Introduction
Craniopharyngioma is a common benign tumor type of
the central nervous system. It accounts for 3% of total tumors and
4% of cranial tumors in children (1); however, the co-occurrence of
craniopharyngioma and intracranial aneurysm is rarely reported.
Fusiform dilation of the internal carotid artery (ICA) following
resection of craniopharyngioma has been reported previously, but it
has been suggested to occur as a result of surgical manipulation
(2). For the first time,
intracranial aneurysms were described to be associated to a certain
extent with craniopharyngioma by Shida et al (3). In 2013, Takeuchi et al
(4) present a case of anterior
cerebral artery dissecting aneurysm associated with
craniopharyngioma and discussed the relationship between these two
lesions, but they did not perform any treatment of the
craniopharyngioma. However, to the best of our knowledge, the
treatment of both of the co-occurrence lesions purely by endoscopic
endonasal approach (EEA) simultaneously has not been reported so
far (search strategy shown as Data
S1).
Case report
A 62-year-old female was admitted to Huanhu Hospital
(Tianjin, China) in August 2021. The patient had been diagnosed
with intracranial space-occupying lesions 2 years previously with
occasional double vision and had recently developed a headache,
which was getting worse. Neurological examination of the patient
revealed partial temporal visual field defects in both eyes. CT
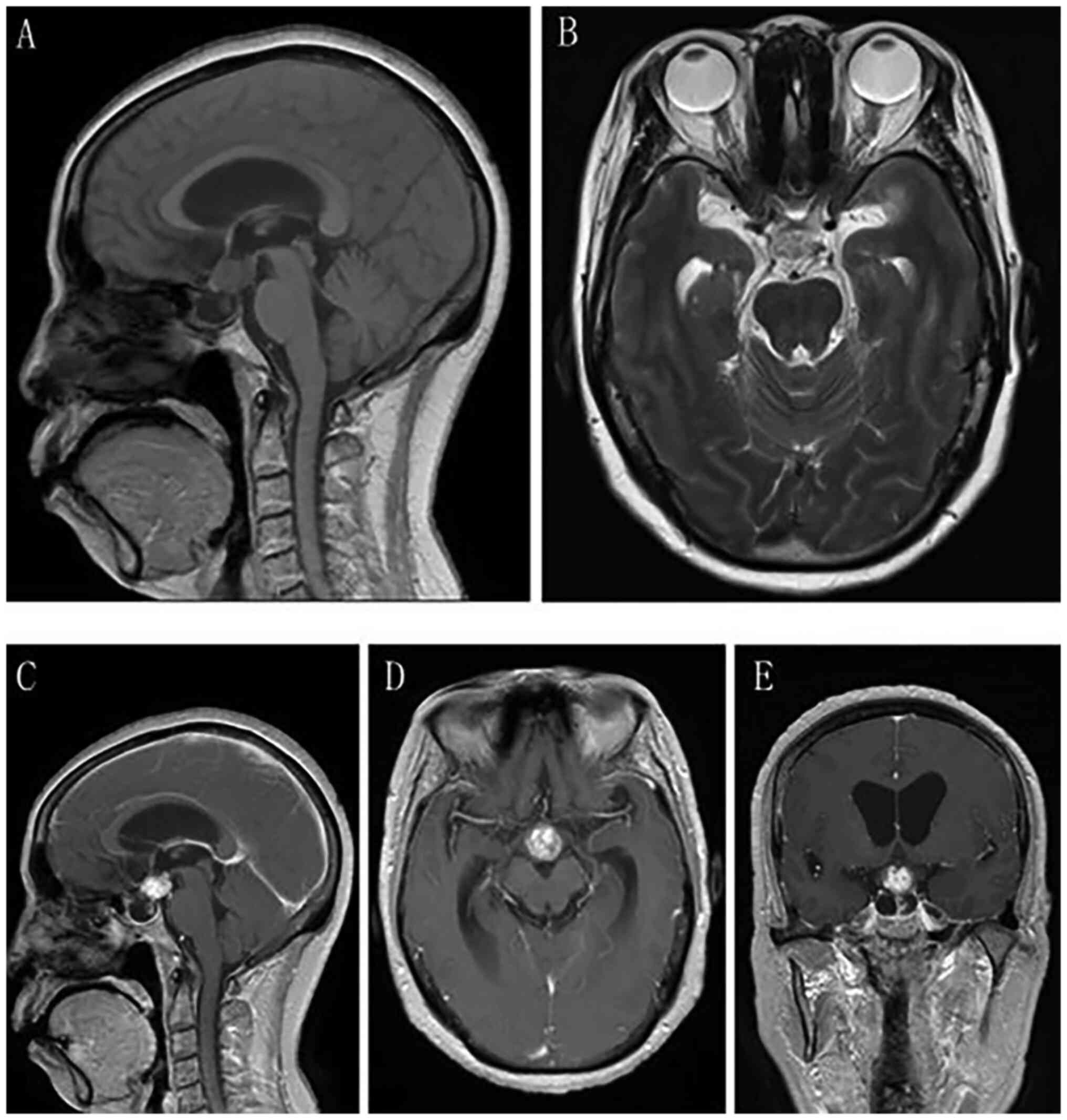
scan indicated a space-occupying lesion in the anterior
tri-ventricle (Fig. 1), and
together with the MRI findings (Fig.
2), a diagnosis of craniopharyngioma was determined.
Specifically, on MRI, the tumor was irregularly defined, with a
cyst-solid intra- and suprasellar cystic lesion expansively growing
into the third ventricle, and indicated to be isointense on T1 and
hyperintense on T2. The optic nerve, optic chiasma and pituitary
stalk were obviously displaced under compression. Enhancement was
inhomogeneous and the imaging specialist diagnosed
craniopharyngioma empirically. CT angiography (CTA) was routinely
scheduled for the patient, which revealed a 6.0×6.0×5.0 mm aneurysm
originating at the C5 segment of the right internal carotid artery
