Introduction
At present, the collective worldwide incidence and
mortality rates of all cancers are high, and have become the second
leading cause of death (1). Among
all cancer types, lung cancer is the most common type of cancer,
which accounted for 11.6% of all cancer cases in 2018 (2). Non-small cell lung cancer (NSCLC)
accounts for ~85% of the total number of lung cancer cases
(3). The 5-year survival rate of
patients with advanced NSCLC is only ~15%, and the recurrence rate
of advanced NSCLC following radical treatment is >40% (4). In the past two decades, an increasing
number of therapies have been widely considered and studied to
improve the survival rate and quality of life for patients with
advanced or metastatic NSCLC. These treatments include surgery,
radiotherapy, chemotherapy, targeted therapy, immunotherapy and
combined therapies. Among these treatment options, immunotherapy
has become the optimum choice, particularly for patients with
advanced or metastatic NSCLC. Immunotherapy can effectively control
disease progression and improve survival rates (5), and the use of immune checkpoint
inhibitors (ICIs) is an effective immunotherapeutic method
(6). Thus, the aim of the present
review was to investigate the developments in novel immune
checkpoints and immunotherapy, with their associated challenges and
potential solutions to these issues.
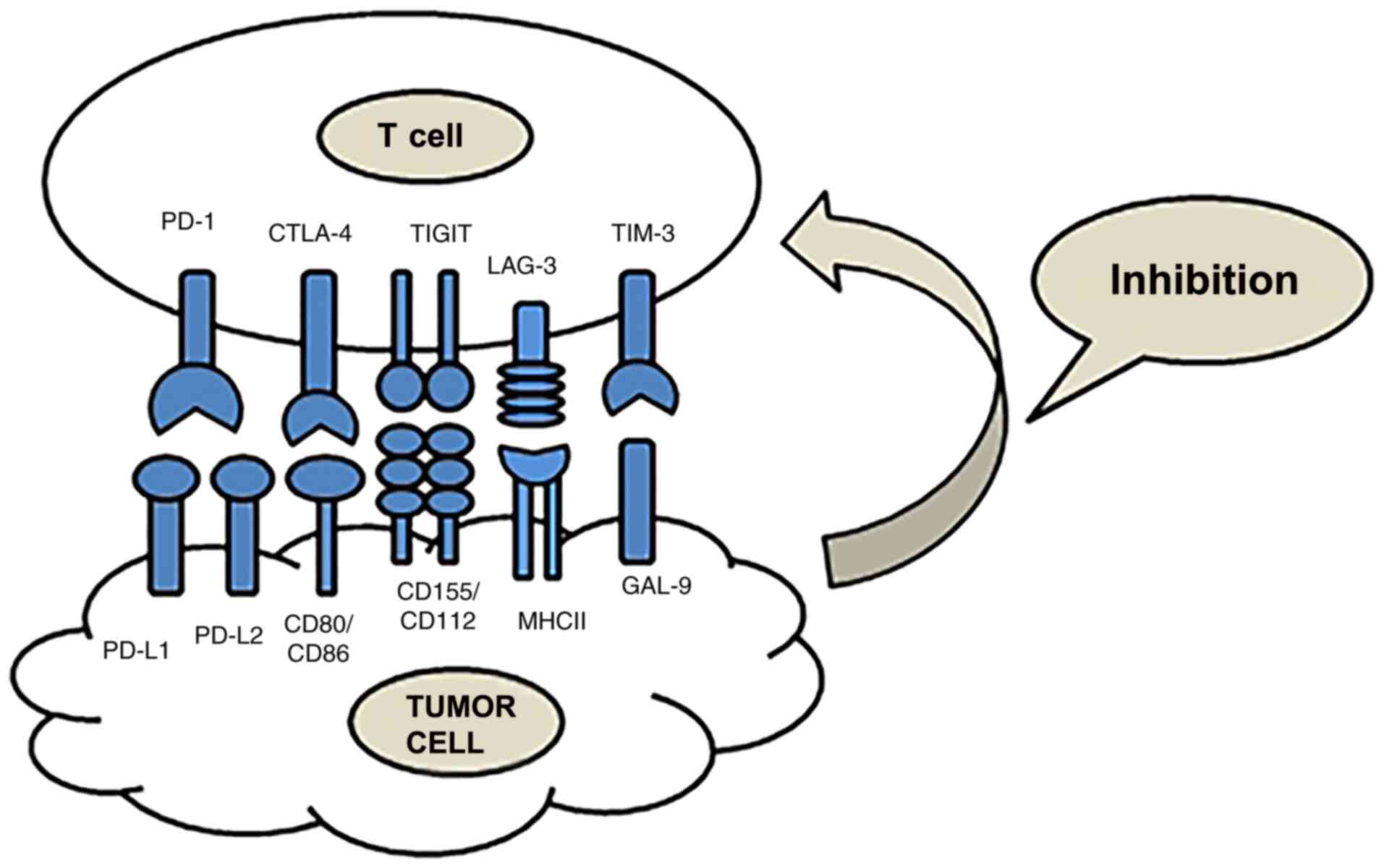
Overview of immune checkpoints in NSCLC
Immunotherapy is a type of treatment that uses ICIs
to block the immune checkpoint signaling pathway and reactivate T
cells, for the purpose of destroying tumors via the immune system
(Fig. 1). The effects of
immunotherapy are superior to those of other traditional therapies,
rendering it an effective and innovative method for treating cancer
(7). However, low response rates and
immune-related adverse events (irAEs) have also been observed in
patients treated with ICIs. Overcoming these challenges is
necessary (8). At present, immune
checkpoints, including cytotoxic T-lymphocyte associated protein 4
(CTLA-4), programmed cell death protein 1 (PD-1), programmed
death-ligand 1 (PD-L1), T cell immunoglobulin and mucin
domain-containing protein 3 (TIM-3), lymphocyte activation gene 3
(LAG-3) and T cell immunoreceptor with Ig and ITIM domains (TIGIT),
are a research hotspot (Table I)
(9).
 | Table I.Summary of immune checkpoints. |
Table I.
Summary of immune checkpoints.
| Checkpoint | Chromosomal
location | Number of amino
acids | Binding
partner | Binding partner
expression location | Receptor
expression | Trial | Drugs |
|---|
| PD-1 |
2q37.3 | 288 | PD-L1 | Cancer cells, | TILs, effector | CheckMate-017 | Nivolumab |
|
|
|
| (B7-H1) | APCs, | T cells, T
cells, | CheckMate-057 | Pembrolizumab |
|
|
|
| PD-L2 | tumor | regulatory B
cells, | KEYNOTE-010 | Atezolizumab |
|
|
|
| (B7-DC) | MDSCs | NK cells | KEYNOTE-021 | Durvalumab |
|
|
|
|
|
|
| KEYNOTE-024 | Avelumab |
|
|
|
|
|
|
| KEYNOTE-189 | PDR001 |
|
|
|
|
|
|
| IMpower-131 | REGN2810 |
|
|
|
|
|
|
| IMpower-150 | Y3300054 |
|
|
|
|
|
|
| Pembro-RT | Tislelizumab |
|
|
|
|
|
|
| CT02008227 | MGA012 |
|
|
|
|
|
|
| NCT02125461 | MEDI4736 |
|
|
|
|
|
|
| NCT02395172 | SHR-1210 |
|
|
|
|
|
|
|
| AB122 |
| CTLA-4 |
2q33 | 239 | B7-1 | APCs | Effector T
cells, | CheckMate227 | Ipilimumab |
|
|
|
| (CD80) | Tumor | Tregs | CheckMate-568 | Tremelimumab |
|
|
|
| B7-2 (CD86) | MDSCs |
|
|
|
| LAG-3 | 12p13.32 | 498 | MHC-II | APCs | Effector T
cells, | NCT03250832 | LAG525 |
|
|
|
| Galectin-3 |
| Tregs, B
cells, | NCT02460224 | TSR-033 |
|
|
|
| LSECtin |
| NK cells, DCs | NCT01968109 | BMS-986016 |
|
|
|
| A-synuclein |
|
| NCT02966548 | REGN3767 |
|
|
|
| FGL1 |
|
| NCT03005782 |
|
|
|
|
|
|
|
| NCT03459222 |
|
| TIM-3 |
5q33.2 | 302 | Galectin-9 | APCs | Effector T
cells, | NCT03099109 | LY3321367 |
|
|
|
| Ceacam-1 | Cancer cells | B cells, Tregs | NCT03744468 | BGB-A425 |
|
|
|
| HMGB-1 |
| DCs, NK cells, | NCT02608268 | MBG453 |
|
|
|
| PtdSer |
| Monocytes | NCT02817633 | TSR-022 |
| TIGIT |
3q13.31 | 244 | CD155 | APCs | T cells, | AB154 | Domvanalimab |
|
|
|
| CD112 | Cancer cells | NK cells | MTIG7192A | Tiragolumab |
|
|
|
|
|
|
| BGB-A1217 | BGB-A1217 |
CTLA-4
In 1995, CTLA-4 was first discovered to deliver
inhibitory immune response signals (10). Leach et al (11) demonstrated that blocking CTLA-4
enhanced the immune-mediated targeting of tumor cells, and that
tumor cells had the ability to upregulate the expression of CTLA-4.
