Introduction
Renal cancer is the most common malignant solid
tumor of the kidney. Its incidence accounts for ~3% of systemic
malignant tumors, with 85% as clear cell carcinomas (1). Of patients with kidney cancer ~30%
have advanced tumors, and ~30% of patients with localized kidney
cancer develop metastasis or recurrence after surgery. The main
treatment for kidney cancer is surgery, and the treatments for
metastatic and advanced cases include radiotherapy, chemotherapy,
immunotherapy and molecular targeted therapy (2). Bladder cancer is a common malignant
tumor in the urinary tract system, mainly comprising urothelial
carcinomas. The pathogenesis of bladder cancer is complex,
regulated by various genes and factors, and closely related to the
activation of oncogenes (3,4). Prostate cancer is one of the most
common malignant tumors in men (5),
with a high incidence in European and American countries, and its
incidence rate continues to rise in China (6). There is no effective clinical
treatment for advanced prostate cancer, leading to a high mortality
rate. Therefore, finding suitable molecular targets is very
important for the early detection of urinary system tumors and the
prognosis of patients.
EZH2 is located on chromosome 7q36.1, has ~40 kb,
and contains 26 exons. The EZH2 gene belongs to the PCG (Polycom b
group) gene family and has a SET (suvar3-9, enhancer of zeste,
trithorax) domain associated with cell proliferation. The EZ gene
can regulate cell transduction pathways through the SET domain,
inhibiting transcription processes (7,8). As a
transcriptional suppressor, EZH2 can significantly inhibit the
expression of tumor suppressor or metastasis-related genes. Thus,
its high expression or abnormal activation can lead to excessive
cell proliferation and malignant transformation and promote tumor
invasion and metastasis (9). EZH2
plays a central role in the PG gene family. EZH2 regulates histone
methyltransferase and histone deacetylase (HDAC) and is involved in
transcriptional suppression (10).
As a transcriptional suppressor, EZH2 can reduce the binding of
transcription factors to DNA, inhibiting the expression of target
genes, and acts on the region where the C-terminus of the pRb2/p130
protein is linked to HDAC1 to restore pRb2/pB0-HDAC1
complex-mediated cyclin A promoter activity, ultimately promoting
cell cycle progression and malignant transformation (11).
Numerous studies have shown that EZH2 is closely
related to the occurrence and development of tumors. EZH2
expression increases in breast, prostate, pancreatic, gastric
cancers, and other malignant tumors. High EZH2 expression promotes
tumor proliferation and metastasis and is closely related to tumor
malignancy and poor prognosis (12–15).
In the present study, meta- and bioinformatics analyses was
conducted to explore the relationship between EZH2 expression and
the clinicopathological characteristics and prognosis of urological
cancers.
Materials and methods
Literature search
The PubMed (https://pubmed.ncbi.nlm.nih.gov/) and CNKI (https://oversea.cnki.net/index/) databases were
utilized on April 22, 2023. The following keywords were used: EZH2
AND (kidney OR bladder OR prostate OR urothelium) AND (cancer OR
carcinoma). Searches were not limited by language or year of
publication. The inclusion criteria were: i) Articles that detected
EZH2 protein levels in kidney, bladder, or prostate cancers by
immunohistochemistry; ii) the relationship between EZH2 expression
and the pathobiological behavior of renal, bladder, or prostate
cancer was assessed. The exclusion criteria included: i) Abstracts,
reviews, reviews, and conferences; ii) duplicate published
articles; iii) EZH2 levels detected by western blot analysis,
reverse transcription PCR, cDNA microarray, or RNA sequencing; and
iv) patients did not receive any medical treatment before
surgery.
Data extraction and quality
assessment
Two reviewers (Y-KB and YW) independently extracted
information from all eligible publications according to the
inclusion criteria. The following information was extracted from
included studies: Name of the first author, year of publication,
country, cancer type, antibody company, number of cases and
controls, and clinicopathological data of patients. Any
disagreements were resolved by discussion until a consensus was
reached between the two reviewers. These two reviewers
independently assessed the quality of included studies according to
the Newcastle-Ottawa Scale (NOS).
Bioinformatics analysis
Using Kaplan-Meier plotter database (https://kmplot.com/analysis), the prognostic
significance of EZH2 mRNA expression was analyzed in kidney and
bladder cancer. The differences in EZH2 mRNA level were compared
between normal tissue and cancer tissue using GEPIA (http://gepia.cancer-pku.cn/) and UALCAN (http://ualcan.path.uab.edu/index.html)
databases. In the present study, the TIMER database (https://cistrome.shinyapps.io/timer/)
was used to analyze the relationship between EZH2 gene expression
and clinical outcomes and immune cell infiltration.
Statistical analysis
Meta-analyses were performed with Revman software
5.3 (https://www.cochrane.org/). The strength
of the association between EZH2 expression and tumor risk was
assessed by odds ratios (ORs) with 95% confidence intervals.
Statistical significance of pooled ORs was determined by Z-test.