(ICA) (Fig. 3A). For further
confirmation, digital subtraction angiography was performed, based
on which the presence of the aneurysm was confirmed (Fig. 3B-D). After sufficient preoperative
preparation, endoscopic endonasal transsphenoidal tumor resection
and aneurysm clipping were carried out. Informed consent was
obtained from the patient for the publication of the study.
After the general anesthesia accompanied by
intravenous infusion of third-generation cephalosporins, the
patient was placed in the supine position with the head elevated at
20° and slightly tilted to the operator's side. Following routine
facial disinfection with chlorhexidine, the nasal cavity was
disinfected with iodophor cotton balls and then filled with
absorbent cotton soaked with epinephrine to facilitate its mucosal
vasoconstriction, thus reducing intraoperative bleeding. A Karl
Storz endoscope was adopted with 0 and 30° lenses, equipped with an
image 1HUB HD camera (Karl Storz SE & Co. KG). A bilateral
nasal access was applied but the surgical approach was with a
predominantly right-sided nasal access. The endoscope with a 0°
lens was inserted into the right nostril to determine the middle
turbinate and middle nasal meatus, and followed the middle nasal
meatus inward to localize the aperture of the sphenoidal sinus
above the root of the middle turbinate at the junction with the
nasal septum, and then a standard nasoseptal flap was prepared (the
upper edge was kept 1 cm away from the olfactory fissure, avoiding
to affect the sense of smell).
Special attention should be paid to the
sphenopalatine artery or its branches in order to preserve the
irrigation of the nasoseptal flap. The anterior wall of the
sphenoid sinus was removed by a grinding drill or biting forceps.
For a smooth operation, the posterior wall of the pterygoid sinus,
also known as the saddle base, was required to be adequately
exposed. The following anatomical landmarks were identified: The
planum sphenoidale, tuberculum sellae, both optic nerve
protuberance, medial and lateral optic-carotid recess, floor of the
sella turcica, clivus and inferior margin of the sphenoid sinus,
cavernous segment of ICA and clinoidal carotid artery protuberance.
