Introduction
Liver cancer is one of the most common malignant
tumors worldwide and is a leading cause of cancer-associate
mortality (1). The therapeutic
strategies for liver cancer include surgery, liver transplantation,
local ablation, interventional chemotherapy and biological drug
targeted therapy (2). However,
chemotherapy drug resistance and chemotoxicity can reduce the
antitumor efficacy and patient tolerance (3–5). It is
therefore crucial to develop novel non-toxic and efficient agents
that could be used in combination with existing therapies for the
treatment of patients with liver cancer.
Survivin, an inhibitor of the apoptosis protein
family, is specifically expressed in tumors whereas it is
under-expressed in normal adult differentiated mature tissues
(6,7). Survivin serves crucial roles in cell
cycle and cell death by inhibiting apoptosis (8). Survivin inhibits apoptosis mainly via
the intrinsic apoptotic pathway. In the intrinsic pathway, survivin
overexpression can stabilize the activation of X-linked inhibitor
of apoptosis protein (XIAP) and inhibit the caspase-3/9 apoptotic
pathway (9). Furthermore, survivin
is closely associated with liver cancer recurrence and therapeutic
resistance. The results from a previous study including 60 patients
demonstrated that the overall survival of patients is significantly
higher in patients with negative expression of survivin at tumor
margins. Furthermore, negative survivin expression in tumor tissues
is associated with fewer extensive operations and the absence of
vascular invasion (10). Therefore,
due to its inhibiting effect on liver cancer cell apoptosis,
survivin may be considered as a potential target for tumor gene
therapy.
Matrine is a component isolated from Sophora
flavescens (11). It has been
reported to have anti-inflammatory, antiviral and antitumor
effects, and has been used for treatment of chronic active
hepatitis and hepatocellular carcinoma (HCC) (12). Previous studies have demonstrated
that matrine can inhibit the proliferation and induce the apoptosis
of SMMC-7721, HepG2 and BEL-7402 tumor cell lines in a
dose-dependent manner (13–15). A recent study reported that the
combination of matrine and cisplatin has some inhibitory effects on
HCC cell proliferation and tumor growth and exhibits a reduced
toxicity (16). However, further
investigation is required to determine the underlying mechanisms of
matrine + cisplatin in liver cancer.
The present study aimed to explore the antitumor
effects of matrine combined with cisplatin in a nude mouse model
transplanted with HepG2 liver cancer cells.
Materials and methods
Main reagents
Matrine was obtained from Guangzhou Baiyun Shan Ming
Xing Pharmaceutical, Co., Ltd. (cat. no. H10950071). Cisplatin was
purchased from Qilu Pharmaceutical Co., Ltd. (cat. no. H37021358).
Rabbit survivin (cat. no. A00379), caspase-3 (cat. no. BM3954),
caspase-7 (cat. no. PA1442), caspase-9 (cat. no. BM4619), XIAP
(cat. no. BA2620), β-actin (cat. no. BM3873) monoclonal primary
antibodies and goat anti-mouse secondary IgG were obtained from
Wuhan Boster Biological Technology, Ltd.
Nude mice
A total of 24 six-weeks-old BALB/c nude mice were
purchased from the the Animal Experimental Center of Guangxi
Medical University (Nanning, China; registration no. SCXK
2010-0002). Mice weighted 16–20 g and were housed under
specific-pathogen-free conditions at a temperature of 22–26°C and a
relative humidity of 40–60%. All efforts were made to minimize
animal suffering. Animal protocol was approved by the Ethical
Committee of the Youjiang Medical University for Nationalities.
Cell culture
The HepG2 cell line was obtained from The
Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences and was authenticated by the manufacturer using the STR
method. HepG2 cells were cultured in RPMI-1640 medium (HyClone;
Cytiva) supplemented with 10% FBS (Zhejiang Tianhang Biotechnology
Co., Ltd.) and placed at 37°C in a humidified incubator containing
5% CO2. The medium was changed once every three
days.
