan revealed a round, circumscribed, hypodense
lesion in the right frontal lobe (Fig.
3). MRI revealed a right frontal non-enhanced lesion (T1
hypointense, T2 and FLAIR slightly hyperintense; Fig. 4). Low-grade glioma was diagnosed at
the initial stage. The patient then underwent right craniotomy. At
the time of this initial surgery, the tumor was fish flesh-like in
appearance and soft, had a rich blood supply and was not distinctly
different from the surrounding brain tissue. Intra-operative frozen
histological analysis also suggested low-grade glioma. The tumor
was completely resected as tumor cells were not detected in the
surgical margin based on the intra-operative histology. The final
pathological assessment (Fig. 5)
revealed infiltrating round or ovoid tumor cells in and under the
subcortex that were partly arranged around blood vessels and
neurons in concentric sleeves and pseudorosettes, demonstrating an
angiocentric and creeping pattern. The tumor was dense with
irregular cell nuclei and new blood vessels. No mitotic figures or
necrotic cells were observed. The Ki-67 proliferative rate was 2%.
The tumor was also immunoreactive for S100, vimentin, neurofilament
and NeuN, but was negative for Olig-2, CD3, CD20, CD34 and CD68.
These features supported the diagnosis of AG (WHO grade I). At the
2.5-year follow-up, the patient continued to be seizure-free and
did not have any complications or neurological deficits. MRI at 5
months post-operatively (Fig. 6)
revealed no recurrence of the tumor.
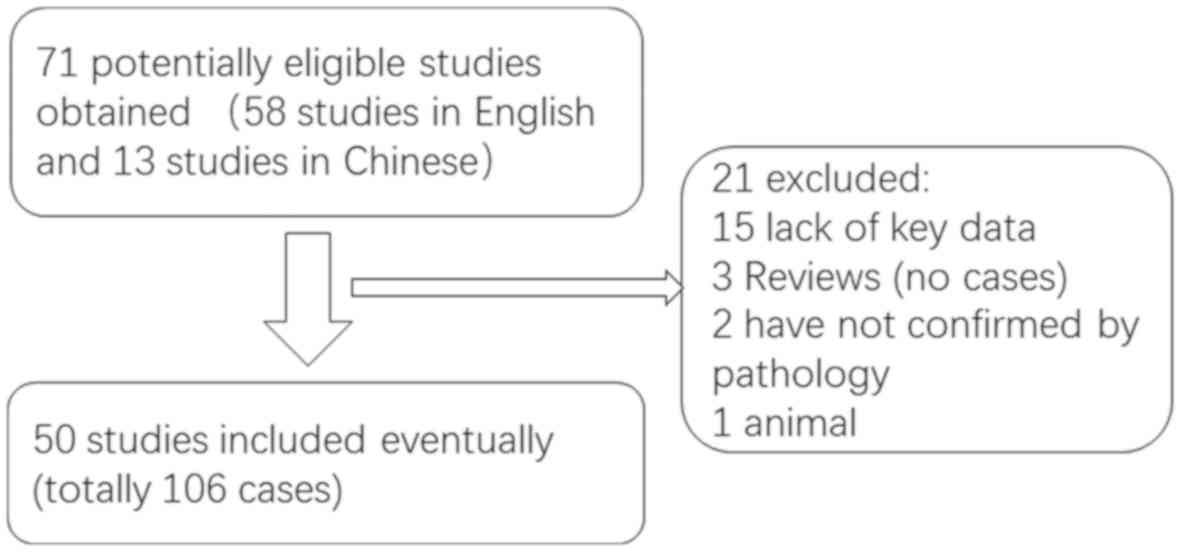
A literature review of studies on AG published in
English or Chinese between January 2005 and December 2019 (Fig. 7), performed using Medline, PubMed and
the China National Knowledge Infrastructure database, revealed that
a total of 108 cases, including the two cases of the present study,
have been reported (Table SI)
(1,2,6–54). Of the 108 patients with AG, 61 were
male and 47 were female (male/female ratio, 1:0.77). The age at the
time of admission ranged between 1.5 and 83 years (median age, 13
years) and the majority of the patients were children and young
adults. A total of 90 patients (85.7%; 90/105) presented with a
long history of several types of intractable seizures (symptoms in
three cases were not described). Only 15 patients exhibited
different symptoms. Of these 15 patients, eight had headaches, four
of which had decreased vision (7,12,47),
three experienced dizziness, one of which had otalgia (9,19,49), two
had ataxia (11,47), two had swallowing disorders (34,37), two
presented with weakness and numbness of the left side of the body
(17,45) and one had strabismus (34). The majority of the AG tumors (94.4%;
102/108) were in a supratentorial location situated within/under
the cerebral cortex and 81.5% (88/108) were located in a single
lobe. A total of six tumors were located in the brainstem (11,34,37,47). In
a total of 46 cases, the tumor was in the left brain and in 43
cases, it was in the right brain. As AG arises in a superficial
location in the cerebrum, which is typically completely removed,
the literature indicated that gross total resection (GTR) of this
lesion is curative. A total of 64.9% (61/94) of the patients with
AG underwent GTR and 27.7% (26/94) underwent subtotal resection
(STR). Furthermore, 7 cases only had a biopsy (7,34,37,47).