Heterogeneity effects were quantified by the I2 test and
classified into low, medium, and high degrees of heterogeneity
according to cut-off values of 25, 50 and 75%. If there is no
significant heterogeneity (P>0.1, I2<50%), a
fixed-effects model will be used. Otherwise, a random effects model
(P<0.1, I2≥50%) will be used. Publication bias was
assessed by funnel plots and quantified by Begg and Egger tests to
assess funnel plot asymmetry (Fig.
3). Two-sided P<0.05 was considered to indicate a
statistically significant difference.
Results
Search results
As shown in Fig. 1,
27 articles on the relationship between EZH2 expression and
urological cancers risk, clinicopathological or prognostic
parameters were retrieved in PubMed and CNKI by
immunohistochemistry for our meta-analysis, including 10 articles
on renal cancer (16–25), 7 articles on bladder cancer
(26–32) and 10 articles on prostate cancer
(33–42). The main characteristics of the
included studies are presented in Table
I.
 | Table I.Main characteristics of eligible
studies. |
Table I.
Main characteristics of eligible
studies.
| First author,
year | Country | Antibody
supplier | Cases | Ctr | Risk to cancer | Quality | (Refs.) |
|---|
| Lv YJ, 2011 | China | Cell Signaling
Technology, Inc. | 50 | 50 | up | 8 | (16) |
| Xu ST, 2010 | China | OriGene
Technologies, Inc. | 65 | 71 | up | 8 | (17) |
| Liu F, 2013 | China | Invitrogen; Thermo
Fisher Scientific, Inc. | 64 | 12 | up | 8 | (18) |
| Li HB, 2009 | China | Signalway Antibody
LLC | 58 | 8 | up | 8 | (19) |
| Dan JF, 2012 | China | Cell Signaling
Technology, Inc. | 55 | 15 | up | 8 | (20) |
| Wang GD, 2013 | China | Signal | 56 | 56 | up | 8 | (21) |
| Wagener N,
2010 | Germany | Agilent
Technologies, Inc. | 768 |
|
| 7 | (22) |
| Lee HW, 2012 | Korea | Zymed; Thermo
Fisher Scientific, Inc. | 171 |
|
| 7 | (23) |
| Eichenauer T,
2021 | Germany | Abnova | 1,809 |
|
| 7 | (24) |
| Wang Y, 2015 | China | Signal | 165 | 80 | up | 8 | (25) |
| Liao B, 2013 | China | OriGene
Technologies, Inc. | 76 | 10 | up | 8 | (26) |
| Xu ST, 2010 | China | OriGene
Technologies, Inc. | 72 | 10 | up | 8 | (27) |
| Li F, 2010 | China | Wuhan Boster
Biological Technology, Ltd. | 80 | 10 | up | 8 | (28) |
| Cai YJ, 2019 | China |
| 60 |
|
| 7 | (29) |
| Li HB, 2008 | China | Wuhan Boster
Biological Technology, Ltd. | 68 | 12 | up | 8 | (30) |
| Zhang YB, 2008 | China |
| 49 | 10 | up | 8 | (31) |
| Arisan S, 2005 | Istanbul | Abcam | 44 | 24 | up | 8 | (32) |
| Li DW, 2010 | China | OriGene
Technologies, Inc. | 62 | 30 | up | 8 | (33) |
| Zheng J, 2012 | China | OriGene
Technologies, Inc. | 30 | 17 | up | 8 | (34) |
| Song L, 2006 | China | Abcam | 60 |
|
| 7 | (35) |
| Su YS, 2014 | China | Santa Cruz
Biotechnology, Inc. | 100 | 30 | up | 8 | (36) |
| Li J, 2010 | China | Zymed; Thermo
Fisher Scientific, Inc. | 48 | 15 | up | 8 | (37) |
| Zhang SG, 2010 | China | OriGene
Technologies, Inc. | 29 | 34 | up | 8 | (38) |
| Li J, 2017 | China | Zymed; Thermo
Fisher Scientific, Inc. | 52 | 60 | up | 8 | (39) |
| Zheng C, 2016 | China |
| 64 | 54 | up | 8 | (40) |
| Ruan Y, 2021 | China | Abcam | 50 | 9 | up | 8 | (41) |
| Melling N,
2015 | Germany | Abnova | 10,168 |
|
| 7 | (42) |
Forest plot of OR for the relationship
between EZH2 expression and clinicopathological parameters of
urological cancer
The present meta-analysis revealed that EZH2
expression was higher in three urological tumor tissues than in
normal tissues (Fig. 2A-C,
P<0.01). A high EZH2 expression was detected in TNM staging
III–IV than in I–II (Table II,
P<0.01). EZH2 overexpression was detected in T3-4 bladder cancer
compared with Tis-2 (Table II,
P<0.01). In three urological tumors, high EZH2 expression was
negatively associated with the pathological grade of patients
(Table II, P<0.01).
 | Table II.The relationship between EZH2
expression and clinicopathological parameters of urological
cancer. |
Table II.