The enlarged posterior wall of the sphenoid sinus was obviously
exposed and then removed. The dura was then prepared for incision
and the dura was then incised around the optic chiasm in an I-beam
pattern after satisfactory dural hemostasis by bipolar
electrocoagulation. After dissection of the arachnoid, the tumor
process was fully removed (Fig.
4).
The next step was to examine the aneurysm (Fig. 5), for which the dura at the right
optic-carotid recess was initially gradually incised and the distal
and proximal rings of the right ICA were then further opened, so as
to clearly expose the C5 segment of the right ICA and the C4-C5
transition, thus obtaining manipulation of the ICA proximal to the
aneurysm. It was further indicated that the apex of the aneurysm
was medially oriented and was in close proximity to the optic
nerve; the adherent arachnoid was gradually separated by a
microdebrider and the aneurysm dome and the aneurysm neck were then
carefully freed. The visualization and control of the aneurysm were
robust and a simulation of the application of a test clip confirmed
the possibility of safe clipping. The aneurysm was initially
clipped with one straight FT 720 T Yaşargil clip (B. Braun
Melsungen AG); however, a small amount of bleeding from the
aneurysm appeared during the clamping process. The C4 segment of
the ICA was temporarily blocked with an aneurysm clip applied for
additional clamping, then the aneurysm was perfectly clamped
without bleeding (Fig. 6).
Finally, the fascia lata of the thigh with adipose tissue was
excised and the skull base was repaired with a combined artificial
dural patch and nasoseptal flap (5).
During the postoperative treatment, the patient did
not report any symptoms of intracranial infection, cerebrospinal
fluid leakage, serious water and electrolyte metabolic disorders or
ischemic symptoms of cerebral tissue. A transient urinary collapse
was reported, which was relieved after symptomatic treatment with
desmopressin tablets. Finally, the diagnosis of craniopharyngioma
was confirmed by pathology (Fig.
7) and a postoperative enhanced MRI indicated the complete
tumor resection (Fig. 8).
Furthermore, CTA indicated complete aneurysm clamping without any
residual aneurysm (Fig. 9). Nasal
endoscopic examination 2 weeks after the surgery indicated a stable
saddle base restoration with abundant blood flow in the nasal
mucosal flap. The patient was discharged from the hospital without
any neurological deficiency. The patient did not receive any
radiotherapy or chemotherapy after surgery, and no tumor recurrence
was observed at the follow-up until now.
Discussion
Craniopharyngioma as a benign tumor type arising
from the epithelial remnants of Rathke's pouch, commonly presenting
in children with non-specific symptoms, accounting for 3% of total
tumors and 4% of cranial tumors in children, with an annual
incidence of 0.05-0.20 per 100,000 individuals (1,6,7).
Unruptured intracranial aneurysms are relatively common in the
general population, with a prevalence of 3.2% (8). With the popularity and application of
non-invasive imaging technology for intracranial vessels, the
diagnosis of unruptured intracranial aneurysms is made with
increasing frequency. However, to the best of our knowledge, the
probability of simultaneous occurrence of craniopharyngioma and
intracranial aneurysm is rather low, and there is a lack of related
literature reporting whether craniopharyngioma has a role in the
occurrence of intracranial aneurysm. Shida et al (3) argued that craniopharyngioma typically
invades the brain, eliciting anaplastic transformations and an
intense glial reaction in subjacent brain and vascular structures,
thus contributing to the development of aneurysm; however, the
hypothesis has also been put forward that the craniopharyngioma
itself or cyst rupture produces chemicals that induce asymptomatic
chemical meningitis, resulting in degeneration of the vessel wall
and development of the dissecting aneurysm (4). However, in any case, scientific and
uniform evidence is still lacking to sufficiently explain the
relationship between craniopharyngioma and aneurysm occurrence. For
selecting the optimal surgical approach, craniopharyngiomas may be
generally classified into the following four subtypes: Intrasellar,
intrasuprasellar, suprasellar and intra-third ventricle (9). Due to its close reach to
hypothalamus, pituitary gland, optic nerve and carotid artery, or
other crucial nerve vessels, it is difficult to be completely
removed, carrying a significant risk for recurrence and
postoperative morbidity despite the general postoperative
radiotherapy. The patient's tumor was completely removed. The team
of clinicians suggested that radiotherapy should be decided at the
follow-up of 3 months according to the results of the review. and
in consideration of the wishes of the patient and the patient's
family, the patient did not receive radiotherapy or chemotherapy.
