Introduction
Immune checkpoint inhibitors of programmed cell
death receptor-1 (PD-1) and programmed cell death ligand-1 (PD-L1)
showed promising efficacy in a variety of malignancies (1). In recent years, the blockade of the
PD-1/PD-L1 pathway with monoclonal antibodies emerged as a
successful target for cancer immunotherapy. At present, several
PD-1/PD-L1 inhibitors are approved worldwide for the treatment of
multiple tumors, leading to a paradigm shift in the treatment of
immuno-oncology therapies that provide durable remissions for
patients with cancer (1). In
particular, PD-1/PD-L1 inhibitors are also used for the treatment
of tumors with high microsatellite instability (MSI-H). This type
of tumor is characterized by deficient mismatch repair (dMMR),
which results in microsatellite instability. In the study performed
by Le et al (2), the
mismatch repair state predicted a clinical benefit of immune
checkpoint blockade therapy with pembrolizumab. Usually, MSI-H
tumors are sensitive to PD-1/PD-L1 blockade.
Envafolimab is a novel recombinant protein of a
humanized single-domain anti-PD-L1 antibody fused with a human IgG1
crystallizable fragment formulated for subcutaneous (SC) injection
(3). The molecular weight of
envafolimab is half that of conventional antibodies. It also shows
fast tumor enrichment, high tissue penetration efficiency,
stability and water solubility (4).
These features provide a theoretical basis for the use of
envafolimab SC injection, making it different from the previously
approved PD-1/PD-L1 inhibitors, which were all administered as an
intravenous infusion. Patients can achieve long-term survival after
receiving drugs such as pembrolizumab; nonetheless, these drugs
still need to be infused in the hospital during the maintenance
phase (5,6). In a context where Corona Virus Disease
2019 is rampant, the shortage of medical resources is a worldwide
concern. Oncology associations and specialists from countries such
as China and Italy recommended that patients should experience the
pandemic with minimal risk to their health. It is recommended that
patients are treated at home to not visit the hospital (7). However, it is difficult to provide
standardized treatments for this group of patients. In this regard,
envafolimab can be used at home under the guidance of a physician
and is more affordable compared to other drugs.
In November 2021, the National Medical Products
Administration (China) approved the marketing of envafolimab
injection (8). Envafolimab became
the first domestic PD-L1 inhibitor approved in China and the first
subcutaneously injectable PD-L1 inhibitor worldwide (8). Although some studies showed that
envafolimab was effective in the treatment of advanced dMMR/MSI-H
solid tumors, it should be highlighted that the studies on the
mechanism of action and clinical trials are currently limited; for
example, only two phase II clinical trials have been completed
(9,10). Therefore further studies are needed.
To the best of our knowledge, there are no systematic meta-analyses
on the use of envafolimab for the treatment of advanced dMMR/MSI-H
solid tumors. Therefore, the present study aimed to perform a
meta-analysis of all the data collected from single-arm trials
using envafolimab for advanced dMMR/MSI-H solid tumors to evaluate
its efficacy and safety.
Materials and methods
Search strategy
A systematic search to retrieve published literature
from PubMed, Web of Science, Cochrane Library, China National
Knowledge Infra-structure and Wan Fang databases was performed from
initiation to October 1, 2022. There was no language restriction in
the present meta-analysis. The keywords searched were ‘envafolimab’
or ‘KN035’.
Selection of the studies
The inclusion criteria were as follows: i) Study
participants were patients with advanced dMMR/MSI-H solid tumors;
ii) patients were diagnosed with MSI-H or dMMR; iii) the age of the
participants and the dosage of the treatment medications were
reported; and iv) studies reporting patients with Eastern
Cooperative Oncology Group performance status of 0 or 1.
The exclusion criteria were as follows: i) Case
report studies; ii) duplicated studies; and iii) studies for which
data could not be extracted separately.
Two reviewers independently screened the titles and
abstracts of all retrieved studies according to the search
strategy. Studies that did not conform to the inclusion criteria
were excluded. Discrepancies were resolved by consensus through
negotiation, with a third reviewer ruling when disputes arose. Two
independent reviewers assessed the final set of articles'
characteristics of the included studies that are summarized as
follows: First author name; year of publication; study type; the
number of cases; patient age; envafolimab dosages; and outcome
parameters.