Tumor model and treatment
To generate the tumor model, nude mice were
subcutaneously injected with a 200 µl cell suspension containing
5×106 HepG2 cells into the axillary region. The tumors
grew to almost 6 mm after 10 days of inoculation, and a successful
HepG2 tumor model was confirmed by cell morphology observation with
hematoxylin-eosin (H&E) staining. Subsequently, mice were
randomly divided into four groups of six mice as follows: i) normal
saline (NS) group, mice administered with NS; ii) matrine group,
mice intraperitoneally injected with 100 mg/kg matrine; iii)
cisplatin group, mice intraperitoneally injected with 2 mg/kg
cisplatin; and iv) matrine + cisplatin group, mice
intraperitoneally injected with 100 mg/kg matrine and 2 mg/kg
cisplatin. Drugs were intraperitoneally injected five days per week
for three weeks. The reactivity and behavior of mice in the cages
were observed. The length and width of the tumors were measured on
days 7, 10, 12, 14, 17, 19 and 21 using a Vernier caliper, and the
weight of the mice was recorded. The tumor volume was calculated
using the formula: Tumor volume=0.5× length × (width)2.
Mice were sacrificed by cervical dislocation at the end of the
treatment period. The sacrifice of mice was confirmed by the sound
of cervical spine fracture and the absence of breathing. The tumors
were excised, one part was stored at −80°C for western blotting and
another part was fixed for further staining. The tumor inhibition
rate was analyzed using the following formula: Tumor inhibition
rate (%)=(1-average weight of tumors in treatment group/average
weight of tumors in control group) ×100.
Histological analysis
Tumor tissues were harvested and fixed in 4%
formaldehyde for 24 h at room temperature. The samples were then
dehydrated using increasing ethanol gradient (75, 85, 95% and
anhydrous ethanol), embedded in paraffin and cut into 4-µm
sections. Subsequently, dried slices were dewaxed using xylene
twice for 5 min followed by 100, 95, 85 and 75% ethanol for 2 min.
Sections were stained by hematoxylin for 8–15 min and
differentiated by 1% hydrochloric alcohol for 10 sec. Finally,
sections were blocked for H&E staining after bluing under 50°C
water. Histopathological examination was performed under a light
microscope (Leica Microsystems, Inc.).
Immunohistochemical (IHC)
staining
IHC staining was performed according to the
manufacturers' instructions. Briefly, the tumor tissue slices were
incubated with 0.3% hydrogen peroxide for 10 min at room
temperature and washed with PBS. Sections were incubated with goat
anti-mouse secondary IgG (1:500) at 4°C overnight, washed three
times with PBS and incubated with secondary antibody (1:4,000) at
room temperature for 30 min. The tumor sections were then
visualized for 3–15 min using DBA solution. Eventually, slides were
counterstained with hematoxylin for 8–15 min at room temperature.
Cells positively stained were colored as brownish-yellow and were
counted in 10 random fields per section using a light microscope
(magnification, ×400). The rate of positive cells was calculated
using the following formula: Positive cell rate (%)=number of
positive cells/tumor cells ×100%.
Western blotting
Tumor samples were thawed and cut into small pieces.
Tissues were lysed using a western blotting lysis kit (cat. no.
P0013B; Beyotime Institute of Biotechnology) at 4°C. Protein
concentration was detected using the BCA method and proteins were
separated by 10% SDS-PAGE and transferred onto PVDF membranes.
Membranes were incubated with primary antibodies against survivin,
caspase-3, caspase-7, caspase-9, XIAP and β-actin diluted in
blocking buffer (1:10,000) overnight at 4°C, and with
HRP-conjugated secondary antibody for 1 h at room temperature.
Bands were developed on X-ray films using enhanced
chemiluminescence substrate (Super Signal West Pico; Pierce; Thermo
Fisher Scientific, Inc.). Densitometric analysis was performed
using a UV light box (Gel Doc XR+; Bio-Rad Laboratories, Inc.).
Statistical analysis
Data were presented as the means ± standard
deviation. Statistical analysis was performed using SPSS 16.0 (SPSS
Inc.) and GraphPad Prism v7.0 (GraphPad Software, Inc.).