The follow-up time ranged between 0.25 and 168 months (mean, 23.5
months). Of the patients with resection, 93.1% (81/87) were free of
seizures during the follow-up time and the 6 patients with seizure
recurrence had all undergone STR. A total of 10 patients received
adjuvant therapy including radiation or chemotherapy, of which six
received STR and four had a biopsy as the lesions were located in
the brainstem (6,14,34,37).
Histopathological evaluation revealed that the tumors were WHO
grade I, with one exception, which was WHO grade III–IV (17).
The present case study reported on two patients that
were surgically treated at the Chinese PLA General Hospital
(Beijing, China). The cases were similar to the ones reported in
the literature, regardless of age, symptoms, tumor location and
prognosis. The 8- and 16-year-old patients of the present study
presented with intractable seizures and the 16-year-old patient
also presented with headaches and vomiting. In the two patients,
the tumor was located in the superficial cerebrum, in the left
temporal lobe and in the right frontal lobe, respectively. The two
patients underwent GTR. During the follow-up period of 42 and 30
months, the patients were seizure-free and did not experience any
tumor recurrence.
As there is a lack of specific clinical
manifestations and radiological features for AG, diagnosis still
depends primarily on histopathological examination. Monomorphic,
diffusely infiltrating bipolar spindled cells are commonly arranged
around cortical blood vessels or neurons in concentric sleeves and
pseudorosettes, which was the typical angiocentric gliomas pattern
(15). Immunohistochemical staining
results are generally positive for GFAP, S-100 and vimentin, and
EMA has a dot-like pattern (8).
Based on these findings, the main entities that were considered in
the differential diagnosis were ependymoma and AG (6). The neuronal markers NeuN, Syn and
chromogranin A (CgA) were usually negative. The Ki-67 proliferative
index was ~1% and not >5%. This tumor type is also similar to
other benign brain tumor types, including focal cortical dysplasia,
ganglioglioma and certain neuroepithelial neoplasms, including
pilomyxoid astrocytoma and supratentorial cortical ependymoma.
Differences between AG and various easily misdiagnosed brain tumors
are presented in Table I (5,55–61).
While AG has been established as a distinct tumor
type, its cytogenesis remains elusive. Wang et al (2) posited that AG arises from ependymoma
and astroblastoma. Lellouch-Tubiana et al (1) suggested that AG has radial glia or
neuronal origin. Bandopadhayay et al (62) and Qaddoumi et al (63) observed that an myeloblastosis quaking
(MYB-QKI) gene rearrangement occurred in the majority of tumors,
which contributed to the tumorigenesis, and this rearrangement was
specific to angiocentric gliomas. Therefore, MYB-QKI gene fusion
may be a defining genetic alteration typical for AG. While
mutations of isocitrate dehydrogenase-1 most commonly result in the
replacement of arginine at position 132 by histidine (R132H) in WHO
grade II and III diffuse gliomas and secondary glioblastomas, this
was not identified in AG (20,34,43).
Therefore, this may facilitate the differential diagnosis of AG
from tumors with a higher potential for recurrence.