CTLA-4 belongs to the immunoglobulin superfamily and its gene is
localized to chromosome 2q33 in humans (12). The structure of CTLA-4 is homologous
to that of CD28, with which it shares two ligands, CD80 and CD86
(13). Therefore, CTLA-4 and CD28
exert opposing functions. CTLA-4 induces inhibitory signals in T
cells and blocks T cell responses by competing with CD28 for ligand
binding (14). CTLA-4 also regulates
T-cell activation through several mechanisms, including the
inhibition of T-cell proliferation, differentiation, IL-2
production and cell cycle progression. Therefore, CTLA-4 serves a
significant role in immunotherapy, and has achieved satisfactory
results in clinical treatment (15,16).
PD-1/PD-L1
PD-1 is a member of the CD28 superfamily involved in
programmed cell death (17,18), that is preferentially expressed by T
and B cells, but also expressed in other cell subsets, such as
dendritic cells, natural killer (NK) cells and monocytes. PD-1
forms conjugates with PD-L1 and PD-L2, which belong to the B7
protein family, where PD-L1 is its primary ligand (19). PD-1 interacts with PD-L1 to inhibit
the activation of T cells and promote immune escape, since Src
homology region 2-containing protein tyrosine phosphatase 2
inhibits kinases involved in T-cell activation (20). Although anti-PD-1/PD-L1 inhibitors
(that block the binding of PD-1 and PD-L1) have shown encouraging
results in the clinic (21), certain
challenges remain, including irAEs and low response rates (22).
LAG-3
LAG-3 (also known as CD223) was discovered in 1990
and includes Ig-like domains 1–4. Domain 1 contains an additional
sequence of ~30 amino acids known as the ‘extra loop’ (23). LAG-3 is primarily expressed on
CD4+ and CD8+ T cells, though plasmacytoid
dendritic cells, regulatory T cells (Tregs), activated B cells and
NK T cells also express cell surface LAG-3 (24,25).
Stable peptide-major histocompatibility complex class II (MHC-II)
is considered to be a ligand of LAG-3 (26), and liver sinusoidal endothelial cell
lectin (LSECtin), galectin-3 and fibrinogen-like protein 1 have
also been reported as potential LAG-3 ligands (27,28).
LAG-3 possesses a similar tumor immune escape mechanism to that of
PD-1, and is considered the most important tumor treatment target
after PD-1. At present, clinical trials to verify the efficacy of
LAG-3 ICIs, alone and combined with other ICIs, are in
progress.
TIM-3
TIM-3 is a member of the TIM family of
immunoregulatory proteins, first discovered in 2002 (29). TIM-3 is a type I transmembrane
protein expressed on T cells, B cells, NK cells, dendritic cells
(DCs) and monocytes (30). Ligands
of TIM-3 have been reported to include galectin 9,
phosphatidylserine, carcinoembryonic antigen-related cell adhesion
molecule 1 (Ceacam1) and high mobility group protein B1 (31). The interaction between TIM-3 and its
ligands (galectin-9 or Ceacam1) induces Tyr256 and Tyr263
phosphorylation in the intracellular domain of TIM-3, releasing BAG
cochaperone 6 (BAG6) from the TIM-3 tail. The release of BAG6
allows the recruitment of Src kinases (including, but not limited
to Lck and Fyn) and promotes the subsequent negative regulation of
T cell receptor signaling (32,33).
When TIM-3 is not bound by a ligand, BAG6 is bound to its
unphosphorylated cytoplasmic tail, and maintains T-cell activation
through Lck recruitment (9). TIM-3
is also co-expressed with PD-1, and the co-blockade of PD-1 and
TIM-3 can exert synergistic effects, restoring T cell effector
function and killing tumor cells (34).
TIGIT
TIGIT (also known as WUCAM, Vstm3 and VSIG9) belongs
to a constantly expanding family of poliovirus receptor like
proteins that plays a critical role in limiting immune functions
(35). In 2009, TIGIT was first
identified by three groups as an immune checkpoint that inhibits NK
and T-cell activation. TIGIT is composed of an extracellular
immunoglobulin variable domain, a type I transmembrane domain and
two inhibitory motifs of the cytoplasmic tail; one immunoreceptor
tyrosine-based inhibitory motif and one immunoglobulin tyrosine
tail (ITT)-like motif (36,37). TIGIT has three ligands, CD112, CD113
and CD155, though it binds to CD155 with the highest affinity. In
humans, TIGIT is expressed by activated CD4+ T and
CD8+ T cells, Tregs, NK cells and follicular T helper
cells. NK cytotoxicity is inhibited by ITT phosphorylation when
TIGIT binds to its ligands in NK cells (38). Furthermore, TIGIT-mediated inhibition
of effector T and NK cells is also achieved by interfering with
DNAX accessory protein-1 co-stimulation, which directly delivers
inhibitory signals to the effector cell (39).
Immunotherapy in NSCLC
In the current clinical treatment of NSCLC,
immunotherapy is primarily centered around two checkpoint
inhibitors that target CTLA-4 and PD-1/PD-L1 (40). CTLA-4 is the first ICI to be used in
the clinic. In previous years, researchers have paid more attention
to PD-1/PD-L1, leading to progress in basic and clinical research.
Due to the high toxicity of iplimumab, an ICI of the CTLA-4
signaling pathway, the probability of irAEs increases (41). The drugs approved by the Food and
Drug Administration (FDA) for the treatment of NSCLC include
pembrolizumab, nivolumab, avelumab, atezolizumab and durvalumab. In
addition, there are several novel ICIs in the developmental and
clinical research stages (42,43). In
conclusion, immunotherapy is associated with both benefits and
challenges. It is therefore crucial to overcome the difficulties in
expanding the number of individuals who are able to receive
treatment, and to improve the efficacy of immunotherapy.
Challenges of immunotherapy in NSCLC
Although immunotherapy presents a promising
treatment option, not all patients can benefit from it, and the
benefits may not be long-lasting. In the clinic, the objective
response rates (ORRs) of PD-1 treatment without the prior screening
of patients is only 15–20%, thus only a proportion of patients with
NSCLC are suitable for, and benefit from, immunotherapy (44). The primary reason for the low
response rate is that suitable patients are not accurately selected
prior to treatment. Therefore, it is essential to identify
biomarkers that can predict the efficacy of immunotherapy in
patients (45). Of the patients that
do benefit from immunotherapy, the majority develop resistance,
which has a marked impact on future treatment. Therefore, selecting
suitable biomarkers and overcoming resistance is necessary for
improving therapeutic effects (46).