Statistical differences between groups were analyzed using one-way
ANOVA followed by Tukey's post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Growing of HepG2-transplanted nude
mice
Nodules were detected at the inoculation site ~7
days after HepG2 cell injection, and tumors grew to almost 6 mm in
size after 10 days of inoculation, with a 100% success rate.
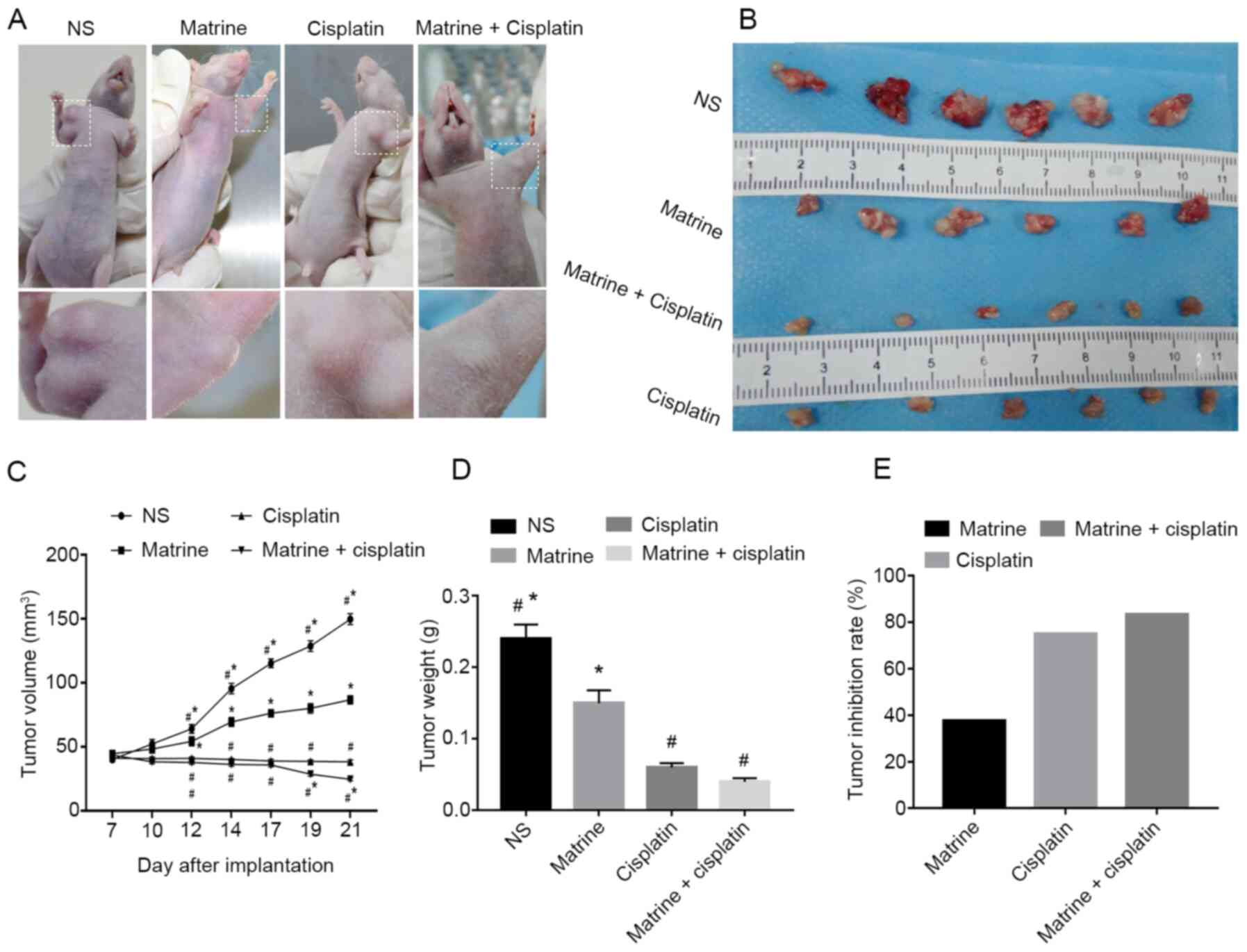
As presented in Fig.