AG is a slow-growing, stable tumor and lesions in
the cerebral cortex are generally benign and may be cured by
surgical excision alone. Adjuvant therapy, including chemotherapy
or radiation, are not typically required (45). However, angiocentric gliomas arising
in structures that are not amenable to surgical resection,
including the brainstem, may require stereotactic biopsies and
adjuvant therapy (37). Seizure
control was dependent on the degree of tumor resection. According
to the present review, AG is associated with a more favorable
prognosis, with low mortality and incidence of disability. In two
cases of tumor recurrence (2,42), the
histopathological evaluation revealed a malignant neoplasm (WHO
grade III) that was ultimately fatal.
AG is a recently described rare tumor of the CNS and
exhibits radiological features on MRI that usually resemble those
of diffuse low-grade glioma. AG tends to be non-malignant and
curable and typically has a favorable prognosis. However, certain
tumors may undergo malignant transformation. Longer follow-up
periods are required to accurately establish the time to
recurrence, to determine whether additional treatment is required
and to establish the overall survival time.
Not applicable.
No funding was received.
All data generated or analyzed during this study
were included in this published article.
GQH, JSZ and YM conceived the present study. GQH
performed the data analysis. QPG and SY provided and analyzed the
imaging and pathological data. GQH performed software analysis of
the data and figures. YM supervised the research. GQH and JSZ
drafted and reviewed the initial manuscript, and performed the
literature review. YM edited the manuscript. All authors read and
approved the manuscript.
Not applicable.
Written informed consent for publication was
provided by the patients' guardians as the patients were both under
the age of 18.
The authors declare that they have no competing
interests.
|
1
|
Lellouch-Tubiana A, Boddaert N, Bourgeois
M, Fohlen M, Jouvet A, Delalande O, Seidenwurm D, Brunelle F and
Sainte-Rose C: Angiocentric neuroepithelial tumor (ANET): A new
epilepsy-related clinicopathological entity with distinctive MRI.
Brain Pathol. 15:281–286. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang M, Tihan T, Rojiani AM, Bodhireddy
SR, Prayson RA, Iacuone JJ, Alles AJ, Donahue DJ, Hessler RB, Kim
JH, et al: Monomorphous angiocentric glioma: A distinctive
epileptogenic neoplasm with features of infiltrating astrocytoma
and ependymoma. J Neuropathol Exp Neurol. 64:875–881. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brat DJ, Scheithauer BW, Fuller GN and
Tihan T: Newly codified glial neoplasms of the 2007 WHO
classification of tumours of the central nervous system:
Angiocentric glioma, pilomyxoid astrocytoma and pituicytoma. Brain
Pathol. 17:319–324. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 world health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mott RT, Ellis TL and Geisinger KR:
Angiocentric glioma: A case report and review of the literature.
Diagn Cytopathol. 38:452–456. 2010.PubMed/NCBI
|
|
7
|
Marburger T and Prayson R: Angiocentric
glioma: A clinicopathologic review of 5 tumors with identification
of associated cortical dysplasia. Arch Pathol Lab Med.
135:1037–1041. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alexandru D, Haghighi B and Muhonen MG:
The treatment of angiocentric glioma: Case report and literature
review. Perm J. 17:e100–e102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rho GJ, Kim H, Kim HI and Ju MJ: A case of
angiocentric glioma with unusual clinical and radiological
features. J Korean Neurosurg Soc. 49:367–369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fulton SP, Clarke DF, Wheless JW, Ellison
DW, Ogg R and Boop FA: Angiocentric glioma-induced seizures in a
2-year-old child. J Child Neurol. 24:852–856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Covington DB, Rosenblum MK, Brathwaite CD
and Sandberg DI: Angiocentric glioma-like tumor of the midbrain.