Biomarkers
PD-L1 expression in tumors
PD-L1 is a potential tumor biomarker, and its
predictive value varies in different tumors (47). Pembrolizumab has been approved by the
FDA as a first-line treatment for patients with NSCLC with PD-L1
expression ≥50%, and without EGFR or anaplastic lymphoma kinase
(ALK) gene mutations (48). A
meta-analysis revealed that the higher the expression level of
PD-L1, the greater its benefit in patients using anti-PD-1/PD-L1
(49). However, patients with
squamous NSCLC are treated with nivolumab regardless of PD-L1
expression level (50). As a
biomarker, PD-L1 expression is not omnipotent. At present, PD-L1
detection is widely employed in the clinic, and can be used as a
clinical auxiliary or supplementary diagnostic tool to predict the
efficacy of immunotherapy (51,52);
however, it is not without its challenges.
PD-L1 is a continuous variable, the levels of which
can be altered by induction under constant conditions, for example,
by cisplatin. Therefore, accurately detecting the level of PD-L1 is
challenging (53). Furthermore,
surgical resection and biopsy can impact PD-L1 expression, due to
the heterogeneity of the tumor (54). The expression of PD-L1 in
surgically-removed sections and lung biopsies was analyzed by SP142
immunohistochemistry (IHC) in 160 patients with NSCLC. The results
showed that the expression of PD-L1 in lung biopsies was not
consistent with that in the surgically-removed sections (overall
inconsistency rate, 48%), and the expression of PD-L1 in the
biopsies was generally lower than that in the sections (55). Finally, the difference between IHC
assays also has important implications on PD-L1 detection, as
results are inconsistent among different assays. Currently, four
IHC assays have been approved by the FDA (28-8, SP263, SP142 and
22C3), and selecting the most suitable one for each patient is
crucial. In addition, the scoring system for PD-L1 expression has
not been standardized (56,57). In conclusion, clinical detection of
PD-L1 demands standardization and improved accuracy.
Tumor mutation burden (TMB)
TMB is a potential biomarker for immunotherapy,
which is defined as the total number of detected somatic gene
coding, base substitution, gene insertion or deletion errors in
every million bases (Mb). The principle of TMB in predicting the
efficacy of immunotherapy is that tumors with a high TMB may
express a greater variety of antigens and have stronger
immunogenicity, thus increasing their recognition by, and the
killing effect of, cytotoxic CD8+ T cells. Therefore,
tumors with a high TMB are suitable for immunotherapy (58). In terms of clinical trials,
CheckMate-568 revealed that patients with a TMB of >10 mutations
(mut)/Mb had higher ORR and longer progression-free survival (PFS)
than those with a TMB of <10 mut/Mb, regardless of PD-L1
expression level. The results showed that TMB could be used as a
prospective biomarker for patients treated with nivolumab combined
with ipilimumab (59). However, the
use of TMB as a biomarker has certain limitations.
In terms of detection methods, whole genome
sequencing or whole-exome sequencing (WES) are the standard methods
for calculating the TMB (58); their
disadvantages include the need for high sample quality, long
detection times and high cost, and as such, their wide application
in clinical practice is limited (60). A promising and convenient alternative
is next-generation sequencing (NGS) (61). In a study by Samstein et al
(62), integrated mutation profiling
of actionable cancer targets (MSK-IMPACT™) was used to sequence the
genome. The results showed that for the majority of tumors treated
with ICIs, patients with a higher TMB exhibited a higher survival
rate (62). The relevant data also
showed that the results of quantification of TMB detected by NGS
and WES were correlated (63). The
FDA has approved two NGS panels (FoundationOne®CDx and
MSK-IMPACT) to evaluate TMB. Although NGS has its advantages,
in-depth research is required in order to improve accuracy in
clinical practice before it can become the standard method for TMB
determination (64). Secondly, there
is no fixed standard TMB cut-off value. Generally speaking, a TMB
of >20 mut/Mb is considered high, whereas a TMB of <10 mut/Mb
is considered low. Therefore, it is necessary to determine the
optimal cut-off value of each tumor type in more prospective
clinical studies and clinical practice (65).
Additionally, 30% of cancer patients face further
challenges, including the inability to obtain tumor tissue,
insufficient tumor tissue samples and tumor tissue content not
meeting the requirements for detection (66). Therefore, a blood-based assay was
developed to measure blood tumor mutation burden (bTMB). Blood
detection is safer, less costly, and blood samples are easier to
obtain than tumor biopsies. Related studies have confirmed that the
bTMB can predict the efficacy of atezolizumab in patients with
advanced NSCLC (67). However, other
studies have shown that the bTMB cannot evaluate the results of
immunotherapy (PFS and ORR). Patients with a low bTMB may also
benefit from immunotherapy (68). In
addition, recent studies have reported that the maximum somatic
allele frequency combined with bTMB has a higher predictive effect
than bTMB alone in patients with advanced NSCLC treated with
atezolizumab (69). bTMB is
therefore not a mature biomarker, and further experimentation is
required for clinical verification. Although both TMB and bTMB can
predict the efficacy of immunotherapy, there are still several
obstacles that need to be overcome, including the specification of
the detection platform, and the lack of standardization in the
evaluation of TMB and bTMB.
At present, there is no single biomarker that can
accurately predict the efficacy and prognosis of immunotherapy. In
a clinical setting, PD-L1 and TMB are the most commonly used
biomarkers, but there are certain limitations regarding their
clinical use. It is therefore crucial to continue investigating
existing, and identify novel, biomarkers. For example,
tumor-infiltrating lymphocytes (TIL), epithelial-to-mesenchymal
transition/inflammation signature score, intestinal flora,
microsatellite instability high/deficient mismatch repair, gene
expression signatures and tumor-specific genotypes are potential
biomarkers currently being explored (70,71).
However, the identification of a ‘perfect’ biomarker is unlikely;
therefore, assessing the suitability of patients for immunotherapy
based on the comprehensive evaluation of multiple indicators is
currently the most effective strategy. A study has shown that a
combination of human leukocyte antigen (HLA) class I,
CD8+ T cell infiltration, PD-L1 expression and tumor
mutational load is a promising biomarker for predicting the
efficacy of anti-PD-1 in the treatment of NSCLC (72). However, this combination still
requires considerable clinical verification to confirm its
predictive ability. Therefore, the focus of future studies should
be not only on identification of novel biomarkers, but also the
investigation of the most effective combination of various existing
biomarkers.
Immunotherapy-induced resistance
With the development of immunotherapy, the global
use of ICIs has increased; however, the development of drug
resistance remains a considerable challenge (73). Resistance can be categorized as
acquired or primary, with the probability of primary resistance at
~60% (74). Most patients develop
resistance following immunotherapy, which reduces its anticancer
effects. Therefore, overcoming resistance is important in improving
immunotherapeutic efficacy. Existing literature suggest that the
current understanding of resistance mechanisms is limited; the
potential mechanisms of resistance (based on existing data) are
summarized herein.
Firstly, tumor immunogenicity and TMB are associated
with the mechanism of resistance. The necessary conditions for
effective immunotherapy include tumor expression of the appropriate
antigens and the generation of tumor antigen-specific T cells
(75). Tumors with a high
immunogenicity (NSCLC, renal cell carcinoma and human melanoma) are
more sensitive to immunotherapy (76,77).
However, tumors with a low TMB produce fewer tumor antigens and
have poor immunogenicity, which negatively impacts the activation
of effector T cells, further leading to resistance (78). Prostate cancer has poor
immunogenicity and low expression of PD-L1, which is the primary
reason for drug resistance (79).