1, the weight of nude mice treated with NS increased by 1–3 g
every two days, and stabilized at 26–28 g. The weight of nude mice
in the matrine group was ~27 g at the end of the experiment. Mice
in both NS and matrine groups presented with no abnormal mental
state, indicating that matrine had no significant adverse effects
on the development of HepG2-transplanted nude mice. Conversely,
nude mice treated with cisplatin showed poor mental health and a
less active behavior than those treated with NS. Furthermore,
treatment with cisplatin significantly decreased the body weight to
~17 g. However, the body weight in matrine + cisplatin group was
higher than that in the cisplatin group at the end of the
experiment (~22 g).
Matrine in combination with cisplatin
inhibits tumor growth
Matrine + cisplatin was administered in
HepG2-transplanted nude mouse model to observe its effect on tumor
progression. The results demonstrated that treatment with matrine
significantly decreased tumor volume compared with NS or cisplatin
groups. In addition, the tumor growth rate was significantly
decreased in mice treated with matrine + cisplatin compared with NS
group (Fig. 2A-C). Furthermore, as
presented in Fig. 2D, the tumor
weight of mice treated with matrine (0.15±0.018 g), cisplatin
(0.06±0.006 g) and matrine + cisplatin (0.04±0.005 g) was
significantly decreased compared with NS group (0.24±0.02 g).
Furthermore, treatment with matrine + cisplatin resulted in tumor
inhibition rate of 83.3%, whereas treatment with matrine- and
cisplatin-alone resulted in inhibition rates of 37.5 and 75%,
respectively, in HepG2-transplanted nude mice (Fig. 2E).
Tumor tissue morphology
Morphology of the tumor tissue under the microscope
was similar to that of the primary liver tumor. The tumor cells
were of various sizes. Nuclei were stained intensely. The
nucleocytoplasmic ratio was enlarged, and most of the nuclei were
divided. Few tumor cells showed fatty degeneration and a
transparent cytoplasm. In the NS group, a few apoptotic tumor cells
were scattered in the tumor tissue. Furthermore, more apoptotic
cells were seen in tumor tissue of mice treated with matrine, where
sheet-like focal areas of coagulative necroses could be seen. In
addition, increased apoptosis and coagulative necroses were
observed in the cisplatin group, in particular in the combination
group. These results suggested that matrine may promote tumor cell
apoptosis when combined with cisplatin (Fig. 3).
Survivin is downregulated following
mice treatment with matrine and cisplatin
Since survivin is associated with recurrence and
therapeutic resistance in liver cancer, the present study analyzed
whether matrine and cisplatin could affect survivin expression. The
results demonstrated that individual treatment with matrine and
cisplatin decreased the number of cells positive to survivin to
62.50±8.09 and 38.67±8.26%, respectively, compared with NS group
(83.26±15.56%; Fig. 4A and B).
Furthermore, the expression of survivin was significantly decreased
in mice treated with matrine + cisplatin (19.58±4.52%) compared
with those treated with cisplatin only (Fig. 4C and D). The results from western
blotting were similar to that of IHC. In addition, the expression
of XIAP, a downstream protein to survivin, was significantly
downregulated following combined treatment with matrine and
cisplatin (Fig. 4C and D).
 | Figure 4.Matrine in combination with cisplatin
regulated the expression of survivin, XIAP, caspase-3, caspase-7
and caspase-9 in HepG2 transplanted nude mice. (A and B)
Expression of survivin, caspase-3, caspase-7 and caspase-9 in tumor
tissues was assessed by immunohistochemistry (magnification, ×400;
scale bar, 20 µm) and quantitatively analyzed (n=6 per group). (C
and D) Expression of survivin, XIAP, caspase-3, caspase-7 and
caspase-9 in tumor samples was analyzed by western blotting and
quantified (n=6 per group). #P<0.05 vs. matrine group
and *P<0.05 vs. cisplatin group. NS, normal saline; XIAP,
X-linked inhibitor of apoptosis protein. |
Matrine in combination with cisplatin
activates the caspase apoptotic pathway
To investigate the molecular mechanism underlying
the anticancer role of matrine in liver cancer, the protein
expression profiles of caspase-3, caspase-7 and caspase-9 was
investigated in tumor tissues from mice treated with matrine,
cisplatin or both. The results demonstrated that the rate of cells
positive to caspase-3, caspase-7 and caspase-9 in the NS group were
21.15±3.68, 20.61±5.87 and 25.39±6.80%, respectively. However, this
rate was significantly increased in the matrine (35.13±10.57,
38.45±12.08 and 28.79±10.05%, respectively), cisplatin (65.88±4.8,
47.33±10.65 and 42.09±9.19%, respectively) and combination
treatment (78.26±6.09, 68.13±13.01 and 82.37±14.11%, respectively)
groups compared with the NS group (Fig.