Quality assessment
The Canadian Institute of Health Economics (IHE)
(11) quality assessment tool was
used to assess the quality of single-arm studies. The list gives
corresponding options for each item to enhance the objectivity of
scoring. Following the IHE recommendation, meeting ≥14 (>70%) of
the 20 items indicated an acceptable quality.
Data extraction and analysis
Clinical outcomes included objective remission rate
(ORR), disease control rate (DCR), median progression-free survival
(mPFS), median overall survival (mOS) and adverse reactions. All
adverse reactions were classified into grades ‘any’ and ‘3–4’.
Joint analysis was performed using STATA 14.0 software (StataCorp
LP). The heterogeneity of the data was quantified using the
I2 statistics. I2≥50% was considered to
indicate a significant heterogeneity. For I2≥50%, the
combined proportion and 95% CI for the outcomes of interest were
calculated using the random effects model, whereas the fixed
effects model was used for I2<50%. The heterogeneity
of results was reduced through sensitivity analysis using a
one-by-one elimination method and subgroup analysis according to
different trial stages. P<0.05 was considered to indicate a
statistically significant difference.
Results
Search results
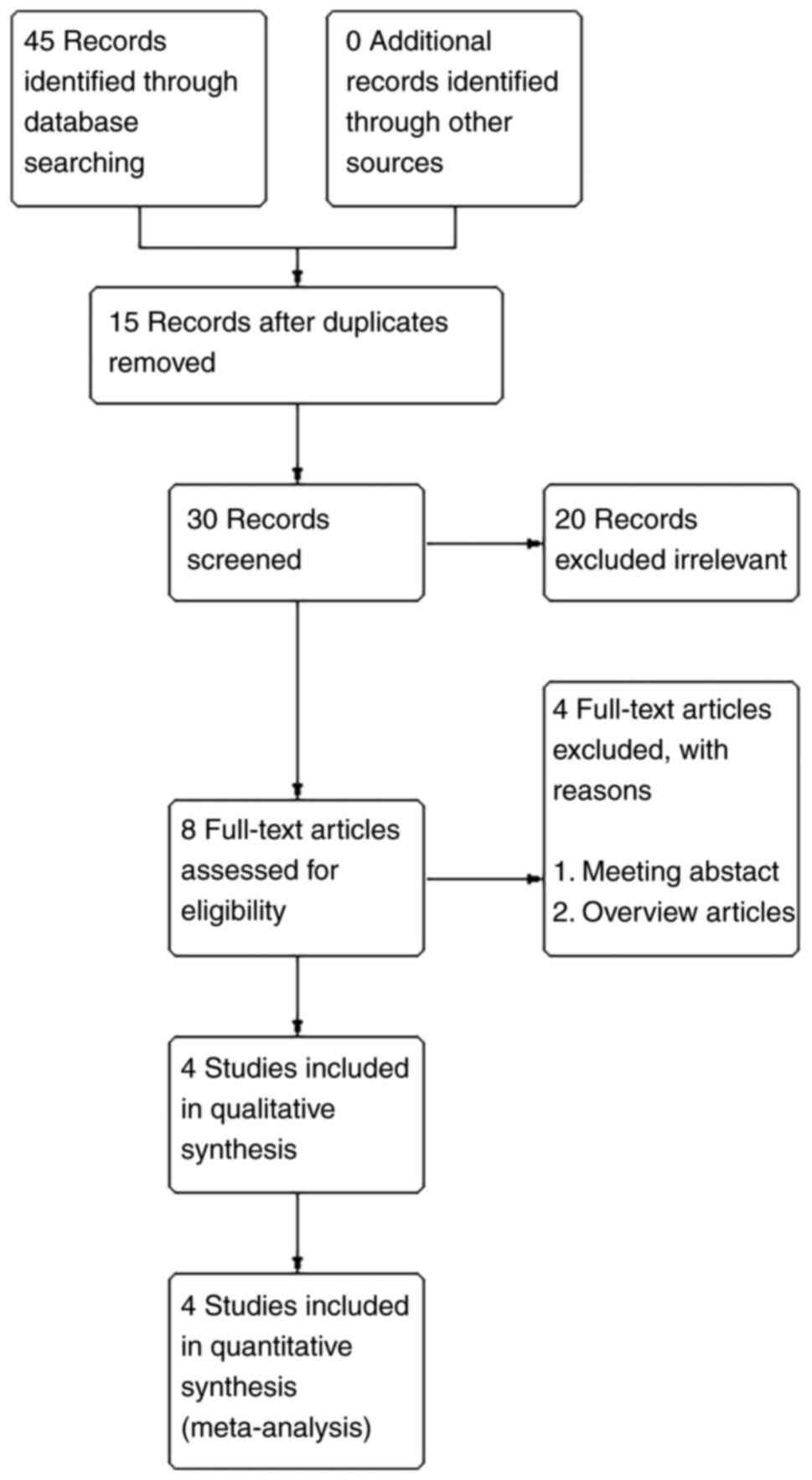
A total of 45 articles were retrieved, of which 15
were duplicates. After reading the title and abstract, 22 articles
were excluded because not relevant to the aim of the present
meta-analysis. By reading the full text of the remaining
literature, four studies (3,9,10,12)