The relationship between EZH2
expression and clinicopathological parameters of urological
cancer.
|
|
|
| Test for overall
effect |
|---|
|
| Heterogeneity |
|
|---|
|
|
| Odds ratio (95%
confidence interval) |
|
|---|
| Clinicopathological
features |
I2(%) | P-value | P-value |
|---|
| Renal cancer |
|
|
|
|
|
Subtypes (Clear cell
RCC/Papillary RCC) | 35 | 0.21 | 1.02
(0.72-1.44) | 0.91 |
| TNM
staging (I–II/III–IV) | 68 | <0.01 | 0.38
(0.23-0.61) | <0.01 |
| Lymph
node metastasis (LN+/LN−) | 71 | 0.02 | 1.57
(0.42-5.85) | 0.50 |
| Distant
metastasis (M+/M−) | 73 | <0.01 | 1.35
(0.60-3.03) | 0.47 |
|
Pathological grade
(G1-2/G3-4) | 32 | 0.15 | 0.51
(0.41-0.64) | <0.01 |
| Bladder cancer |
|
|
|
|
| Depth
of invasion (T1-2/T3-4) | 0 | 0.92 | 0.23
(0.14-0.40) | <0.01 |
|
Pathological grade
(I–II/III) | 0 | 0.81 | 0.13
(0.06-0.26) | <0.01 |
| Prostate
cancer |
|
|
|
|
| TNM
staging (I–II/III–IV) | 48 | 0.09 | 0.63
(0.58-0.68) | <0.01 |
|
Pathological grade
(High/Low) | 65 | <0.01 | 0.23
(0.10-0.51) | <0.01 |
Publication bias
As demonstrated in Fig.
3, a heterogeneity test was performed on the included articles.
One study at a time was removed from the pooled analysis, the
sensitivity of the results was looked at, and this approach was
used to coagulate the effect of individual studies on the pooled
results. Data that significantly affected heterogeneity were not
found in the present study.
Relationship between EZH2 expression
and bioinformatics analysis in kidney cancer patients
According to the Kaplan-Meier plotter, higher EZH2
mRNA levels were negatively associated with the OS rates of kidney
clear cell renal and papillary renal cell carcinoma (RCC) (Fig. 4A and B, P<0.01). Higher EZH2
expression was negatively correlated with the relapse-free survival
rates in clear cell RCC (Fig. 4C,
P<0.01), while it was positively correlated in papillary RCC
(Fig. 4D, P<0.01). In GEPIA
databases, EZH2 mRNA levels were higher in kidney clear cell renal
and papillary RCC than in normal tissues (Fig. 4E and I, respectively, P<0.05).
EZH2 mRNA levels were negatively correlated with the TNM stage of
kidney clear cell RCC but not in kidney papillary RCC compared with
normal tissues (Fig. 4F and J,
respectively, P<0.05). Moreover, higher EZH2 mRNA levels were
negatively associated with overall survival rates of kidney clear
cell RCC and disease-free survival rates of kidney papillary RCC
(Fig. 4G and L, P<0.05) and not
associated with disease-free survival rates of kidney clear cell
RCC (Fig. 4H, P>0.05) or overall
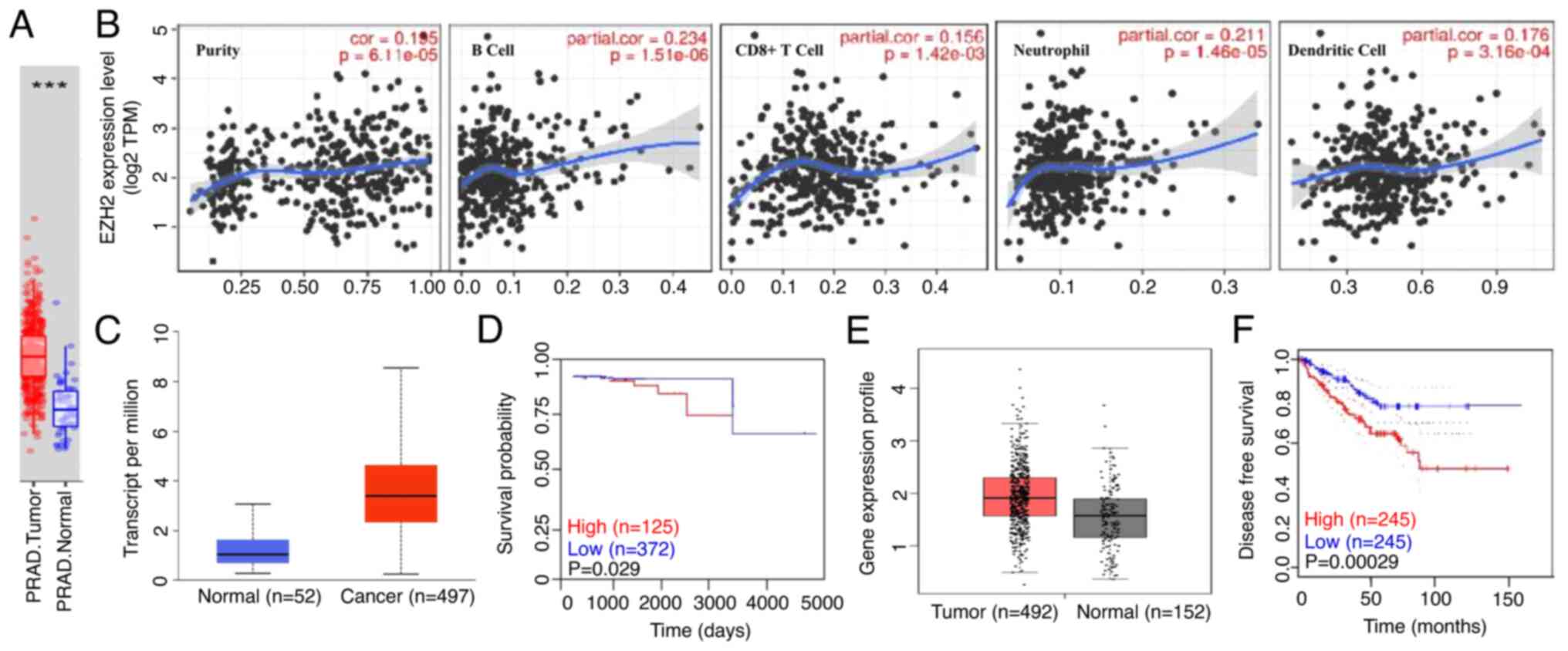
survival rates of kidney papillary RCC (Fig. 4K, P>0.05) The TIMER database
demonstrated that EZH2 expression was higher in kidney clear cell
renal and papillary RCC than in normal tissues (Fig. 5A, P<0.05). Additionally, EZH2