4B). The results from western blotting were consistent with
that of IHC, indicating that the caspase apoptotic pathway may be
activated following mice treatment with matrine and cisplatin.
Discussion
Chemoresistance and chemotoxicity affect the
antitumor efficacy and patient tolerance to chemotherapy. Drug
combination therapy is therefore a common and efficient strategy
for cancer treatment. Matrine, an active component of Sophora
flavescens, has antitumor effects in breast, ovarian, non-small
cell lung and colorectal cancers, similarly to that of cisplatin in
liver cancer (17–19). A study by Li et al (20) reported that treatment with matrine in
combination with docetaxel inhibits the proliferation and migration
of tumor cells in androgen-resistant prostate cancer, indicating
the potential antitumor effects of matrine in combination with
chemotherapeutic drugs. A previous study from our laboratory
demonstrated that matrine in combination with cisplatin exerts a
favorable antitumor effect in liver cancer (16). However, the underlying mechanisms
remain unknown.
The subcutaneously transplanted mouse tumor model is
widely used to establish tumor models and study the efficacy of
drugs (21). In the present study,
the liver cancer mice model was successfully established following
subcutaneous injection of HepG2 cells, with a tumor success rate of
100%.
Survivin is a member of the IAPs family that has
been reported to be strongly associated with cell proliferation,
cell cycle control and apoptosis (22,23).
Previous studies have determined the medical roles of survivin in
numerous malignancies, including liver cancer. A study by Ikeguchi
et al (24) reported higher
recurrence rates and lower one- and three-year survival rates in
survivin-positive HCC patients compared with survivin-negative
patients. Furthermore, the proliferation of hepatoma cells is
significantly inhibited following survivin knockdown, promoting
hepatoma cell apoptosis and inhibition of tumor growth in nude mice
(25). Survivin serves therefore a
crucial role in the progression of liver cancer. A previous study
demonstrated that the proliferation index in survivin-positive
liver cancer is higher than that in survivin-negative liver cancer,
which may be related to the inhibitory effect of survivin in the
caspase pathway (26). In the
present study, matrine + cisplatin significantly inhibited the
expression of survivin, resulting in the inhibition of tumor growth
and lower mice tumor weights. The overexpression of survivin leads
to formation of complex with XIAP that inhibit apoptosis via
binding with members of the caspase family, in particular
caspase-3, caspase-7 and caspase-9 (8), suggesting that inhibition of survivin
could contribute to tumor apoptosis. In addition, overexpression of
procaspase can lead to proenzyme activation, indicating that
caspase proenzyme promotes auto-activation at high concentrations
(27). These studies indicate that
inhibiting surviving expression may be considered as an effective
antitumor strategy. In the present study, IHC and western blotting
were performed to analyze the regulatory effect of matrine +
cisplatin on surviving expression. The results demonstrated that
treatment with matrine in combination with cisplatin inhibited the
overexpression of survivin, reduced the inhibitory effect of
survivin on the downstream caspase pathway and promoted tumor cell
apoptosis.
In the present study, matrine presented some
antitumor effect similar to that of cisplatin. Furthermore, the
effects of matrine + cisplatin were better than that of cisplatin
alone. In addition, the changes in mice weight and behavioral and
psychological observations indicated that matrine may reduce the
side effects of cisplatin in liver cancer nude mice.