were finally included according to the inclusion and exclusion
criteria (Fig. 1). As shown in
Table I, all articles meet ≥70% of
the items according to the IHE quality assessment tool.
 | Table I.Quality evaluation of the article of
included studies using IHE case series quality assessment tool. |
Table I.
Quality evaluation of the article of
included studies using IHE case series quality assessment tool.
|
| First author/s,
year |
|---|
|
|
|
|---|
| IHE items | Liu et al,
2022 | Li et al,
2021 | Papadopoulos et
al, 2021 | Shimizu et al,
2022 |
|---|
| (1) | 1 | 1 | 1 | 1 |
| (2) | 1 | 1 | 1 | 1 |
| (3) | 1 | 1 | 0 | 1 |
| (4) | 1 | 1 | 0 | 1 |
| (5) | 1 | 1 | 0 | 1 |
| (6) | 1 | 1 | 1 | 1 |
| (7) | 1 | 1 | 1 | 1 |
| (8) | 1 | 0 | 0 | 0 |
| (9) | 1 | 1 | 1 | 1 |
| (10) | 1 | 1 | 1 | 1 |
| (11) | 0 | 0 | 0 | 0 |
| (12) | 1 | 1 | 1 | 1 |
| (13) | 1 | 1 | 1 | 1 |
| (14) | 1 | 1 | 1 | 1 |
| (15) | 1 | 1 | 1 | 1 |
| (16) | 1 | 1 | 1 | 1 |
| (17) | 1 | 1 | 1 | 1 |
| (18) | 0 | 0 | 1 | 1 |
| (19) | 1 | 1 | 1 | 1 |
| (20) | 0 | 1 | 0 | 0 |
| Total score | 17 | 17 | 14 | 17 |
| (Refs.) | (9) | (10) | (3) | (12) |
All four eligible articles were single-arm studies,
including two phase I and two phase II clinical trials.
Finally, a total of 181 patients with advanced
dMMR/MSI-H solid tumors were included in the present meta-analysis.
The characteristics of all patients are shown in Table II, while the distribution of the
tumor types is shown in Table
III.
 | Table II.Study information and patient
characteristics. |
Table II.
Study information and patient
characteristics.