expression was closely associated with the infiltration of seven
immune cells (B cells, CD8+ T cells, CD4+ T
cells, macrophages, neutrophils and dendritic cells) in kidney
clear cell RCC and kidney papillary RCC (Fig. 5B and D). EZH2 expression was also
negatively correlated with patient prognosis (Fig. 5C and E). According to the UALCAN
database, EZH2 expression was higher in kidney clear cell RCC, and
EZH2 expression was elevated in patients with lymph node metastases
(Fig. 6A and B, P<0.05). Higher
EZH2 expression was negatively associated with OS rates of all
cancer patients considering tumor grade (Fig. 6C and D, P<0.05). EZH2 expression
was also higher in kidney papillary RCC (Fig. 6E, P<0.05). A higher EZH2
expression was negatively correlated with OS rates of all cancer
patients regardless of race and sex (Fig. 6F-H, P<0.05).
 | Figure 5.Partial correlation of EZH2
expression levels with immune cells (CD4+ T cells, macrophages, B
cells, CD8+ T cells, neutrophils and dendritic cells) in RCC. (A)
According to TIMER database, EZH2 expression was higher in clear
cell RCC and papillary RCC than normal tissues. (B and D) EZH2
expression closely associated with the proportion of 7 immune cell
types (B cells, CD8+ T cells, CD4+ T cells, macrophages,
neutrophils and dendritic cells) infiltrates in clear cell RCC and
papillary RCC. (C and E) EZH2 expression was negatively correlated
with patient prognosis. ***P<0.05. EZH2, zeste enhancer homolog
2 gene; RCC, renal cell carcinoma. |
Relationship between EZH2 expression
and bioinformatics analysis in patients with bladder cancer
The TIMER database demonstrated that EZH2 expression
was higher in bladder cancer than in normal tissues (Fig. 7A, P<0.05). EZH2 expression was
closely associated with the proportion of immune cell infiltrates
in bladder cancer (Fig. 7B). The
Kaplan-Meier plotter results are shown in Fig. 7C-E. According to the UALCAN and
GEPIA databases, EZH2 expression was higher in bladder cancer than
in normal tissues (Fig. 7F and G,
P<0.05). Higher EZH2 expression was negatively correlated with
disease-free survival rates of bladder cancer (Fig. 7H, P<0.05).
Relationship between EZH2 expression
and bioinformatics analysis in patients with prostate cancer
Furthermore, the TIMER results indicated that EZH2
expression was higher in prostate cancer than in normal tissues
(Fig. 8A, P<0.05). EZH2
expression was closely associated with the proportion of immune
cell infiltrates in prostate cancer (Fig. 8B). The UALCAN and GEPIA results
revealed that EZH2 expression was higher in prostate cancer than in
normal tissues (Fig. 8C and E,
P<0.05). Furthermore, higher EZH2 expression was negatively
correlated with the OS rates of prostate cancer (Fig. 8D, P<0.05) and also negatively
correlated with disease-free survival rates of prostate cancer
(Fig. 8F, P<0.05).