Pediatr Neurosurg. 45:429–433. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shakur SF, McGirt MJ, Johnson MW, Burger
PC, Ahn E, Carson BS and Jallo GI: Angiocentric glioma: A case
series. J Neurosurg Pediatr. 3:197–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sugita Y, Ono T, Ohshima K, Niino D, Ito
M, Toda K and Baba H: Brain surface spindle cell glioma in a
patient with medically intractable partial epilepsy: A variant of
monomorphous angiocentric glioma? Neuropathology. 28:516–520. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Preusser M, Hoischen A, Novak K, Czech T,
Prayer D, Hainfellner JA, Baumgartner C, Woermann FG, Tuxhorn IE,
Pannek HW, et al: Angiocentric glioma: Report of clinico-pathologic
and genetic findings in 8 cases. Am J Surg Pathol. 31:1709–1718.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Buccoliero AM, Castiglione F,
Degl'innocenti DR, Moncini D, Spacca B, Giordano F, Genitori L and
Taddei GL: Angiocentric glioma: Clinical, morphological,
immunohistochemical and molecular features in three pediatric
cases. Clin Neuropathol. 32:107–113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arsene D, Ardeleanu C, Ogrezeanu I and
Danaila L: Angiocentric glioma: Presentation of two cases with
dissimilar histology. Clin Neuropathol. 27:391–395. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aguilar HN, Hung RW, Mehta V and Kotylak
T: Imaging characteristics of an unusual, high-grade angiocentric
glioma: A case report and review of the literature. J Radiol Case
Rep. 6:1–10. 2012.PubMed/NCBI
|
|
18
|
Qi X, Duan Z, Yao K and Liu C:
Clinicopathological features of angiocentric glioma: A report of
two cases. J Diag Pathol. 266–269. 2012.
|
|
19
|
Hu XW, Zhang YH, Wang JJ, Jiang XF, Liu JM
and Yang PF: Angiocentric glioma with rich blood supply. J Clin
Neurosci. 17:917–918. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu JT, Wang L, Chen GQ and Jin YJ:
Angiocentric glioma with refractory epelepsy in children. Chin J
Stereotact Funct Neurosurg. 25:109–114. 2012.
|
|
21
|
Sun FH, Piao YS, Wang W, Chen L, Wei LF,
Yang H and Lu DH: Brain tumors in patients with intractable
epilepsy: A clinicopathologic study of 35 cases. Zhonghua Bing Li
Xue Za Zhi. 38:153–157. 2009.(In Chinese). PubMed/NCBI
|
|
22
|
Ma XM, Liu HM, Li YL, He J, WANG LZ, Xu Y
and Chen B: Hippocampus glioma with temporal lobe epilepsy as the
main manifestation: The clinicopathologic properties. Acad J Second
Mil Med Univ. 31:60–62. 2010. View Article : Google Scholar
|
|
23
|
Li JY, Langford LA, Adesina A, Bodhireddy
SR, Wang M and Fuller GN: The high mitotic count detected by
phospho-histone H3 immunostain does not alter the benign behavior
of angiocentric glioma. Brain Tumor Pathol. 29:68–72. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Raghunathan A, Olar A, Vogel H, Parker JR,
Coventry SC, Debski R, Albarracin CT, Aldape KD, Cahill DP III,
Powell SZ and Fuller GN: Isocitrate dehydrogenase 1 R132H mutation
is not detected in angiocentric glioma. Ann Diagn Pathol.
16:255–259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyahara H, Toyoshima Y, Natsumeda M,
Uzuka T, Aoki H, Nakayama Y, Okamoto K, Fujii Y, Kakita A and
Takahashi H: Anaplastic astrocytoma with angiocentric ependymal
differentiation. Neuropathology. 31:292–298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takada S, Iwasaki M, Suzuki H, Nakasato N,
Kumabe T and Tominaga T: Angiocentric glioma and surrounding
cortical dysplasia manifesting as intractable frontal lobe
epilepsy-case report. Neurol Med Chir (Tokyo). 51:522–526. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pokharel S, Parker JR, Parker JC Jr,
Coventry S, Stevenson CB and Moeller KK: Angiocentric glioma with
high proliferative index: Case report and review of the literature.
Ann Clin Lab Sci. 41:257–261. 2011.PubMed/NCBI
|
|
28
|
Rosenzweig I, Bodi I, Selway RP, Crook WS,
Moriarty J and Elwes RD: Paroxysmal ictal phonemes in a patient
with angiocentric glioma. J Neuropsychiatry Clin Neurosci.