|
|
|
|
| ECOG PS, n |
|
|
|
|---|
| First author/s,
year | Research type | Number of patients
(male/female) | Age (range),
years |
| Envafolimab
treatment regimen | Research
quality | (Refs.) |
|---|
| 0 | 1 |
|---|
| Liu et al,
2022 | Phase II clinical
trial | 15 (11/4) | 56 (31–66) | 3 | 12 | 5 mg/kg Q2W and
mFOLFOX6 | 17 | (9) |
| Li et al,
2021 | Phase II clinical
trial | 103 (65/38) | 53(22–77) | 27 | 76 | 150 mg QW | 17 | (10) |
| Papadopoulos et
al, 2021 | Phase I clinical
trial | 28 (20/8) | 66(35–79) | 6 | 22 | 0.01, 0.03, 0.1,
0.3, 1.0, 2.5, 5, 10 mg/kg QW increasing to 300 mg Q4W | 14 | (3) |
| Shimizu et
al, 2022 | Phase I clinical
trial | 35 (15/20) | 65 (31–78) | 17 | 18 | 1.0 mg/kg QW | 17 | (12) |
|
|
|
|
|
|
| 2.5 mg/kg QW |
|
|
|
|
|
|
|
|
| 5.0 mg/kg QW |
|
|
|
|
|
|
|
|
| 2.5 mg/kg Q2W |
|
|
|
|
|
|
|
|
| 5.0 mg/kg Q2W |
|
|
|
|
|
|
|
|
| 300 mg Q4W |
|
|
 | Table III.Distribution of tumor types. |
Table III.
Distribution of tumor types.
|
| First author/s,
year |
|---|
|
|
|
|---|
| Type of cancer
diagnosis | Liu et al,
2022 | Li et al,
2021 | Papadopoulos et
al, 2021 | Shimizu et
al, 2022 |
|---|
| Prostate cancer,
n | - | 1 | 6 | - |
| Colorectal cancer,
n | - | 65 | 5 | 3 |
| Intraheptic biliary
tract cancer, n | - | - | 3 | - |
| Non-small cell lung
cancer, n | - | 1 | 2 | - |
| Breast cancer,
n | - | - | 2 | - |
| Cervical cancer,
n | - | 1 | 2 | 2 |
| Bladder cancer,
n | - | 1 | 1 | - |
| Esophageal cancer,
n | - | 1 | 1 | 5 |
| Head and neck
cancer, n | - | - | 1 | - |
| Liver cancer,
n | - | 4 | 1 | 3 |
| Melanoma, n | - | - | 1 | - |
| Neuroendocrine
tumor, n | - | - | 1 | 2 |
| Gastrointestinal
stromal tumor, n | - | - | 1 | 1 |
| Pancreatic cancer,
n | - | - | 1 | 3 |
| Gastric cancer,
n | 12 | 18 | - | - |
| Esophagogastric