Discussion
EZH2 is the core catalytic subunit of polycomb
repressive complex 2 (PRC2). EZH2 is a tumor-related protein that
exhibits histone methyltransferase activity and is commonly present
in early embryonic development. EZH2, SUZ12, and EED form the PRC2
complex, which mediates gene silencing by catalyzing histone 3
trimethylation at position 27 (H3K-27me3) and is involved in X
chromosome inactivation, cell differentiation, and regulation of
embryonic development (43). As a
catalytically active subunit, EZH2 is fully involved in the H3K27
process, and EED and SUZ12 jointly maintain PRC2 homeostasis
(44). The establishment and
maintenance of epigenetic silencing is the basis of cell function.
The basic epigenetic systems involved in gene activity suppression
are the PcG protein and DNA methylation system. EZH2 interacts with
DNA methyltransferase (DNMT) in PRC2 and PRC3, and DNMTs interact
and correlate with DNMT activity in vivo (45).
Previous studies have indicated that EZH2 expression
increases in tumor tissues compared with normal tissues (46). Higher EZH2 expression in tumor
tissue leads to inevitably higher severe tumor malignancy and worse
prognosis. EZH2 is highly expressed in tumor cells. After
interfering with EZH2 inhibition, NK4a/ARF inhibition by PeG will
be relieved, resulting in cell senescence. If PeG function is
restored, senescent cells will show a naive phenomenon again
(18). EZH2 expression is also
increased in breast cancer tissues, participating in lymph node
metastasis, in the invasion of breast cancer and is closely related
to the occurrence of ER-negative breast cancer (47). In breast epithelial cells,
abnormally high EZH2 expression can promote cell growth and enhance
invasive ability. EZH2 induces G1-S phase cells to enter the
division phase, promoting cancer cell proliferation (48). The bioinformatics analysis
identified that EZH2 is abnormally highly expressed in
hepatocellular carcinoma (HCC) stages 1 to 3. EZH2 can mediate the
increase in the modification of histone H3K27me3 near the start
site of gene transcription, exerting an epigenetic regulation
effect. The EZH2 inhibitor GSK343 impairs HepG2 cell viability and
cell proliferation in vitro (49).
EZH2 is highly expressed in gastrointestinal
malignancies including esophageal, gastric, colorectal and
pancreatic cancer (15,50–52).
High EZH2 expression can promote the proliferation and invasion of
esophageal cancer cells (53). EZH2
induces the epithelial-mesenchymal transition of gastric cancer
cells by binding to the PTEN promoter, enhancing migration and
invasion ability of cancer cells (54). Particularly, EZH2 enhances the
spheroidization ability of gastric cancer cells, indicating its
enrichment in cancer stem cells. Additionally, EZH2 inhibits
microRNA (miR)-22 through epigenetic modification and regulates
H3K27 on the miR-22 promoter, downregulating the galactoprotein-9
and promoting the proliferation and progression of HCC cells.
Therefore, inhibiting EZH2 expression can delay the metastatic
spread of HCC cells (55).
EZH2 expression is elevated in colorectal cancer
tissues, and EZH2 protein is positive in patients with distant
metastases. Positive expression rate of EZH2 in tissues of
colorectal cancer patients with distant metastasis is higher than
in patients without distant metastasis. The multivariate analysis
showed that EZH2 protein levels were an independent influencing
factor of poor prognosis in patients with colorectal cancer.
According to the KM-plotter database, an inverse association
between EZH2 expression and poor OS was found in patients with
kidney clear cell renal and papillary RCC. In addition, EZH2
overexpression in colorectal cancer tissue is related to the
proliferation activity of tumor cells (51). In pancreatic cancer, ANLN-induced
EZH2 can upregulate enhancers and regulate cancer cell progression,
partially reversing the tumor-suppressive effect of actin-binding
protein (ANLN) downregulation (52). By interfering with bladder
cancer-related transcription factor 1 (bladder cancer-associated
transcript 1, BLACAT1) to block EZH2 recruitment, promote
cyclin-dependent kinase inhibitor 1C (cyclin-dependent kinase
inhibitor 1C, CDKN1C) expression, and inhibit cyclin expression,
thereby inhibiting pancreatic cancer cell proliferation, migration,
and aerobic glycolysis (56). The
present findings also demonstrated that EZH2 upregulation was
negatively associated with TNM staging and pathological grade in
kidney and prostate cancers. EZH2 expression was also negatively
related to the invasion depth and pathological grade in bladder
cancer.
The TIMER database only contains the data of kidney
cancer, while the relationship between prostate cancer, bladder
cancer and immune cells needs further research in the future, the
lack of the use of TISIDB database or other databases as a
limitation on this specific aspect, which is also the limitation of
the present study.
In conclusion, EZH2 is upregulated in urological
cancers. Its high expression was negatively correlated with the
invasion depth, TNM staging, and pathological grade of urological
cancers at both mRNA and protein levels. The expression of EZH2 was
identified to be closely related to four types of immune cells in
kidney cancer, which helps with future research. However, the
specific mechanism is not yet clear and whether immunotherapy can
be considered requires further verification in the future.