Therefore, tumor immunogenicity and TMB are closely associated with
the mechanism of resistance. Secondly, immunotherapy restores T
cell function by blocking immune checkpoints. However, if tumor
cells cannot be identified by T cells, drug resistance ensues. The
expression of tumor cell MHC-I is necessary for identification of
tumor cells by T cells, and MHC-I loss or downregulation results in
tumor immune escape. β2 m is an integral part of the HLA
class I molecule, and is necessary for antigen presentation.
Mutations in β2 m limit the recognition of
CD8+ T cells, which results in T cell failure (80). Therefore, a promising solution is to
generate synthetic long peptides from tumor-associated antigens, as
well as DNA and RNA, in order to develop novel vaccines. The
associated mechanism is that the vaccine is transported to MHC-I
and MHC-II molecules of antigen-presenting cells, thus promoting
CD8 and CD4 T cell responses to overcome resistance (81). In addition, radiotherapy can lead to
the independent upregulation of MHC-I, recover antigen presentation
and overcome resistance (82).
Finally, the pathway of immunosuppression is not only associated
with PD-1/PD-L1, but also other pathways with similar functions,
such as those of TIM-3, LAG-3, CTLA-4 and B- and T-lymphocyte
attenuator (BTLA). In the event of the combined action of these
pathways, blockade of one may not be sufficient, as the others
pathways will likely compensate for the loss of immunosuppressive
signals. Therefore, the combination of multiple ICIs may improve
their therapeutic effects (83). In
clinical and mouse models, the expression of TIM-3 was increased
following anti-PD-1 resistance, and the combination of anti-TIM-3
and anti-PD-1 was found to improve survival rates (84,85).
Therefore, the combination of multiple ICIs may improve tumor
control and overcome resistance.
Future solutions and research directions for
immunotherapy in NSCLC
Although its affects remain controversial, obtaining
a deeper understanding of immunotherapy is necessary for its
improvement. Future research directions can be divided into a basic
and a clinical aspect. With regard to basic research, key
directions may include the identification of novel targets, the
effective combination of various biomarkers, and the investigation
of resistance mechanisms. Based on current basic research findings,
the purpose of clinical research is to combine existing treatment
methods to obtain the optimum therapeutic effect. Several studies
are currently in progress, which have achieved promising results
(Table II), and are described in
the following sections.
 | Table II.First-line treatment of NSCLC. |
Table II.
First-line treatment of NSCLC.
| Study | Immunological
pathway | Study design | Phase | Condition or
disease | Objective response
rate, % | Median
progression-free survival, months | Median overall
survival, months |
|---|
| CheckMate-227 | CTLA-4/PD-1 | Nivolumab plus
ipilimumabvs. chemotherapy | III | Squamous or
non-squamous | 45.3 | 7.2 | NR |
| CheckMate-568 | CTLA-4/PD-1 | Nivolumab plus
ipilimumab | II | Advanced/metastatic
NSCLC | 30 | 4.2 | NR |
| Keynote-021 | PD-1 | Pembrolizumab plus
pemetrexed carboplatin vs. pemetrexed carboplatin | II | Non-squamous | 56.7 | 24 | NR |
| Keynote-189 | PD-1 | Pembrolizumab
platinum-pemetrexed vs. placebo-platinum pemetrexed | II | Non-squamous | 47.6 | 8.8 | NR |
| IMpower-131 | PD-L1 | Atezolizumab +
carboplatin+ paclitaxel vs. atezolizumab+ paclitaxel vs.
atezolizumab+ carboplatin + nab-paclitaxel vs. carboplatin +
nab-paclitaxel | III | Squamous | 49 | 6.3 | 14 |
| IMpower-150 | PD-L1 | Atezolizumab plus
carboplatin plus paclitaxel vs. Atezolizumab plus bevacizumab plus
carboplatin plus paclitaxel vs. Bevacizumab plus carboplatin plus
paclitaxel | III | Non-squamous | 69.3 | 8.3 | 19.2 |
| Pembro-RT | PD-1 | Pembrolizumab-SBRT
vs. Pembrolizumab | III | Metastatic
NSCLC | 36 | 6.6 | 15.9 |
Combination of multiple treatment options
in clinical research
Combination of multiple ICIs for NSCLC
therapy
PD-1/PD-L1 is one of the signaling pathways of
several immune checkpoints, but various other immune checkpoints
perform similar functions. The anti-tumor effect can be improved
using a combination of multiple ICIs. The combination of anti-PD-1
and anti-CTLA-4 has been widely studied and shown to yield positive
results. CheckMate 012 showed that the efficacy of nivolumab +
ipilimumab as a first-line treatment was superior to that of
nivolumab alone (86). The phase III
clinical study CheckMate 227 confirmed that the efficacy of
nivolumab + ipilimumab as a first-line treatment was superior to
that of traditional chemotherapy. It was also shown that, in
patients with a high TMB, the median PFS of the nivolumab +
ipilimumab group was longer than that of the chemotherapy group
(7.2 vs. 5.5 months) (87).
Furthermore, CheckMate 568 revealed an association between the
efficacy of nivolumab + ipilimumab as a first-line treatment, and
the expression of PD-L1 and TMB. The results indicated that
patients with a TMB of >10 mut/Mb had a higher ORR and longer
PFS, regardless of PD-L1 expression, providing evidence for the use
of TMB as a potential biomarker (5).
In addition to the combination of anti-PD-1 and anti-CTLA-4, a
number of novel immunotherapy combinations are in the experimental
stages.
ICIs combined with chemotherapy
Chemotherapy is a traditional treatment for advanced
cancer, which can increase tumor antigen presentation, enhance the
activity of effector T cells, and increase the expression of PD-L1
in tumors (88). In addition, the
regulatory effect of a variety of chemotherapeutic drugs on immune
function has been reported. For example, cisplatin upregulates
PD-L1 expression in tumor cells through the PI3K/AKT signaling
pathway (53), and pemetrexed can
promote the activation of NK cells, increasing the production of
IFN-γ (89). Also, docetaxel
selectively reduces the number of Tregs and prevents immune
suppression (90). Therefore,
chemotherapy and immunotherapy have a synergistic effect,
suggesting that combination therapy has a superior effect to
monotherapy.
A number of studies have shown that as a first-line
treatment, the therapeutic benefits of immunotherapy combined with
chemotherapy are greater than those of chemotherapy alone.
KEYNOTE-021 studied the difference in efficacy between
pembrolizumab + pemetrexed-carboplatin (PC) and PC alone. The
results showed that the ORR of the combination group was 56.7%,
while that of the PC group was 30.2%. Compared with PC alone, PFS
and overall survival (OS) following combination therapy also
improved significantly (91).
Patients were divided into three groups based on first-line
treatment: Atezolizumab + carboplatin + paclitaxel, atezolizumab +
bevacizumab + carboplatin + paclitaxel (ABCP) and bevacizumab +
carboplatin + paclitaxel (BCP) as. In the ITT-WT population
(patients with EGFR or ALK alterations were excluded), the mPFS of
the ABCP group was significantly higher than that of the BCP group
(8.3 vs. 6.8 months). In the WT population which had high
expression of an effector T cell (Teff) gene signature in the tumor
(Teff-high WT) cases, the mPFS of the two groups was 11.3 and 6.8
months. The results showed that adding atezolizumab to bevacizumab
+ chemotherapy for non-squamous metastatic NSCLC improved OS and
PFS, regardless of PD-L1 expression. In December 2018, the FDA
approved atezolizumab + carboplatin + paclitaxel + bevacizumab as a
first-line treatment option for patients with advanced metastatic
NSCLC without EGFR and ALK mutations (92).
