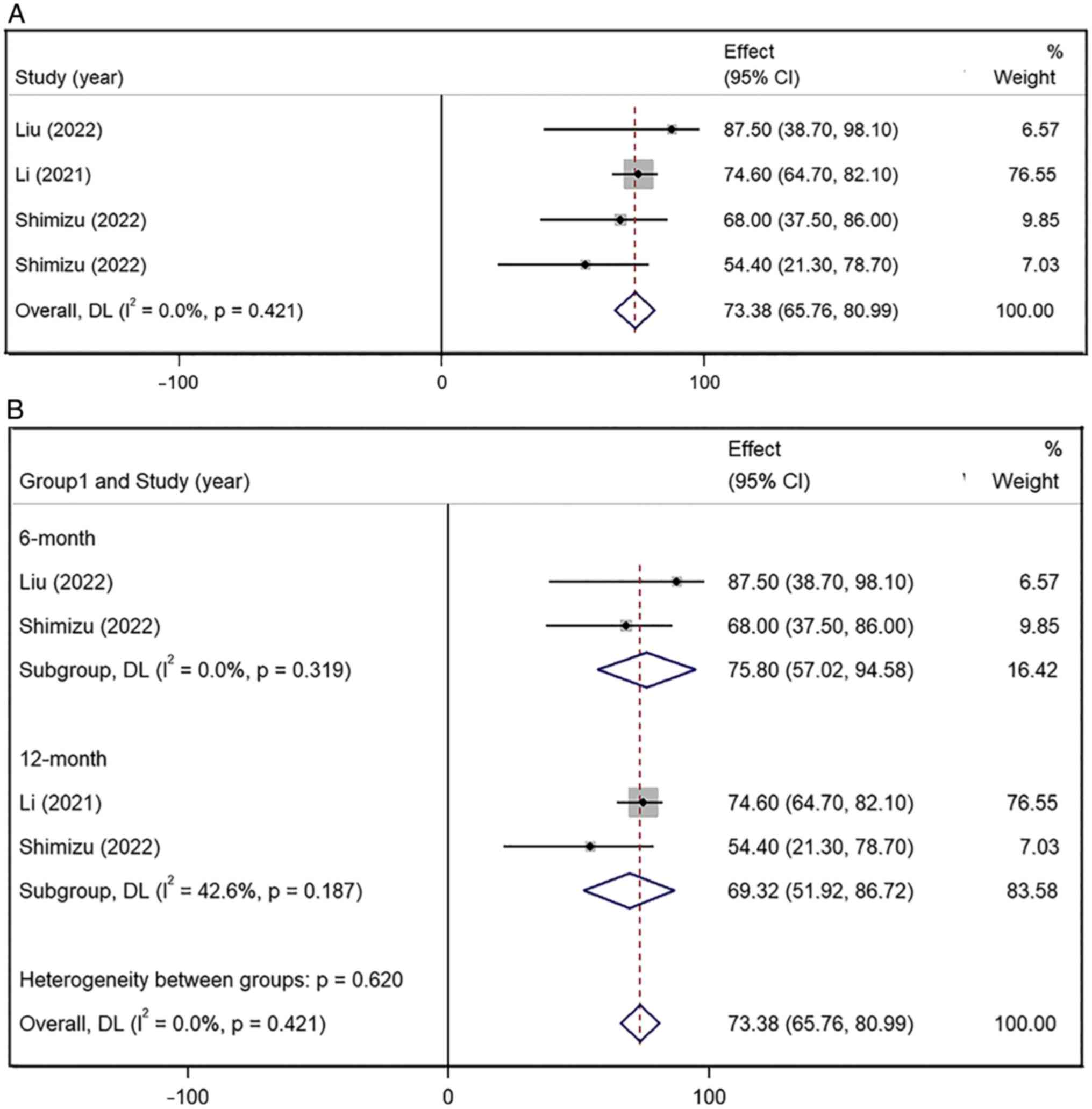
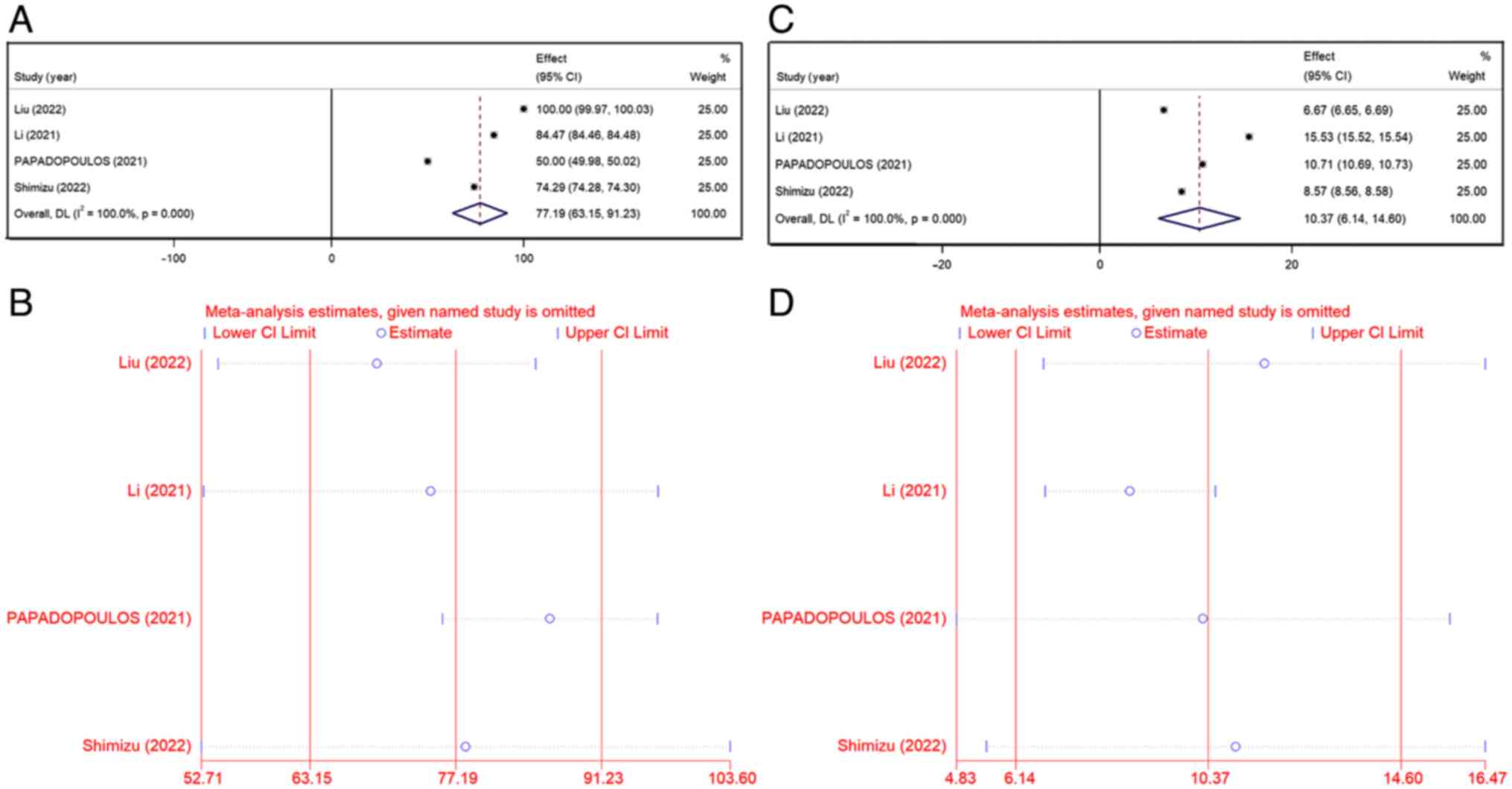
|
1
|
Das S and Johnson DB: Immune-related
adverse events and anti-tumor efficacy of immune checkpoint
inhibitors. J Immunother Cancer. 7:3062019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Le DT, Durham JN, Smith KN, Wang H,
Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et
al: Mismatch repair deficiency predicts response of solid tumors to
PD-1 blockade. Science. 357:409–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Papadopoulos KP, Harb W, Peer CJ, Hua Q,
Xu S, Lu H, Lu N, He Y, Xu T, Dong R, et al: First-in-human phase I
study of envafolimab, a novel subcutaneous single-domain anti-PD-L1
antibody, in patients with advanced solid tumors. Oncologist.
26:e1514–e1525. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Markham A: Envafolimab: First approval.