Moreover, EZH2 expression may be a favorable potential marker for
the worse prognosis of urological cancer patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the Kaplan-Meier plotter (https://kmplot.com/analysis/), TIMER database
(https://cistrome.shinyapps.io/timer/), GEPIA (Gene
Expression Profiling Interactive Analysis) database(http://gepia.cancer-pku.cn/) and UALCAN (http://ualcan.path.uab.edu/index.).
Authors' contributions
YKB and YW designed the study. YKB and JS prepared
figures and tables, interpreted the data and wrote the main
manuscript. QLX, YSW and NZ participated in the research of the
study and performed the statistical analysis. YKB and YW confirm
the authenticity of all the raw data. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shah AR, Lazar EL and Atlas AB: Tracheal
diverticula after tracheoesophageal fistula repair: Case series and
review of the literature. Pediatr Surg. 44:2107–2111. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Escudier B, Goupil MG, Massard C and
Fizazi K: Sequential therapy in renal cell carcinoma. Cancer. 115
(10 Suppl):S2321–S2326. 2009. View Article : Google Scholar
|
|
3
|
Fleshner NE, Herr HW, Stewart AK, Murphy
GP, Mettlin C and Menck HR: The national cancer data base report on
bladder carcinoma. The American college of surgeons commission on
cancer and the American Cancer Society. Cancer. 78:1505–1513. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim KH and Roberts CWM: Targeting EZH2 in
cancer. Nat Med. 22:128–134. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen M and Li CM: MRI diagnosis points and
clinical treatment options of prostate cancer. Chin J Magn Reson
Imaging. 2:82011.(In Chinese).
|
|
6
|
Ding HM and Li L: The application of
prostate specific antigen in the diagnosis of prostate cancer. Int
Med Health Guid News. 13:32007.(In Chinese).
|
|
7
|
Chen H, Rossier C and Antonarakis SE:
Cloning of a human homolog of the Drosophila enhancer of zeste gene
(EZH2) that maps to chromosome 21q22.2. Genomics. 38:30–37. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bachmann IM, Halvorsen OJ, Collett K,
Stefansson IM, Straume O, Haukaas SA, Salvesen HB, Otte AP and
Akslen LA: EZH2 expression is associated with high proliferation
rate and aggressive tumor subgroups in cutaneous melanoma and
cancers of the endometrium, prostate, and breast. J Clin Oncol.
24:268–273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Varambally S, Dhanasekaran SM, Zhou M,
Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt
RG, Otte AP, et al: The polycomb group protein EZH2 is involved in
progression of prostate cancer. Nature. 419:624–629. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuzmichev A, Jenuwein T, Tempst P and
Reinberg D: Different EZH2-containing complexes target methylation
of histone H1 or nucleosomal histone H3. Mol Cell. 14:183–193.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tonini T, Bagella L, D'Andrilli G, Claudio
PP and Giordano A: Ezh2 reduces the ability of HDAC1-dependent
pRb2/p130 transcriptional repression of cyclin A. Oncogene.
23:4930–4937. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jang SH, Lee JE, Oh MH, Lee JH, Cho HD,
Kim KJ, Kim SY, Han SW, Kim HJ, Bae SB and Lee HJ: High EZH2
protein expression is associated with poor overall survival in
patients with luminal a breast cancer. J Breast Cancer. 19:53–60.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu C, Jin X, Yang J, Yang Y, He Y, Ding L,
Pan Y, Chen S, Jiang J and Huang H: Inhibition of EZH2 by chemo-
and radiotherapy agents and small molecule inhibitors induces cell
death in castration-resistant prostate cancer. Oncotarget.
7:3440–3452. 2015. View Article : Google Scholar
|
|
14
|
Kuroki H, Hayashi H, Okabe H, Hashimoto D,
Takamori H, Nakahara O, Nakagawa S, Fukushima Y, Chikamoto A, Beppu
T, et al: EZH2 is associated with malignant behavior in pancreatic
IPMN via p27Kip1 downregulation. PLoS One. 9:e1009042014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo L, Yang TF, Liang SC, Guo JX and Wang
Q: Role of EZH2 protein expression in gastric carcinogenesis among
Asians: A meta-analysis. Tumour Biol. 35:6649–6656. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lv YJ, Ren M, Wang B, Ge RQ and Zhang JD:
Expressions of CTGF and EZH2 in renal clear cell carcinoma and
their clinical significance. J Shandong Univ (Health Sci).
49:85–89. 2011.
|
|
17
|
Xu ST, Yan CY, Huang YH and Ding X:
Research about expression of EZH2 and P53 in human renal cell
carcinoma. Chin J Mod Med. 20:736–737. 2010.(In Chinese).
|
|
18
|
Liu F, Xiao RH, Hong ZD, Lu XB, Shi ZM, Xi
XQ and Pan ZY: Expression of EZH2 and P57 in renal cell carcinoma
and its clinical significance. Pract Clin Med. 14:23–25. 2013.(In
Chinese).
|
|
19
|
Li HB, Wang H and Wu GJ: Expression of
EZH2 and PTEN in renal clear cell carcinoma and their correlation.