22:123.E18–E20. 2010. View Article : Google Scholar
|
|
29
|
Koral K, Koral KM and Sklar F:
Angiocentric glioma in a 4-year-old boy: Imaging characteristics
and review of the literature. Clin Imaging. 36:61–64. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miyata H, Ryufuku M, Kubota Y, Ochiai T,
Niimura K and Hori T: Adult-onset angiocentric glioma of
epithelioid cell-predominant type of the mesial temporal lobe
suggestive of a rare but distinct clinicopathological subset within
a spectrum of angiocentric cortical ependymal tumors.
Neuropathology. 32:479–491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Varikatt W, Dexter M, Mahajan H, Murali R
and Ng T: Usefulness of smears in intra-operative diagnosis of
newly described entities of CNS. Neuropathology. 29:641–648. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Adamek D, Siwek GP, Chrobak AA,
Herman-Sucharska I, Kwiatkowski S, Morga R, Radwańska E and
Urbanowicz B: Angiocentric glioma from a perspective of A-B-C
classification of epilepsy associated tumors. Folia Neuropathol.
54:40–49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ampie L, Choy W, DiDomenico JD, Lamano JB,
Williams CK, Kesavabhotla K, Mao Q and Bloch O: Clinical attributes
and surgical outcomes of angiocentric gliomas. J Clin Neurosci.
28:117–122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chan E, Bollen AW, Sirohi D, Van Ziffle J,
Grenert JP, Kline CN, Tihan T, Perry A, Gupta N and Solomon DA:
Angiocentric glioma with MYB-QKI fusion located in the brainstem,
rather than cerebral cortex. Acta Neuropathol. 134:671–673. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chatterjee D, Gupta K, Singla N and
Radotra BD: Angiocentric glioma of hippocampus-report of a rare
intractable epilepsy-related tumor. Neurol India. 64:340–343. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cheng S, Lü Y, Xu S, Liu Q and Lee P:
Cystoid angiocentric glioma: A case report and literature review. J
Radiol Case Rep. 9:1–9. 2015.PubMed/NCBI
|
|
37
|
D'Aronco L, Rouleau C, Gayden T, Crevier
L, Décarie JC, Perreault S, Jabado N, Bandopadhayay P, Ligon KL and
Ellezam B: Brainstem angiocentric gliomas with MYB-QKI
rearrangements. Acta Neuropathol. 134:667–669. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ersen A, Canda MS, Men S, Yucesoy K,
Kalemci O and Canda T: Angiocentric glioma: The infiltrative glioma
with ependymal differentiation. Turk Patoloji Derg. 33:251–255.
2017.PubMed/NCBI
|
|
39
|
Grajkowska W, Matyja E, Daszkiewicz P,
Roszkowski M, Peregud-Pogorzelski J and Jurkiewicz E: Angiocentric
glioma: A rare intractable epilepsy-related tumour in children.
Folia Neuropathol. 52:253–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kakkar A, Sharma MC, Suri V, Kaushal S,
Chandra SP, Garg A and Sarkar C: Angiocentric glioma: A treatable
cause of epilepsy: Report of a rare case. Neurol India. 62:677–679.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Keser H, Barnes M, Moes G, Lee HS and
Tihan T: Well-differentiated pediatric glial neoplasms with
features of oligodendroglioma, angiocentric glioma and
dysembryoplastic neuroepithelial tumors: A morphological diagnostic
challenge. Turk Patoloji Derg. 30:23–29. 2014.PubMed/NCBI
|
|
42
|
McCracken JA, Gonzales MF, Phal PM and
Drummond KJ: Angiocentric glioma transformed into anaplastic
ependymoma: Review of the evidence for malignant potential. J Clin
Neurosci. 34:47–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ni HC, Chen SY, Chen L, Lu DH, Fu YJ and
Piao YS: Angiocentric glioma: A report of nine new cases, including
four with atypical histological features. Neuropathol Appl
Neurobiol. 41:333–346. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sajjad J, Kaliaperumal C, Bermingham N,
Marks C and Keohane C: ‘Unusual brain stone’: Heavily calcified
primary neoplasm with some features suggestive of angiocentric
glioma. J Neurosurg. 123:1256–1260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gonzalez-Quarante LH, Fernández Carballal
C, Agarwal V, Vargas Lopez AJ, Gil de Sagredo Del Corral OL and
Sola Vendrell E: Angiocentric glioma in an elderly patient: Case
report and review of the literature. World Neurosurg.