Transcranial approaches have always served as the main treatment
for craniopharyngioma. In recent years, with the development of
endoscope equipment and the enhancement of surgical techniques, the
EEA has been widely adopted in the treatment of craniopharyngioma
(10,11), despite its unique disadvantages,
including cerebrospinal fluid (CSF) leakage and high incidence of
intracranial infection, as well as the requirement of a nasal
septum mucosal flap to repair the skull base and lumbar cisterna
drainage to reduce the postoperative CSF leak, it has increased in
popularity all over the world (12). Microsurgical clipping and
endovascular coiling have always been adopted as the two main
treatments for aneurysms (13).
The pace of the progress in neurosurgeons' treatment and
technological innovation for aneurysms have never stopped. EEA is a
contributing technique for midline lesions of the skull base;
however, to the best of our knowledge, only dozens of cases of
intracranial aneurysm clipped by intranasal endoscopy have been
reported so far (14,15) (Table
I). As early as 2006, Kassam et al (16) successfully treated an intracranial
aneurysm using an expanded EEA. Back in 2011, Fischer et al
(17) reported that endoscopic
enhancement of the visual field provided by the endoscope prior to,
during and after microsurgical aneurysm occlusion may serve as a
safe and effective application to increase the quality of
treatment. In 2015, Gardner et al (18) reported a series of patients
undergoing EEA for microsurgical clipping of intracranial aneurysm.
In 2018, EEA clipping in 7 patients with 12 anterior circulatory
aneurysms was reported by the group of Professor Hong Tao of the
First Affiliated Hospital of Nanchang University (Nanchang, China)
(15). However, craniopharyngioma
complicated with aneurysm has been rather rarely reported and no
previous case of craniopharyngioma complicated with intracranial
aneurysm purely treated by EEA has been reported at once so far, to
the best of our knowledge. The present case was the first in which
the patient was admitted for a craniopharyngioma and prepared for
endoscopic resection according to the established treatment plan;
however, the presence of aneurysm was incidentally discovered
during the preoperative examination. The considerations for
simultaneous treatment of the two lesions under pure endoscopy were
as follows: First, the aneurysm was located in the deep C5 segment
of the ICA and preoperative cerebral angiography indicated that the
tip of the aneurysm was facing medially; thus, it was difficult to
maximize the exposure of the lesion, which, however, was resolved
by adopting an endoscopic approach, which provided excellent
access. Furthermore, the craniopharyngioma is mostly located in the
anterior tri-ventricle, which is not contraindicated for the EEA
according to the ‘Kassam type’ (19). Finally, it was a wide-necked
aneurysm with the size of 6.0×6.0×5.0 mm; if adopting an
interventional method, endovascular stent placement may be required
for auxiliary embolization, which means the patient requires to
take oral antiplatelet drugs after the interventional procedure,
resulting in the impossibility to perform tumor resection in a
short time, thus missing the best time for tumor treatment. The
anatomical exposure technique of the cavernous sinus segment of the
ICA under endoscopy is sophisticated but is feasible to perform by
a skilled surgeon, so it was possible to confidently perform
proximal flow control of the aneurysm without any significant risk
of serious adverse effects such as intraoperative hemorrhage and
postoperative cerebral infarction. Ultimately, in the present
study, two lesions were successfully managed in one operation,
which also further demonstrated that skull base lesions (tumor or
vascular disorders) located in the midline area may be completely
resolved by transnasal endoscopic surgery, depending on an adequate
preoperative evaluation. However, this requires skilled
neuro-endoscopic surgical experience and extensive knowledge of
endoscopic anatomy, as well as advanced endoscopic surgical
equipment and perfect teamwork. There is still a long way to go
before the indications and contraindications of the procedure are
fully demonstrated and the technique is widely used.
 | Table I.Summary of previously published cases
of aneurysm clipped via the endonasal approach. |
Table I.