J Shanxi Med Univ. 40:199–201. 2009.(In Chinese).
|
|
20
|
Dan JF, Zhuang QY, Liu F and Peng EJ:
Expression and correlation of EZH2 and RUNX3 in Renal clear cell
carcinoma. J Clin Urol. 27:567–571. 2012.(In Chinese).
|
|
21
|
Wang G, Qin W, Zheng J, Wei M, Zhou X,
Wang H and Wen HW: Expressions of EZH2 and RUNX3 in renal cell
carcinoma and their clinical significance. Xi Bao Yu Fen Zi Mian Yi
Xue Za Zhi. 29:82–84. 2013.(In Chinese). PubMed/NCBI
|
|
22
|
Wagener N, Macher-Goeppinger S, Pritsch M,
Hüsing J, Hoppe-Seyler K, Schirmacher P, Pfitzenmaier J, Haferkamp
A, Hoppe-Seyler F and Hohenfellner M: Enhancer of zeste homolog 2
(EZH2) expression is an independent prognostic factor in renal cell
carcinoma. BMC Cancer. 10:5242010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee HW and Choe M: Expression of EZH2 in
renal cell carcinoma as a novel prognostic marker. Pathol Int.
62:735–741. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eichenauer T, Simmendinger L, Fraune C,
Mandelkow T, Blessin NC, Kluth M, Hube-Magg C, Möller K, Clauditz
T, Weidemann S, et al: High level of EZH2 expression is linked to
high density of CD8-positive T-lymphocytes and an aggressive
phenotype in renal cell carcinoma. World J Urol. 9:481–490. 2021.
View Article : Google Scholar
|
|
25
|
Wang Y, Chen Y, Geng H, Qi C, Liu Y and
Yue D: Overexpression of YB1 and EZH2 are associated with cancer
metastasis and poor prognosis in renal cell carcinomas. Tumour
Biol. 36:7159–7166. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liao B and Yang ZB: Expression and
clinical significance of EZH2, FHIT and VEGF in bladder urothelial
carcinoma. Chin J Oncol Prev Treat. 5:142–144. 2013.(In
Chinese).
|
|
27
|
Xu ST, Shao XF, Jiang T and Liu F:
Expression and significance of EZH2 and Ki67 in human bladder
transitional cell carcinoma. Mod Med J Chin. 12:72–74. 2010.(In
Chinese).
|
|
28
|
Liu F, Peng EJ, Li YY, Qi Y, Du LH and
Zhuang QY: Correlation of EZH2 and PTEN gene expression with
disease free survival of bladder transitional cell carcinoma
patients. Chin J Clin Oncol. 37:1220–1223. 2010.(In Chinese).
|
|
29
|
Cai YJ, Zhang XJ, Ke M and Hong T:
Expression of EZH2 gene in bladder urothelial carcinoma and its
relationship with clinicopathological parameters. Mod Chin Doctor.
57:1–3. 2019.(In Chinese).
|
|
30
|
Li HB, Wang H, Wu GJ and Zhou ZM:
Expression of EZH2gene in urothelial carcinoma of bladder and its
clinical value. J Shanxi Med Univ. 39:530–533. 2008.(In
Chinese).
|
|
31
|
Zhang YB, Niu HT and Chang JW: Expression
and clinical significance of EZH2 in transitional cell carcinoma of
bladder. Clin Med China. 24:193–195. 2008.(In Chinese).
|
|
32
|
Arisan S, Buyuktuncer ED, Palavan-Unsal N,
Caşkurlu T, Cakir OO and Ergenekon E: Increased expression of EZH2,
a polycomb group protein, in bladder carcinoma. Urol Int.
75:252–257. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li WD, Wu Q, Ma CM and Wang HQ: Expression
of EZH2 in prostate cancer and its clinicopathologic significance.
Acta Unive Med Anhui. 45:562–564. 2010.(In Chinese).
|
|
34
|
Zheng J, Li XG and Jin TX: Expression and
clinical significance of EZH2 and P27 in prostate carcinoma and
benign prostatic hyperplasia. J Med Sci Yanbian Univ. 35:272–276.
2012.(In Chinese).
|
|
35
|
Song L, Cui XJ, Su DJ and Tan GL:
Expression and correction of EZH2 and PTEN in prostate cancer. J
Fourth Mil Med Univ. 27:994–996. 2006.(In Chinese).
|
|
36
|
Sun YS, Li FJ, Gao Y, Jia HB, Wang QY, Qin
WJ, Wen HW and Wang H: The expression of EZH2 in the prostate
cancer tissues and its clinical significance. J Mod Oncol.
22:1351–1353. 2014.
|
|
37
|
Li J, Fan QH, Fan XS, Zhou W, Qiu Y and
Qiu L: EZH2 expression in human prostate cancer and its
clinicopathologic significance. Zhonghua Nan Ke Xue. 16:123–128.