In summary, the present study demonstrated that
matrine in combination with cisplatin exerted inhibitory effects on
progression of liver cancer progression by promoting apoptosis via
suppression of survivin and activation of the caspase pathway.
Although our work has confirmed the efficacy of matrine in
combination with cisplatin on liver cancer treatment, further
investigation is required to confirm the results on apoptosis. To
do so, future work will focus on the detection of apoptosis-related
indicators, such as TUNEL staining, in vitro and in
vivo, and the determination of the underlying mechanism of
matrine + cisplatin on cell apoptosis. The results from the present
study may significantly contribute to the literature, and further
investigation in patients with liver cancer may validate the
efficacy of matrine in preventing chemotherapy resistance.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Guangxi Zhuang Autonomous Region (grant no.
2014GXNSFAA118143), the Science and Technology Base and Talents
Special Project of Guangxi (Research Project of Guangxi Clinical
Medical Research Center for Hepatobiliary Diseases; grant no. Guike
AD17129025), the 2017 Medical and Health Self-financing Project of
Guangxi (grant no. Z20170224) and the Innovation Project of Guangxi
Graduate Education (grant no. YCSW2020235).
Availability of data and materials
The data sets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
ZH and CCe substantially contributed to the
conception and design of the study. GH and CCa performed
experiments, analyzed data and drafted the manuscript. ZD, JL and
XZ performed experiments and interpreted data. All authors read and
approved the final version.
Ethics approval and consent to
participate
Animal experiments were approved by the Ethical
Committee of Youjiang Medical University For Nationalities (Baise,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chan SL and Yeo W: Development of systemic
therapy for hepatocellular carcinoma at 2013: Updates and insights.
World J Gastroenterol. 20:3135–3145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei YP, Shen NJ, Wang ZC, Yang GS, Yi B,
Yang N, Qiu YH and Lu JH: Sorafenib sensitizes hepatocellular
carcinoma cell to cisplatin via suppression of Wnt/β-catenin
signaling. Mol Cell Biochem. 381:139–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jung SH, Kim HJ, Oh GS, Shen A, Lee S,
Choe SK, Park R and So HS: Capsaicin ameliorates cisplatin-induced
renal injury through induction of heme oxygenase-1. Mol Cells.
37:234–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jin J, Ye MC, Wang LP, Li RX, Zhou Y, Wang
Y, Zhu WZ, Zuo Y and Liu SH: Grade IV Myelosuppression after
induction chemotherapy of TPF on oral cancer: Clinical analysis of
29 cases. Shanghai Kou Qiang Yi Xue. 23:219–223. 2014.(In Chinese).
PubMed/NCBI
|
|
6
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fenstermaker RA, Figel SA, Qiu JX, Barone
TA, Dharma SS, Winograd EK, Galbo PM, Wiltsie LM and Ciesielski MJ:
Survivin monoclonal antibodies detect survivin cell surface
expression and inhibit tumor growth in vivo. Clin Cancer Res.
24:2642–2652. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Altieri DC: Survivin in apoptosis control
and cell cycle regulation in cancer. Prog Cell Cycle Res.
5:447–452. 2003.PubMed/NCBI
|
|
9
|
Dohi T, Okada K, Xia F, Wilford CE, Samuel
T, Welsh K, Marusawa H, Zou H, Armstrong R, Matsuzawa S, et al: An
IAP-IAP complex inhibits apoptosis. J Biol Chem. 279:34087–34090.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kapiris I, Nastos K, Karakatsanis A,
Theodosopoulos T, Karandrea D, Pafiti AK and Contis J: Survivin
expression in hepatocellular carcinoma. Correlation with
clinicopathological characteristics and overall survival. J BUON.
24:1934–1942. 2019.PubMed/NCBI
|
|
11
|
Chu YJ, Ma WD, Thome R, Ping JD, Liu FZ,
Wang MR, Zhang ML, Zhang GX and Zhu L: Matrine inhibits CNS
autoimmunity through an IFN-β-dependent mechanism. Front Immunol.