Summary of previously published cases
of aneurysm clipped via the endonasal approach.
| Author (year) | Patient age, sex | Clinical
presentation | Location/size,
mm | Complications | Outcome | (Refs.) |
|---|
| Kassam (2006) | 51, F | Focal deficit | Verteb/11 | None | Complete
recovery | 16 |
| Kassam (2007) | 56, F | Incidental
finding | Sup Hyp/5 | None | Complete
recovery | 20 |
| Masahiko (2007) | 58, F | Incidental
finding | Acom/n.a | None | Complete
recovery | 21 |
| Ensenat (2015) | 74, F | SAH | PICA/1.2 | CSF Leak | Complete
recovery | 22 |
| Froelich S
(2011) | 55, M | Incidental
finding | Acom/7 | None | Complete
recovery | 23 |
| Germanwala
(2011) | 42, F | SAH | Ophth/5Paracl/10 | None | Complete
recovery | 24 |
| Drazin (2012) | 59, F | SAH | Bas.Tr/4 | None | Repeat surgery for
reclipping | 25 |
| Dehdashti A R
(2015) | 42, F | SAH | Bas.Ap/10 | None | Endovascular coiling
for residual neck | 26 |
| Dehdashti A R
(2015) | 70, F | SAH | Bas.Ap/5 | Lacunar stroke | Neurological
disability | 26 |
| Dehdashti A R
(2015) | 35, M | Focal deficits | PCA/9.4 | Stroke CSF leak
Meningitis | Neurological
disability | 26 |
| Dehdashti A R
(2015) | 50, M | SAH | Bas.Tr/9 | None | Complete
recovery | 26 |
| Gardner (2015) | 42, F | Incidental
finding | Ophth/3.5 | None | Complete
recovery | 18 |
| Gardner (2015) | 74, M | CN palsy | PCA/19 | CSF leak | Mild disability | 18 |
|
|
|
|
| Meningitis |
|
|
|
|
|
|
| Lacunar stroke |
|
|
| Gardner (2015) | 43, F | Incidental
finding | Sup Hyp/5 | CSF leak | Complete
recovery | 18 |
| Gardner (2015) | 47, F | Incidental
finding | Bas.Ap/9 | Lacunar stroke | Complete
recovery | 18 |
| Gardner (2015) | 45, M | Vision loss
hypopituitarism | Ophth/giant
Ophth/5 | None | Complete
recovery | 18 |
| Gardner (2015) | 73, F | Incidental
finding | Ophth/6 | CSF leak
Meningitis | Complete
recovery | 18 |
| Gardner (2015) | 45, F | SAH | Ophth/7 | None | Complete
recovery | 18 |
| Gardner (2015) | 34, F | Incidental
finding | Ophth/4 | None | Complete
recovery | 18 |
| Gardner (2015) | 55, F | Incidental
finding | Sup.Hyp/NA | None | Complete
recovery | 18 |
| Gardner (2015) | 42, F | Incidental
finding | Sup.Hyp/NA | None | Complete
recovery | 18 |
| Yildirim
(2015) | 72, F | Incidental
finding | Acom/NA | None | Complete
recovery | 27 |
| Xiao (2018) | 42, M | SAH | Acom/7.2 | None | Complete
recovery | 15 |
| Xiao (2018) | 63, F | Incidental
finding |
R.para/13.3a L.para/7.2 Acom/3.1 | None | Endovascular
coiling of the right large paraclinoid aneurysm | 15 |
| Xiao (2018) | 61, F | Incidental
finding |
L.cav-ICA/7.9a R.par/10 | None | Complete
recovery | 15 |
| Xiao (2018) | 52, M | Incidental
finding | Acom.an/3.5 | None | Complete
recovery | 15 |
| Xiao (2018) | 50, M | Incidental
finding | Acom/5.7 | None | Complete
recovery | 15 |
| Xiao (2018) | 45, F | Incidental
finding | Acom/2.8 | None | Complete
recovery | 15 |
| Xiao (2018) | 47, F | Incidental
finding | L.para/4.2
L.oph/2.2L. cav-ICA/2.4a | None | Complete
recovery | 15 |
| Present case | 62, F | Double vision;
headache | R.C5-ICA
(R.para) | Transient urinary
collapse | Complete
recovery | / |
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The patient's surgery was performed by JYC and JMK.