2010.(In Chinese). PubMed/NCBI
|
|
38
|
Zhang SG, Qiu Y, Qiu L, Wu Y and Ding Y:
Expressions and significance of P504S‚EZH2and pim-1in prostate
cancer. J Contemp Urol Reprod Oncol. 2:354–356. 2010.
|
|
39
|
Li J, Qiu Y and Qiu L: Significance of
detection of mixed type antibody EZH2/34βE12/p63 in needle biopsy
specimen of prostatic lesions. Pract Geriatr. 31:458–460. 2017.
|
|
40
|
Zheng C, Wang Z and Cao YG: Drosophila
Zeste gene enhancer 2, phosphatase and tensin in prostate
adenocarcinoma tissue homologous protein expression and its effect
on Ki67 proliferation index. Chin J Gerontol. 6:2946–2947. 2016.(In
Chinese).
|
|
41
|
Ruan Y, Xu H, Ji X and Zhao J: BLM
interaction with EZH2 regulates MDM2 expression and is a poor
prognostic biomarker for prostate cancer. Am J Cancer Res.
11:1347–1368. 2021.PubMed/NCBI
|
|
42
|
Melling N, Thomsen E, Tsourlakis MC, Kluth
M, Hube-Magg C, Minner S, Koop C, Graefen M, Heinzer H, Wittmer C,
et al: Overexpression of enhancer of zeste homolog 2 (EZH2)
characterizes an aggressive subset of prostate cancers and predicts
patient prognosis independently from pre- and postoperatively
assessed clinicopathological parameters. Carcinogenesis.
36:1333–1340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li X, Wen ZL and Tu XL: Research progress
on EZH2. J Clin Ration Drug Use. 10:32017.(In Chinese).
|
|
44
|
Simon JA and Lange CA: Roles of the EZH2
histone methyltransferase in cancer epigenetics. Mutat Res.
647:21–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Viré E, Brenner C, Deplus R, Blanchon L,
Fraga M, Didelot C, Morey L, Van Eynde A, Bernard D, Vanderwinden
JM, et al: The polycomb group protein EZH2 directly controls DNA
methylation. Nature. 439:871–874. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhou L, Mudianto T, Ma X, Riley R and
Uppaluri R: Targeting EZH2 enhances antigen presentation, antitumor
immunity, and circumvents Anti-PD-1 resistance in head and neck
cancer. Clin Cancer Res. 26:290–300. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yao F, Liu C, Fang Y, et al: The
expression and clinical significance of EZH2 in breast cancer. Chin
J Microecol. 25:42013.(In Chinese).
|
|
48
|
Bracken AP, Pasini D, Capra M, Prosperini
E, Colli E and Helin K: EZH2 is downstream of the pRB-E2F pathway,
essential for proliferation and amplified in cancer. EMBO J.
22:5323–5335. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhao YS, Ji MD, Dong LC, Guo X, Song BY
and Chen Y: Epigenetic regulation mechanism of EZH2 gene in
hepatocellular carcinoma. Basic Clin Med. 42:864–870. 2022.
|
|
50
|
Peng Y, Chen HB, Su ZJ, Chen L, Zhang JK
and Xu LY: Expression of EZH2 protein in esophageal cancer tissue.
Acta Anat Sin. 37:62006.(In Chinese).
|
|
51
|
Hou FH, Su YH, Bu YJ, Wang X and Hou ZB:
Prognostic value of EZH2,Ki-67 and CD34 expression in patients with
surgically treated colorectal cancer. J Sun Yat-Sen Univ (Med Sci).
866–872. 2017.
|
|
52
|
Wang A, Dai H, Gong Y, Zhang C, Shu J, Luo
Y, Jiang Y, Liu W and Bie P: ANLN-induced EZH2 upregulation
promotes pancreatic cancer progression by mediating
miR-218-5p/LASP1 signaling axis. J Exp Clin Cancer Res. 38:3472019.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Huang WN, Gu LN, Liu F and Sang M: Effect
of EZH2 gene on proliferation of human esophageal cancer cells.
Chin J Cancer Biother. 24:960–965. 2017.(In Chinese).
|
|
54
|
Gan L, Xu M, Hua R, Tan C, Zhang J, Gong
Y, Wu Z, Weng W, Sheng W and Guo W: The polycomb group protein EZH2
induces epithelial-mesenchymal transition and pluripotent phenotype
of gastric cancer cells by binding to PTEN promoter. J Hematol
Oncol. 11:92018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen S, Pu J, Bai J, Yin Y, Wu K, Wang J,
Shuai X, Gao J, Tao K, Wang G and Li H: EZH2 promotes
hepatocellular carcinoma progression through modulating
miR-22/galectin-9 axis. J Exp Clin Cancer Res. 37:32018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhou X, Gao W, Hua H and Ji Z:
LncRNA-BLACAT1 facilitates proliferation, migration and aerobic
glycolysis of pancreatic cancer cells by repressing CDKN1C via
EZH2-induced H3K27me3. Front Oncol. 10:5398052020. View Article : Google Scholar : PubMed/NCBI
|