ICIs combined with radiotherapy
For patients with advanced NSCLC, radiotherapy is
one of the most conventional and effective treatments, and improves
survival rate by increasing the control of primary tumour (93). Radiotherapy can increase tumor
antigen release, enhance antigen presentation and promote T-cell
infiltration into the tumor tissue, thus enhancing the systemic
anti-tumor immune response and altering the tumor microenvironment
(94). Radiotherapy can induce
immunogenic cell death, enhance the TIL repertoire and upregulate
MHC and PD-L1 expression (95,96). In
immunotherapy, the expression of PD-L1 plays an important role in
predicting the therapeutic effect. A previous study revealed that
radiotherapy increases the expression of PD-L1 in tumor cells by
activating the PI3K/AKT and STAT3 signaling pathways. In addition,
radiotherapy is less toxic, and thus a more favorable option for
combined immunotherapy (97).
At present, there are few NSCLC clinical studies on
radiotherapy combined with ICIs. PEMBRO-RT is a recent multi-center
phase II randomized clinical trial, the purpose of which was to
determine whether the use of stereotactic body radiotherapy before
pembrolizumab treatment increases the anti-tumor response of
patients with metastatic NSCLC. The patients in the experimental
arm were treated with pembrolizumab following stereotactic body
radiotherapy at a single tumor site, while the control arm were
treated with pembrolizumab only. The results showed that the ORR at
12 weeks in the experimental arm was significantly higher than that
of the control arm (36 vs. 18%). The mPFS of the experimental arm
was 6.6 months, and that of the control arm was 1.9 months. The
median OS was 7.6 in the control arm, and 15.9 months in the
experimental arm. Although the experimental results showed that the
ORR following combination therapy was significantly higher than
that of the control arm, the results did not reach the expected
standard. In addition, different patient PD-L1 expression levels
may also have effeced the experimental results. Therefore, these
findings require further experimental confirmation (98). However, based on the existing
experimental outcomes, immunotherapy combined with radiotherapy is
superior to radiotherapy alone, and does not increase the number of
adverse reactions.
ICIs combined with Traditional Chinese
Medicine (TCM)
In patients with NSCLC, combined immunotherapy can
compensate for the deficiencies of immunotherapy alone, allowing
increased benefit to a greater number of patients. Combined
immunotherapy appears promising, but the current thinking cannot be
limited by the existing programs when investigating this research
area. TCM has a long history of use in China (99). During the novel coronavirus outbreak
in 2020, the majority of hospitals in China used a combination of
western and TCM to treat patients, with successful results
(100). China has also achieved
encouraging results in providing TCM treatment for patients in
other countries, which has become an important player in global
disease treatment (101). The tumor
environment is an important consideration when treating tumors, as
changes therein may directly affect tumor cell proliferation rate,
and subsequently, the effects of immunotherapy and occurrence of
resistance (102). For the
treatment of cancer, TCM has the potential to improve the tumor
environment for increased treatment efficacy. Most TCM agents are
derived from plants, and thus, have the advantages of low toxicity
and multiple targets(diversity of compounds). In addition, TCM
includes a specific class of drugs for the improvement of
self-immunity, which improve the tumor environment; as such, the
primary purpose of systematic treatment for patients with
metastatic NSCLC is to reduce the burden of cancer symptoms, and to
improve survival, while also maintaining patient quality of life
(103). Currently, traditional
treatments and immunotherapy cannot achieve satisfactory results in
patients with metastatic NSCLC. Therefore, the use of TCM combined
with these other treatment types is a promising option for patients
with NSCLC, and research into the role of TCM in immunotherapy is
ongoing.
Astragalus polysaccharide (PG2) is the active
component of the dried roots of Astragalus membranaceus. A
study showed that PG2 increased the M1/M2 macrophage polarization
ratio of H441 and H1299 cells. It also increased the T
cell-mediated antitumor immune response by promoting the functional
maturity of DCs (104). Adding PG2
to cisplatin, a conventional chemotherapeutic drug, can
synergistically increase the antitumor effect (105). According to the above theory, the
effective results of immunotherapy combined with PG2 in NSCLC were
expected (106). Phytolacca
acinosa polysaccharides I (PAP-1) is a compound of
Phytolacca, which has been proven to affect immune functions
in mice. It can also enhance the production of IL-2 and NK
cytotoxic factor, and exerts antitumor activity (107). It has also been reported that total
glucosides of paeony can regulate the expression of PD-1 and PD-L1
in peripheral blood monocytes (108). Thus to conclude, TCM has the
potential to positively influence immunotherapy. In addition,
various other TCM agents can regulate immunity and antitumor
effects. At present, research on the antitumor effects of combining
TCM with immunotherapy is being conducted, the findings of which
may provide patients with further treatment options and superior
benefits.
Conclusions
For future clinical trials in cancer, several
challenges need to be overcome, including irAEs, low patient
response rates and tumor cell resistance to treatment. Although
immunotherapy is currently the best option, more promising
treatments are expected to arise from ongoing basic and clinical
research. Until then, solutions to these problems can be found
through the continuous identification of immune checkpoints, and
the creation of more favorable combination treatment methods. The
research and application of immunotherapy have broad prospects; the
discovery of new immune checkpoints and continuous attempts at
combination treatments, including that of TCM and immunotherapy,
may well be hotspots of future research on NSCLC.
Acknowledgements
No applicable.
Funding
The author(s) disclose receipt of the following
financial support for the research, authorship, and/or publication
of this article: The present study was funded by The Science and
Technology Development Fund, Macau SAR (grant no. 0011/2021/A) and
The Faculty Research Grant Projects of Macau University of Science
and Technology (grant no. FRG-19-028-FC).
Availability of data and materials
No applicable.
Authors' contributions
PYY and ELHL conceived and designed the study. LRM
and JXL wrote the paper. RZL, LT, JSY and AS revised the paper for
important intellectual content. Data authentication is not
applicable. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
No applicable.
Patient consent for publication
No applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gridelli C, Rossi A, Carbone DP, Guarize
J, Karachaliou N, Mok T, Petrella F, Spaggiari L and Rosell R:
Non-small-cell lung cancer. Nat Rev Dis Primers. 1:150092015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng YW, Li RM, Zhang XW and Ren XB:
Current adoptive immunotherapy in non-small cell lung cancer and
potential influence of therapy outcome. Cancer Invest. 31:197–205.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bagchi S, Yuan R and Engleman EG: Immune
checkpoint inhibitors for the treatment of cancer: Clinical impact
and mechanisms of response and resistance. Annu Rev Pathol.
16:223–249. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Somasundaram A and Burns TF: The next
generation of immunotherapy: Keeping lung cancer in check. J
Hematol Oncol. 10:872017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi T, Ma Y, Yu L, Jiang J, Shen S, Hou Y
and Wang T: Cancer immunotherapy: A focus on the regulation of
immune checkpoints. Int J Mol Sci. 19:13892018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Joller N and Kuchroo VK: Tim-3, Lag-3, and
TIGIT. Curr Top Microbiol Immunol. 410:127–156. 2017.PubMed/NCBI
|
|
10
|
Tivol EA, Borriello F, Schweitzer AN,
Lynch WP, Bluestone JA and Sharpe AH: Loss of CTLA-4 leads to
massive lymphoproliferation and fatal multiorgan tissue
destruction, revealing a critical negative regulatory role of
CTLA-4. Immunity. 3:541–547. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leach DR, Krummel MF and Allison JP:
Enhancement of antitumor immunity by CTLA-4 blockade. Science.
271:1734–1736. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ostrov DA, Shi W, Schwartz JC, Almo SC and
Nathenson SG: Structure of murine CTLA-4 and its role in modulating
T cell responsiveness. Science. 290:816–819. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iida T, Ohno H, Nakaseko C, Sakuma M,
Takeda-Ezaki M, Arase H, Kominami E, Fujisawa T and Saito T:
Regulation of cell surface expression of CTLA-4 by secretion of
CTLA-4-containing lysosomes upon activation of CD4+ T
cells. J Immunol. 165:5062–5068. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sharpe AH and Freeman GJ: The B7-CD28
superfamily. Nat Rev Immunol. 2:116–126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Buchbinder EI and Desai A: CTLA-4 and PD-1
pathways: Similarities, differences, and implications of their
inhibition. Am J Clin Oncol. 39:98–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Miguel M and Calvo E: Clinical
challenges of immune checkpoint inhibitors. Cancer Cell.