MCZ, JWL, JYC, HY and JM were involved in manuscript preparation
and in data interpretation. All authors approved the final version
of the manuscript. JWL and HY confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Informed consent was obtained from the patient for
the publication of the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Erfurth EM, Holmer H and Fjalldal SB:
Mortality and morbidity in adult craniopharyngioma. Pituitary.
16:46–55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sutton LN, Gusnard D, Bruce DA, Fried A,
Packer RJ and Zimmerman RA: Fusiform dilatations of the carotid
artery following radical surgery of childhood craniopharyngiomas. J
Neurosurg. 74:695–700. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shida N, Nakasato N, Mizoi K, Kanaki M and
Yoshimoto T: Symptomatic vessel narrowing caused by spontaneous
rupture of craniopharyngioma cyst-case report. Neurol Med Chir
(Tokyo). 38:666–668. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takeuchi S, Wada K, Sakakibara F,
Nawashiro H and Mori K: Anterior cerebral artery dissecting
aneurysm associated with untreated craniopharyngioma. Br J
Neurosurg. 27:102–104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Conger A, Zhao F, Wang X, Eisenberg A,
Griffiths C, Esposito F, Carrau RL, Barkhoudarian G and Kelly DF:
Evolution of the graded repair of CSF leaks and skull base defects
in endonasal endoscopic tumor surgery: Trends in repair failure and
meningitis rates in 509 patients. J Neurosurg. 130:861–875. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ostrom QT, Gittleman H, Farah P, Ondracek
A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C and Barnholtz-Sloan JS:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 15
(Suppl 2):ii1–ii56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gupta S, Bi WL, Giantini Larsen A,
Al-Abdulmohsen S, Abedalthagafi M and Dunn IF: Craniopharyngioma: A
roadmap for scientific translation. Neurosurg Focus. 44:E122018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bunin GR, Surawicz TS, Witman PA,
Preston-Martin S, Davis F and Bruner JM: The Descriptive
epidemiology of craniopharyngioma. Neurosurg Focus. 3:e11997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lei C, Chuzhong L, Chunhui L, Peng Z,
Jiwei B, Xinsheng W, Yazhuo Z and Songbai G: Approach selection and
outcomes of craniopharyngioma resection: A single-institute study.
Neurosurg Rev. 44:1737–1746. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qiao N: Endocrine outcomes of endoscopic
versus transcranial resection of craniopharyngiomas: A system
review and meta-analysis. Clin Neurol Neurosurg. 169:107–115. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Algattas H, Setty P, Goldschmidt E, Wang
EW, Tyler-Kabara EC, Snyderman CH and Gardner PA: EEndoscopic
endonasal approach for craniopharyngiomas with intraventricular
extension: Case series, long-term outcomes, and review. World
Neurosurgery. 144:e447–e459. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zwagerman NT, Wang EW, Shin SS, Chang YF,
Fernandez-Miranda JC, Snyderman CH and Gardner PA: Does lumbar
drainage reduce postoperative cerebrospinal fluid leak after
endoscopic endonasal skull base surgery? A prospective, randomized
controlled trial. J Neurosurg. Oct 1–2018.doi:
10.3171/2018.4.JNS172447 (Epub Ahead of Print).