Drugs. 82:235–240. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Machiels JP, Tao Y, Burtness B, Tahara M,
Licitra L, Rischin D, Waldron J, Simon C, Gregoire V, Harrington K,
et al: Pembrolizumab given concomitantly with chemoradiation and as
maintenance therapy for locally advanced head and neck squamous
cell carcinoma: KEYNOTE-412. Future Oncol. 16:1235–1243. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Galsky MD, Mortazavi A, Milowsky MI,
George S, Gupta S, Fleming MT, Dang LH, Geynisman DM, Walling R,
Alter RS, et al: Randomized double-blind phase II study of
maintenance pembrolizumab versus placebo after first-line
chemotherapy in patients with metastatic urothelial cancer. J Clin
Oncol. 38:1797–1806. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ömeroğlu Şimşek G: Lung cancer management
in COVID-19 pandemic. Turk Thorac J. 21:340–344. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
National Medical Products Administration
(NMPA), . Envafolimab Injection. Full prescribing information.
NMPA; Beijing: 2021
|
|
9
|
Liu R, Yin X, Deng Y, Xu N, Xiang S, Zhang
Y, Gong Y, Liu D and Xu J: Safety and efficacy of envafolimab
combined with FOLFOX as first-line treatment in patients with
locally advanced or metastatic gastric/gastroesophageal junction
adenocarcinoma in a phase II clinical trial. Chin J New Drugs.
031:1502–1508. 2022.(In Chinese).
|
|
10
|
Li J, Deng Y, Zhang W, Zhou AP, Guo W,
Yang J, Yuan Y, Zhu L, Qin S, Xiang S, et al: Subcutaneous
envafolimab monotherapy in patients with advanced defective
mismatch repair/microsatellite instability high solid tumors. J
Hematol Oncol. 14:952021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moga C, Guo B, Schopflocher D and Harstall
C: Development of a quality appraisal tool for case series studies
using a modified delphi technique. 2012.
|
|
12
|
Shimizu T, Nakajima TE, Yamamoto N,
Yonemori K, Koyama T, Kondo S, Sunakawa Y, Izawa N, Horie Y, Xiang
S, et al: Phase I study of envafolimab (KN035), a novel
subcutaneous single-domain anti-PD-L1 monoclonal antibody, in
Japanese patients with advanced solid tumors. Invest New Drugs.
40:1021–1031. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Diaz LA Jr, Shiu KK, Kim TW, Jensen BV,
Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs
P, et al: Pembrolizumab versus chemotherapy for microsatellite
instability-high or mismatch repair-deficient metastatic colorectal
cancer (KEYNOTE-177): Final analysis of a randomised, open-label,
phase 3 study. Lancet Oncol. 23:659–670. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marabelle A, Le DT, Ascierto PA, Di
Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M,
Penel N, Hansen AR, et al: Efficacy of pembrolizumab in patients
with noncolorectal high microsatellite instability/mismatch
repair-deficient cancer: Results from the phase II KEYNOTE-158
study. J Clin Oncol. 38:1–10. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Overman MJ, McDermott R, Leach JL, Lonardi
S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, et al:
Nivolumab in patients with metastatic DNA mismatch repair-deficient
or microsatellite instability-high colorectal cancer (CheckMate
142): An open-label, multicentre, phase 2 study. Lancet Oncol.
18:1182–1191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chongqing T, Sini L, Xiaohui Z, Liubao P,
Ye P, Shuxia Q, Liting W, Meiyu W and Xiaomin W: Cost-effectiveness
of first-line versus second-line pembrolizumab or chemotherapy in
patients with microsatellite-instability-high/mismatch
repair-deficient advanced colorectal cancer. Front Pharmacol.
12:8029422021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu T, Liu S, Guan S, Tai Y, Jin Y and
Dong M: Cost-effectiveness analysis of pembrolizumab versus
chemotherapy for microsatellite instability-high or mismatch
repair-deficient metastatic colorectal cancer. J Chemother. Jan
2–2023.(Epub ahead of print). View Article : Google Scholar
|
|
18
|
Shu Y, Ding Y and Zhang Q:
Cost-effectiveness of nivolumab plus chemotherapy vs chemotherapy
as first-line treatment for advanced gastric
cancer/gastroesophageal junction cancer/esophagel adenocarcinoma in
China. Front Oncol. 12:8515222022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salem ME, Bodor JN, Puccini A, Xiu J,
Goldberg RM, Grothey A, Korn WM, Shields AF, Worrilow WM, Kim ES,
et al: Relationship between MLH1, PMS2, MSH2 and MSH6 gene-specific
alterations and tumor mutational burden in 1057 microsatellite
instability-high solid tumors. Int J Cancer. 147:2948–2956. 2020.
View Article : Google Scholar : PubMed/NCBI
|