38:326–333. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishida Y, Agata Y, Shibahara K and Honjo
T: Induced expression of PD-1, a novel member of the immunoglobulin
gene superfamily, upon programmed cell death. EMBO J. 11:3887–3895.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamazaki T, Akiba H, Iwai H, Matsuda H,
Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, et al:
Expression of programmed death 1 ligands by murine T cells and APC.
J Immunol. 169:5538–5545. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patel SP and Kurzrock R: PD-L1 expression
as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther.
14:847–856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yokosuka T, Takamatsu M,
Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M and Saito T:
Programmed cell death 1 forms negative costimulatory microclusters
that directly inhibit T cell receptor signaling by recruiting
phosphatase SHP2. J Exp Med. 209:1201–1217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
O'Kane GM, Labbé C, Doherty MK, Young K,
Albaba H and Leighl NB: Monitoring and management of immune-related
adverse events associated with programmed cell death protein-1 axis
inhibitors in lung cancer. Oncologist. 22:70–80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang L, Ma Q, Yao R and Liu J: Current
status and development of anti-PD-1/PD-L1 immunotherapy for lung
cancer. Int Immunopharmacol. 79:1060882020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huard B, Mastrangeli R, Prigent P,
Bruniquel D, Donini S, El-Tayar N, Maigret B, Dréano M and Triebel
F: Characterization of the major histocompatibility complex class
II binding site on LAG-3 protein. Proc Natl Acad Sci USA.
94:5744–5749. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Anderson AC, Joller N and Kuchroo VK:
Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized
functions in immune regulation. Immunity. 44:989–1004. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Byun HJ, Jung WW, Lee DS, Kim S, Kim SJ,
Park CG, Chung HY and Chun T: Proliferation of activated
CD1d-restricted NKT cells is down-modulated by lymphocyte
activation gene-3 signaling via cell cycle arrest in S phase. Cell
Biol Int. 31:257–262. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maruhashi T, Okazaki IM, Sugiura D,
Takahashi S, Maeda TK, Shimizu K and Okazaki T: LAG-3 inhibits the
activation of CD4+ T cells that recognize stable pMHCII
through its conformation-dependent recognition of pMHCII. Nat
Immunol. 19:1415–1426. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J, Sanmamed MF, Datar I, Su TT, Ji L,
Sun J, Chen L, Chen Y, Zhu G, Yin W, et al: Fibrinogen-like protein
1 is a major immune inhibitory ligand of LAG-3. Cell.
176:334–347.e12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kouo T, Huang L, Pucsek AB, Cao M, Solt S,
Armstrong T and Jaffee E: Galectin-3 shapes antitumor immune
responses by suppressing CD8+ T cells via LAG-3 and
inhibiting expansion of plasmacytoid dendritic cells. Cancer
Immunol Res. 3:412–423. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang R, Rangachari M and Kuchroo VK:
Tim-3: A co-receptor with diverse roles in T cell exhaustion and
tolerance. Semin Immunol. 42:1013022019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Acharya N, Sabatos-Peyton C and Anderson
AC: Tim-3 finds its place in the cancer immunotherapy landscape. J
Immunother Cancer. 8:e0009112020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Das M, Zhu C and Kuchroo VK: Tim-3 and its
role in regulating anti-tumor immunity. Immunol Rev. 276:97–111.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang YH, Zhu C, Kondo Y, Anderson AC,
Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T, et
al: CEACAM1 regulates TIM-3-mediated tolerance and exhaustion.
Nature. 517:386–390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu C, Anderson AC, Schubart A, Xiong H,
Imitola J, Khoury SJ, Zheng XX, Strom TB and Kuchroo VK: The Tim-3
ligand galectin-9 negatively regulates T helper type 1 immunity.
Nat Immunol. 6:1245–1252. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sakuishi K, Apetoh L, Sullivan JM, Blazar
BR, Kuchroo VK and Anderson AC: Targeting Tim-3 and PD-1 pathways
to reverse T cell exhaustion and restore anti-tumor immunity. J Exp
Med. 207:2187–2194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stanietsky N, Simic H, Arapovic J, Toporik
A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, et al:
The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell
cytotoxicity. Proc Natl Acad Sci USA. 106:17858–17863. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu X, Harden K, Gonzalez LC, Francesco M,
Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, et al: The
surface protein TIGIT suppresses T cell activation by promoting the
generation of mature immunoregulatory dendritic cells. Nat Immunol.
10:48–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Boles KS, Vermi W, Facchetti F, Fuchs A,
Wilson TJ, Diacovo TG, Cella M and Colonna M: A novel molecular
interaction for the adhesion of follicular CD4 T cells to
follicular DC. Eur J Immunol. 39:695–703. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
He Y, Peng H, Sun R, Wei H, Ljunggren HG,
Yokoyama WM and Tian Z: Contribution of inhibitory receptor TIGIT
to NK cell education. J Autoimmun. 81:1–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Johnston RJ, Comps-Agrar L, Hackney J, Yu
X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, et al:
The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T
cell effector function. Cancer Cell. 26:923–937. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Herzberg B, Campo MJ and Gainor JF: Immune
checkpoint inhibitors in non-small cell lung cancer. Oncologist.
22:81–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang P, Xiong X, Rolfo C, Du X, Zhang Y,
Yang H, Russo A, Devenport M, Zhou P, Liu Y and Zheng P: Mechanism-
and immune landscape-based ranking of therapeutic responsiveness of
22 major human cancers to next generation anti-CTLA-4 antibodies.
Cancers (Basel). 12:2842020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kazandjian D, Suzman DL, Blumenthal G,
Mushti S, He K, Libeg M, Keegan P and Pazdur R: FDA approval
summary: Nivolumab for the treatment of metastatic non-small cell
lung cancer with progression on or after platinum-based
chemotherapy. Oncologist. 21:634–642. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Akinleye A and Rasool Z: Immune checkpoint
inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol.
12:922019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mathew M, Enzler T, Shu CA and Rizvi NA:
Combining chemotherapy with PD-1 blockade in NSCLC. Pharmacol Ther.
186:130–137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cyriac G and Gandhi L: Emerging biomarkers
for immune checkpoint inhibition in lung cancer. Semin Cancer Biol.
52:(Pt 2):269–277. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yarchoan M, Albacker LA, Hopkins AC,
Montesion M, Murugesan K, Vithayathil TT, Zaidi N, Azad NS, Laheru
DA, Frampton GM and Jaffee EM: PD-L1 expression and tumor
mutational burden are independent biomarkers in most cancers. JCI
Insight. 4:e1269082019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pai-Scherf L, Blumenthal GM, Li H,
Subramaniam S, Mishra-Kalyani PS, He K, Zhao H, Yu J, Paciga M,
Goldberg KB, et al: FDA approval summary: Pembrolizumab for
treatment of metastatic non-small cell lung cancer: First-line
therapy and beyond. Oncologist. 22:1392–1399. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Abdel-Rahman O: Correlation between PD-L1
expression and outcome of NSCLC patients treated with
anti-PD-1/PD-L1 agents: A meta-analysis. Crit Rev Oncol Hematol.
101:75–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Davis AA and Patel VG: The role of PD-L1
expression as a predictive biomarker: An analysis of all US Food
and Drug Administration (FDA) approvals of immune checkpoint
inhibitors. J Immunother Cancer. 7:2782019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Paver EC, Cooper WA, Colebatch AJ,
Ferguson PM, Hill SK, Lum T, Shin JS, O'Toole S, Anderson L,
Scolyer RA and Gupta R: Programmed death ligand-1 (PD-L1) as a
predictive marker for immunotherapy in solid tumours: A guide to
immunohistochemistry implementation and interpretation. Pathology.