|
|
13
|
Weir B: Unruptured intracranial aneurysms:
A review. J Neurosurg. 96:3–42. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martinez-Perez R, Hardesty DA,
Silveira-Bertazzo G, Albonette-Felicio T, Carrau RL and Prevedello
DM: Safety and effectiveness of endoscopic endonasal intracranial
aneurysm clipping: A systematic review. Neurosurg Rev. 44:889–896.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao LM, Tang B, Xie SH, Huang GL, Wang
ZG, Zeng EM and Hong T: Endoscopic endonasal clipping of anterior
circulation aneurysm: Surgical techniques and results. World
Neurosurg. 115:e33–e44. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kassam AB, Mintz AH, Gardner PA, Horowitz
MB, Carrau RL and Snyderman CH: The expanded endonasal approach for
an endoscopic transnasal clipping and aneurysmorrhaphy of a large
vertebral artery aneurysm: Technical case report. Neurosurgery. 59
(1 Suppl 1):ONSE162–ONSE165. 2006.PubMed/NCBI
|
|
17
|
Fischer G, Oertel J and Perneczky A:
Endoscopy in aneurysm surgery. Neurosurgery. 70:1842011.PubMed/NCBI
|
|
18
|
Gardner PA, Vaz-Guimaraes F, Jankowitz B,
Koutourousiou M, Fernandez-Miranda JC, Wang EW and Snyderman CH:
Endoscopic endonasal clipping of intracranial aneurysms: Surgical
technique and results. World Neurosurg. 84:1380–1393. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hardesty DA, Montaser AS, Beer-Furlan A,
Carrau RL and Prevedello DM: Limits of endoscopic endonasal surgery
for III ventricle craniopharyngiomas. J Neurosurg Sci. 62:310–321.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kassam AB, Thomas AJ, Zimmer LA, Snyderman
CH, Carrau RL, Mintz A and Horowitz M: Expanded endonasal approach:
A fully endoscopic completely transnasal resection of a skull base
arteriovenous malformation. Childs Nerv Syst. 23:491–498. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Masahiko K and Mamoru T: Extended
transsphenoidal approach to anterior communicating artery aneurysm:
Aneurysm incidentally identified during macroadenoma resection:
Technical case report. Neurosurgery. 61 (5 Suppl 2):E299–E300.
2007.PubMed/NCBI
|
|
22
|
Enseñat J, d'Avella E, Tercero A, Valero R
and Alobid I: Endoscopic endonasal surgery for a mesencephalic
cavernoma. Acta Neurochir (Wien). 157:53–55. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Froelich S, Cebula H, Debry C and Boyer P:
Anterior communicating artery aneurysm clipped via an endoscopic
endonasal approach: Technical note. Neurosurgery. 68 (2 Suppl
Operative):S310–S316. 2011.
|
|
24
|
Germanwala AV and Zanation AM: Endoscopic
endonasal approach for clipping of ruptured and unruptured
paraclinoid cerebral aneurysms: Case report. Neurosurgery. 68 (1
Suppl Operative):S234–S240. 2011.PubMed/NCBI
|
|
25
|
Drazin D, Zhuang L, Schievink WI and
Mamelak AN: Expanded endonasal approach for the clipping of a
ruptured basilar aneurysm and feeding artery to a cerebellar
arteriovenous malformation. J Clin Neurosci. 19:144–148. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dehdashti AR: Extended endoscopic
endonasal transclival clipping of posterior circulation
aneurysms-an alternative to the transcranial approach. Acta
Neurochirurgica. 157:2087–2088. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yildirim AE, Divanlioglu D, Karaoglu D,
Cetinalp NE and Belen AD: Pure endoscopic endonasal clipping of an
incidental anterior communicating artery aneurysm. J Craniofac
Surg. 26:1378–1381. 2015. View Article : Google Scholar : PubMed/NCBI
|