97:755.e5–e755.e10. 2017. View Article : Google Scholar
|
|
46
|
Tauziède-Espariat A, Fohlen M,
Ferrand-Sorbets S and Polivka M: A unusual brain cortical tumor:
Angiocentric glioma. Ann Pathol. 35:154–158. 2015.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Weaver KJ, Crawford LM, Bennett JA,
Rivera-Zengotita ML and Pincus DW: Brainstem angiocentric glioma:
Report of 2 cases. J Neurosurg Pediatr. 20:347–351. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Whitehead MT and Vezina G: MR
spectroscopic profile of an angiocentric glioma. Anticancer Res.
35:6267–6270. 2015.PubMed/NCBI
|
|
49
|
Wu CX, Zheng D, Yao K and Liu N: Clinical,
imaging and pathological findings of angiocentric gliomas: An
analysis of 8 cases. Clin J Neuromed. 9:869–873. 2015.
|
|
50
|
Feng LJ, Wen ZB, Wang XL, Yu Y and Jiang
SS: Intracranial angiocentric glioma: Case report. Chin J Med
Imaging Technol. 4:522–524. 2015.
|
|
51
|
Li YY, Lan YQ and Chen YM: A case of
angiocentric glioma. Chin J Magn Reson Imaging. 3:230–232.
2017.
|
|
52
|
Liang Y, Di HJ, Fu J and Leng H: A case of
angiocentric glioma. J Cliff Exp Pathol. 7:831–832. 2016.
|
|
53
|
Liu F, Zhang LY, Guo L, Hu WW and Li Z:
Angiocentric glioma: A case report and review of the literature. J
Clin Exp Pathol. 10:1174–1177. 2016.
|
|
54
|
Xu WJ, Zheng ZY and Liu W: Insular
angiocentric glioma: Report of 1 case. J Clin Exp Pathol.
6:717–719. 2015.
|
|
55
|
Wen PY and Huse JT: 2016 World health
organization classification of central nervous system tumors.
Continuum (Minneap Minn). 23:1531–1547. 2017.PubMed/NCBI
|
|
56
|
Mohaghegh MR, Chitsaz A, Okhovat AA and
Pour EB: Supratentorial cortical ependymoma: An unusual
presentation of a rare tumor. Adv Biomed Res. 4:722015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hammas N, Senhaji N, Alaoui Lamrani MY,
Bennis S, Chaoui EM, El Fatemi H and Chbani L: Astroblastoma-a rare
and challenging tumor: A case report and review of the literature.
J Med Case Rep. 12:1022018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Crino PB: Focal cortical dysplasia. Semin
Neurol. 35:201–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kubicky CD, Sahgal A, Chang EL and Lo SS:
Rare primary central nervous system tumors. Rare Tumors.
6:54492014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ma X, Wang Y, Liu H, Yu H and He J:
Pilomyxoid astrocytomas with rare rosenthal fibers. Brain Tumor
Pathol. 33:35–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Esparragosa I, Diez-Valle R, Tejada S and
Gállego Pérez-Larraya J: Management of diffuse glioma. Presse Med.
47:e199–e212. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bandopadhayay P, Ramkissoon LA, Jain P,
Bergthold G, Wala J, Zeid R, Schumacher SE, Urbanski L, O'Rourke R,
Gibson WJ, et al: MYB-QKI rearrangements in angiocentric glioma
drive tumorigenicity through a tripartite mechanism. Nat Genet.
48:273–282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Qaddoumi I, Orisme W, Wen J, Santiago T,
Gupta K, Dalton JD, Tang B, Haupfear K, Punchihewa C, Easton J, et
al: Genetic alterations in uncommon low-grade neuroepithelial
tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and
align with morphology. Acta Neuropathol. 131:833–845. 2016.
View Article : Google Scholar : PubMed/NCBI
|