53:141–156. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fournel L, Wu Z, Stadler N, Damotte D,
Lococo F, Boulle G, Ségal-Bendirdjian E, Bobbio A, Icard P,
Trédaniel J, et al: Cisplatin increases PD-L1 expression and
optimizes immune check-point blockade in non-small cell lung
cancer. Cancer Lett. 464:5–14. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Velcheti V, Schalper KA, Carvajal DE,
Anagnostou VK, Syrigos KN, Sznol M, Herbst RS, Gettinger SN, Chen L
and Rimm DL: Programmed death ligand-1 expression in non-small cell
lung cancer. Lab Invest. 94:107–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ilie M, Long-Mira E, Bence C, Butori C,
Lassalle S, Bouhlel L, Fazzalari L, Zahaf K, Lalvée S, Washetine K,
et al: Comparative study of the PD-L1 status between surgically
resected specimens and matched biopsies of NSCLC patients reveal
major discordances: A potential issue for anti-PD-L1 therapeutic
strategies. Ann Oncol. 27:147–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Rimm DL, Han G, Taube JM, Yi ES, Bridge
JA, Flieder DB, Homer R, West WW, Wu H, Roden AC, et al: A
prospective, multi-institutional, pathologist-based assessment of 4
immunohistochemistry assays for PD-L1 expression in non-small cell
lung cancer. JAMA Oncol. 3:1051–1058. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Büttner R, Gosney JR, Skov BG, Adam J,
Motoi N, Bloom KJ, Dietel M, Longshore JW, López-Ríos F,
Penault-Llorca F, et al: Programmed death-ligand 1
immunohistochemistry testing: A review of analytical assays and
clinical implementation in non-small-cell lung cancer. J Clin
Oncol. 35:3867–3876. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Vilimas T: Measuring tumor mutational
burden using whole-exome sequencing. Methods Mol Biol. 2055:63–91.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ready N, Hellmann MD, Awad MM, Otterson
GA, Gutierrez M, Gainor JF, Borghaei H, Jolivet J, Horn L, Mates M,
et al: First-line nivolumab plus ipilimumab in advanced
non-small-cell lung cancer (CheckMate 568): Outcomes by programmed
death ligand 1 and tumor mutational burden as biomarkers. J Clin
Oncol. 37:992–1000. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Merino DM, McShane LM, Fabrizio D, Funari
V, Chen SJ, White JR, Wenz P, Baden J, Barrett JC, Chaudhary R, et
al: Establishing guidelines to harmonize tumor mutational burden
(TMB): In silico assessment of variation in TMB quantification
across diagnostic platforms: Phase I of the Friends of cancer
research TMB harmonization project. J Immunother Cancer.
8:e0001472020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wojas-Krawczyk K, Kalinka E, Grenda A,
Krawczyk P and Milanowski J: Beyond PD-L1 markers for lung cancer
immunotherapy. Int J Mol Sci. 20:19152019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Samstein RM, Lee CH, Shoushtari AN,
Hellmann MD, Shen R, Janjigian YY, Barron DA, Zehir A, Jordan EJ,
Omuro A, et al: Tumor mutational load predicts survival after
immunotherapy across multiple cancer types. Nat Genet. 51:202–206.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Rizvi H, Sanchez-Vega F, La K, Chatila W,
Jonsson P, Halpenny D, Plodkowski A, Long N, Sauter JL, Rekhtman N,
et al: Molecular determinants of response to anti-programmed cell
death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in
patients with non-small-cell lung cancer profiled with targeted
next-generation sequencing. J Clin Oncol. 36:633–641. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Doostparast Torshizi A and Wang K:
Next-generation sequencing in drug development: Target
identification and genetically stratified clinical trials. Drug
Discov Today. 23:1776–1783. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sa H, Ma K, Gao Y and Wang D: Predictive
value of tumor mutation burden in immunotherapy for lung cancer.
Zhongguo Fei Ai Za Zhi. 22:380–384. 2019.(In Chinese). PubMed/NCBI
|
|
66
|
Pisapia P, Malapelle U and Troncone G:
Liquid biopsy and lung cancer. Acta Cytol. 63:489–496. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gandara DR, Paul SM, Kowanetz M,
Schleifman E, Zou W, Li Y, Rittmeyer A, Fehrenbacher L, Otto G,
Malboeuf C, et al: Blood-based tumor mutational burden as a
predictor of clinical benefit in non-small-cell lung cancer
patients treated with atezolizumab. Nat Med. 24:1441–1448. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Nie W, Qian J, Xu MD, Gu K, Qian FF, Hu
MJ, Lu J, Gan L, Zhang XY, Cao SH, et al: A non-linear association
between blood tumor mutation burden and prognosis in NSCLC patients
receiving atezolizumab. Oncoimmunology. 9:17310722020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chen YT, Seeruttun SR, Wu XY and Wang ZX:
Maximum somatic allele frequency in combination with blood-based
tumor mutational burden to predict the efficacy of atezolizumab in
advanced non-small cell lung cancer: A pooled analysis of the
randomized POPLAR and OAK studies. Front Oncol. 9:14322019.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zeng DQ, Yu YF, Ou QY, Li XY, Zhong RZ,
Xie CM and Hu QG: Prognostic and predictive value of
tumor-infiltrating lymphocytes for clinical therapeutic research in
patients with non-small cell lung cancer. Oncotarget.
7:13765–13781. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Bodor JN, Boumber Y and Borghaei H:
Biomarkers for immune checkpoint inhibition in non-small cell lung
cancer (NSCLC). Cancer. 126:260–270. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hurkmans DP, Kuipers ME, Smit J, van
Marion R, Mathijssen RHJ, Postmus PE, Hiemstra PS, Aerts JGJV, von
der Thüsen JH and van der Burg SH: Tumor mutational load,
CD8+ T cells, expression of PD-L1 and HLA class I to
guide immunotherapy decisions in NSCLC patients. Cancer Immunol
Immunother. 69:771–777. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
O'Donnell JS, Long GV, Scolyer RA, Teng MW
and Smyth MJ: Resistance to PD1/PDL1 checkpoint inhibition. Cancer
Treat Rev. 52:71–81. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Memon H and Patel BM: Immune checkpoint
inhibitors in non-small cell lung cancer: A bird's eye view. Life
Sci. 233:1167132019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Schumacher TN and Schreiber RD:
Neoantigens in cancer immunotherapy. Science. 348:69–74. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang S, He Z, Wang X, Li H and Liu XS:
Antigen presentation and tumor immunogenicity in cancer
immunotherapy response prediction. Elife. 8:e490202019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Alexandrov LB, Nik-Zainal S, Wedge DC,
Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A,
Børresen-Dale AL, et al: Signatures of mutational processes in
human cancer. Nature. 500:415–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Rizvi NA, Hellmann MD, Snyder A, Kvistborg
P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al: Cancer
immunology. Mutational landscape determines sensitivity to PD-1
blockade in non-small cell lung cancer. Science. 348:124–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Martin AM, Nirschl TR, Nirschl CJ,
Francica BJ, Kochel CM, van Bokhoven A, Meeker AK, Lucia MS, Anders
RA, DeMarzo AM and Drake CG: Paucity of PD-L1 expression in
prostate cancer: Innate and adaptive immune resistance. Prostate
Cancer Prostatic Dis. 18:325–332. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Leclerc M, Mezquita L, Guillebot De
Nerville G, Tihy I, Malenica I, Chouaib S and Mami-Chouaib F:
Recent advances in lung cancer immunotherapy: Input of T-cell
epitopes associated with impaired peptide processing. Front
Immunol. 10:15052019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Garrido C, Paco L, Romero I, Berruguilla
E, Stefansky J, Collado A, Algarra I, Garrido F and Garcia-Lora AM:
MHC class I molecules act as tumor suppressor genes regulating the
cell cycle gene expression, invasion and intrinsic tumorigenicity
of melanoma cells. Carcinogenesis. 33:687–693. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang X, Schoenhals JE, Li A, Valdecanas
DR, Ye H, Zang F, Tang C, Tang M, Liu CG, Liu X, et al: Suppression
of type I IFN signaling in tumors mediates resistance to Anti-PD-1
treatment that can be overcome by radiotherapy. Cancer Res.