11:5695302020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L, Gao C, Yao SK and Xie B: Blocking
autophagic flux enhances matrine-induced apoptosis in human
hepatoma cells. Int J Mol Sci. 14:23212–23230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng YM, Li X, Zhao HY and Zhao JY:
Effect of matrine and oxymatrine on proliferation and expression of
Stat3 and Stat5 in SMMC-7721 cell line. Zhongguo Zhong Yao Za Zhi.
33:2234–2237. 2008.(In Chinese). PubMed/NCBI
|
|
14
|
Qin XG, Hua Z, Shuang W, Wang YH and Cui
YD: Effects of matrine on HepG2 cell proliferation and expression
of tumor relevant proteins in vitro. Pharm Biol. 48:275–281. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu LC, Liu SB, Wei JR, Li D, Liu X, Wang
JY and Wang LS: Synthesis and biological evaluation of matrine
derivatives as anti-hepatocellular cancer agents. Bioorg Med Chem
Lett. 26:4267–4271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu J, Huang ZS, Hu GY and Huang MY:
Oxymatrine combined with cisplatin enhances expression of vascular
endothelial growth factor and CD31 in subcutaneous Xenografts of
human hepatocellular carcinoma in nude mice. J Third Military Med
Univ. 40:136–140. 2018.
|
|
17
|
Li X, Liang T, Chen SS, Wang M, Wang R, Li
K, Wang JC, Xu CW, Du N, Qin S and Ren H: Matrine suppression of
self-renewal was dependent on regulation of LIN28A/Let-7 pathway in
breast cancer stem cells. J Cell Biochem. 121:2139–2149. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pu JT, Tang XJ, Zhuang X, Hu Z, He KM, Wu
YF and Dai TY: Matrine induces apoptosis via targeting CCR7 and
enhances the effect of anticancer drugs in non-small cell lung
cancer in vitro. Innate Immun. 24:394–399. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gu YY, Chen MH, May BH, Liao XZ, Liu JH,
Tao LT, Man-Yuen Sze D, Zhang AL and Mo SL: Matrine induces
apoptosis in multiple colorectal cancer cell lines in vitro and
inhibits tumour growth with minimum side effects in vivo via Bcl-2
and caspase-3. Phytomedicine. 51:214–225. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Q, Xu J, He Z, Wen X, Wang F, Zhang P,
Li J, Song B, Wang Q, Li R and Huang H: The effects of matrine in
combination with docetaxel on castration-resistant
(Androgen-Independent) prostate cancer. Cancer Manag Res.
11:10125–10133. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brognaro E: ‘The development tumor model’
to study and monitor the entire progression of both primary and
metastatic tumors. Tumour Biol. 35:2219–2230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki A, Hayashida M, Ito T, Kawano H,
Nakano T, Miura M, Akahane K and Shiraki K: Survivin initiates cell
cycle entry by the competitive interaction with
Cdk4/p16INK4a and Cdk2/cyclin E complex activation.
Oncogene. 19:3225–3234. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suzuki A, Ito T, Kawano H, Hayashida M,
Hayasaki Y, Tsutomi Y, Akahane K, Nakano T, Miura M and Shiraki K:
Survivin initiates procaspase 3/p21 complex formation as a result
of interaction with Cdk4 to resist Fas-mediated cell death.
Oncogene. 19:1346–1353. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ikeguchi M, Ueda T, Sakatani T, Hirooka Y
and Kaibara N: Expression of survivin messenger RNA correlates with
poor prognosis in patients with hepatocellular carcinoma. Diagn Mol
Pathol. 11:33–40. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang R, Ma L, Zheng M, Ren J, Wang T,
Meng Y, Zhao J, Jia L, Yao L, Han H, et al: Survivin knockdown by
short hairpin RNA abrogates the growth of human hepatocellular
carcinoma xenografts in nude mice. Cancer Gene Ther. 17:275–288.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dai DJ, Lu CD, Lai RY, Guo JM, Meng H,
Chen WS and Gu J: Survivin antisense compound inhibits
proliferation and promotes apoptosis in liver cancer cells. World J
Gastroenterol. 11:193–199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thornberry NA and Lazebnik Y: Caspases:
Enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar : PubMed/NCBI
|