77:839–850. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Thommen DS, Schreiner J, Müller P, Herzig
P, Roller A, Belousov A, Umana P, Pisa P, Klein C, Bacac M, et al:
Progression of lung cancer is associated with increased dysfunction
of T cells defined by coexpression of multiple inhibitory
receptors. Cancer Immunol Res. 3:1344–1355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Curigliano G, Gelderblom H, Mach N, Doi T,
Tai D, Forde PM, Sarantopoulos J, Bedard PL, Lin CC, Hodi FS, et
al: Phase I/Ib clinical trial of sabatolimab, an Anti-TIM-3
antibody, alone and in combination with spartalizumab, an Anti-PD-1
antibody, in advanced solid tumors. Clin Cancer Res. 27:3620–3629.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Koyama S, Akbay EA, Li YY, Herter-Sprie
GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ,
Asahina H, et al: Adaptive resistance to therapeutic PD-1 blockade
is associated with upregulation of alternative immune checkpoints.
Nat Commun. 7:105012016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hellmann MD, Rizvi NA, Goldman JW,
Gettinger SN, Borghaei H, Brahmer JR, Ready NE, Gerber DE, Chow LQ,
Juergens RA, et al: Nivolumab plus ipilimumab as first-line
treatment for advanced non-small-cell lung cancer (CheckMate 012):
Results of an open-label, phase 1, multicohort study. Lancet Oncol.
18:31–41. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hellmann MD, Ciuleanu TE, Pluzanski A, Lee
JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers
S, Salman P, et al: Nivolumab plus Ipilimumab in lung cancer with a
high tumor mutational burden. N Engl J Med. 378:2093–2104. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Bylicki O, Barazzutti H, Paleiron N,
Margery J, Assié JB and Chouaïd C: First-line treatment of
non-small-cell lung cancer (NSCLC) with immune checkpoint
inhibitors. BioDrugs. 33:159–171. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Davis M, Conlon K, Bohac GC, Barcenas J,
Leslie W, Watkins L, Lamzabi I, Deng Y, Li Y and Plate JM: Effect
of pemetrexed on innate immune killer cells and adaptive immune T
cells in subjects with adenocarcinoma of the pancreas. J
Immunother. 35:629–640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Javeed A, Ashraf M, Riaz A, Ghafoor A,
Afzal S and Mukhtar MM: Paclitaxel and immune system. Eur J Pharm
Sci. 38:283–290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Borghaei H, Langer CJ, Gadgeel S,
Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins
RG, Stevenson JP, Jalal SI, et al: 24-Month overall survival from
KEYNOTE-021 Cohort G: Pemetrexed and carboplatin with or without
pembrolizumab as first-line therapy for advanced nonsquamous
non-small cell lung cancer. J Thorac Oncol. 14:124–129. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Socinski MA, Jotte RM, Cappuzzo F, Orlandi
F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D,
Thomas CA, Barlesi F, et al: Atezolizumab for first-line treatment
of metastatic nonsquamous NSCLC. N Engl J Med. 378:2288–2301. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Aupérin A, Le Péchoux C, Rolland E, Curran
WJ, Furuse K, Fournel P, Belderbos J, Clamon G, Ulutin HC, Paulus
R, et al: Meta-analysis of concomitant versus sequential
radiochemotherapy in locally advanced non-small-cell lung cancer. J
Clin Oncol. 28:2181–2190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Jarosz-Biej M, Smolarczyk R, Cichoń T and
Kułach N: Tumor microenvironment as a ‘game changer’ in cancer
radiotherapy. Int J Mol Sci. 20:32122019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Barker HE, Paget JT, Khan AA and
Harrington KJ: The tumour microenvironment after radiotherapy:
Mechanisms of resistance and recurrence. Nat Rev Cancer.
15:409–425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Deng L, Liang H, Burnette B, Beckett M,
Darga T, Weichselbaum RR and Fu YX: Irradiation and anti-PD-L1
treatment synergistically promote antitumor immunity in mice. J
Clin Invest. 124:687–695. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Gong X, Li X, Jiang T, Xie H, Zhu Z, Zhou
F and Zhou C: Combined radiotherapy and anti-PD-L1 antibody
synergistically enhances antitumor effect in non-small cell lung
cancer. J Thorac Oncol. 12:1085–1097. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Theelen WSME, Peulen HMU, Lalezari F, van
der Noort V, de Vries JF, Aerts JGJV, Dumoulin DW, Bahce I,
Niemeijer AN, de Langen AJ, et al: Effect of pembrolizumab after
stereotactic body radiotherapy vs pembrolizumab alone on tumor
response in patients with advanced non-small cell lung cancer:
Results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA
Oncol. 5:1276–1282. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Li JX, Huang JM, Jiang ZB, Li RZ, Sun A,
Lai-Han Leung E and Yan PY: Current clinical progress of PD-1/PD-L1
immunotherapy and potential combination treatment in non-small cell
lung cancer. Integr Cancer Ther. 18:15347354198900202019.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Habas K, Nganwuchu C, Shahzad F, Gopalan
R, Haque M, Rahman S, Majumder AA and Nasim T: Resolution of
coronavirus disease 2019 (COVID-19). Expert Rev Anti Infect Ther.
18:1201–1211. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
DU HZ, Hou XY, Miao YH, Huang BS and Liu
DH: Traditional Chinese Medicine: An effective treatment for 2019
novel coronavirus pneumonia (NCP). Chin J Nat Med. 18:206–210.
2020.PubMed/NCBI
|
|
102
|
Wood SL, Pernemalm M, Crosbie PA and
Whetton AD: The role of the tumor-microenvironment in lung
cancer-metastasis and its relationship to potential therapeutic
targets. Cancer Treat Rev. 40:558–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wang Y, Zhang Q, Chen Y, Liang CL, Liu H,
Qiu F and Dai Z: Antitumor effects of immunity-enhancing
traditional Chinese medicine. Biomed Pharmacother. 121:1095702020.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Liao CH, Yong CY, Lai GM, Chow JM, Cheng
CF, Fang CL, Lin PC, Chang CL, Zheng YM, Chuang SE, et al:
Astragalus polysaccharide (PG2) suppresses macrophage
migration inhibitory factor and aggressiveness of lung
adenocarcinoma cells. Am J Chin Med. 48:1491–1509. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Li W, Hu X, Wang S, Jiao Z, Sun T, Liu T
and Song K: Characterization and anti-tumor bioactivity of
astragalus polysaccharides by immunomodulation. Int J Biol
Macromol. 145:985–997. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Bamodu OA, Kuo KT, Wang CH, Huang WC, Wu
ATH, Tsai JT, Lee KY, Yeh CT and Wang LS: Astragalus
polysaccharides (PG2) enhances the M1 polarization of macrophages,
functional maturation of dendritic cells, and T cell-mediated
anticancer immune responses in patients with lung cancer.
Nutrients. 11:22642019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wang HB, Zheng QY, Qian DH, Fang J and Ju
DW: Effects of Phytolacca acinosa polysaccharides I on
immune function in mice. Zhongguo Yao Li Xue Bao. 14:243–246.
1993.PubMed/NCBI
|
|
108
|
Chen Y, Wang Y, Xu L, Zhu W, Xu C, Xu M,
Guo L, Hu W, Xu D, Jing R, et al: Influence of total glucosides of
paeony on PD-1/PD-L1 expression in primary Sjögren's syndrome. Int
J Rheum Dis. 22:200–206. 2019. View Article : Google Scholar : PubMed/NCBI
|