junction cancer, n | 3 | - | - | - |
| Endometrial cancer,
n | - | 6 | - | - |
| Cholangiocarcinoma,
n | - | 1 | - | 1 |
| Osteosarcoma,
n | - | 1 | - | - |
| Renal pelvic
carcinoma, n | - | 1 | - | 2 |
| Urothelial
carcinoma, n | - | 1 | - | 2 |
| Uterine sarcoma,
n | - | 1 | - | - |
| Soft tissue
sarcoma, n | - | - | - | 3 |
| Ovarian epithelial
cancer, n | - | - | - | 2 |
| Ovarian granular
cell tumor, n | - | - | - | 1 |
| Carcinoma of the
appendix, n | - | - | - | 1 |
| Duodenal cancer,
n | - | - | - | 1 |
| Gallbladder
carcinoma, n | - | - | - | 1 |
| Penis carcinoma,
n | - | - | - | 1 |
| Thymic
adenocarcinoma, n | - | - | - | 1 |
| Peritoneal
carcinoma, n | - | - | - | 1 |
| Carcinoma of
unknown primary focus, n | - | - | - | 1 |
| Total, n | 15 | 103 | 28 | 35 |
| (Refs.) | (9) | (10) | (3) | (12) |
Efficacy assessment
ORR
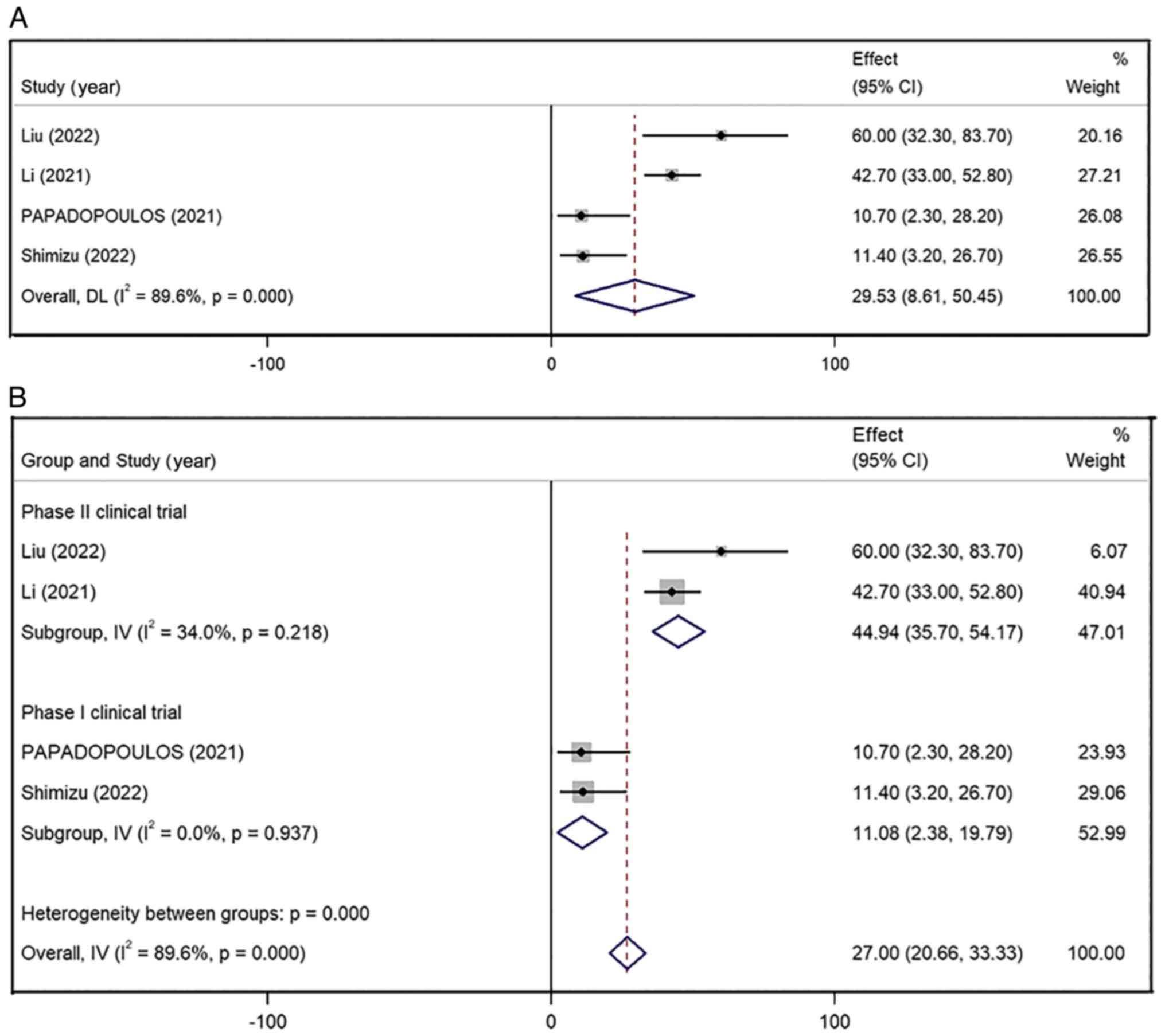
All four studies reported ORR data and the combined
ORR for all the included patients was 29.53% (95% CI, 8.61–50.45%),
with high inter-study heterogeneity (I2=89.6%; P=0.00)
(Fig. 2A). Due to the high
heterogeneity, these data were analyzed using the random effects
model. These four studies had biased data that did not lend
themselves to sensitivity analysis, so a subgroup analysis was
performed. After classifying the articles according to the type of
research design (type I or type II clinical trials), the pooled ORR
of the phase II clinical trial group (44.94%; 95% CI, 35.70–54.17%;
I2=34.0%; P=0.218) was higher than that in the phase I
clinical trial group (11.08%; 95% CI, 2.38–19.79;
I2=0.0%; P=0.937) (Fig.
2B). In addition, there was no significant heterogeneity in
either group of data, which was statistically significant (phase I
clinical trial, P<0.001; phase II clinical trial, P=0.013;
Overall, P=0.006).
DCR
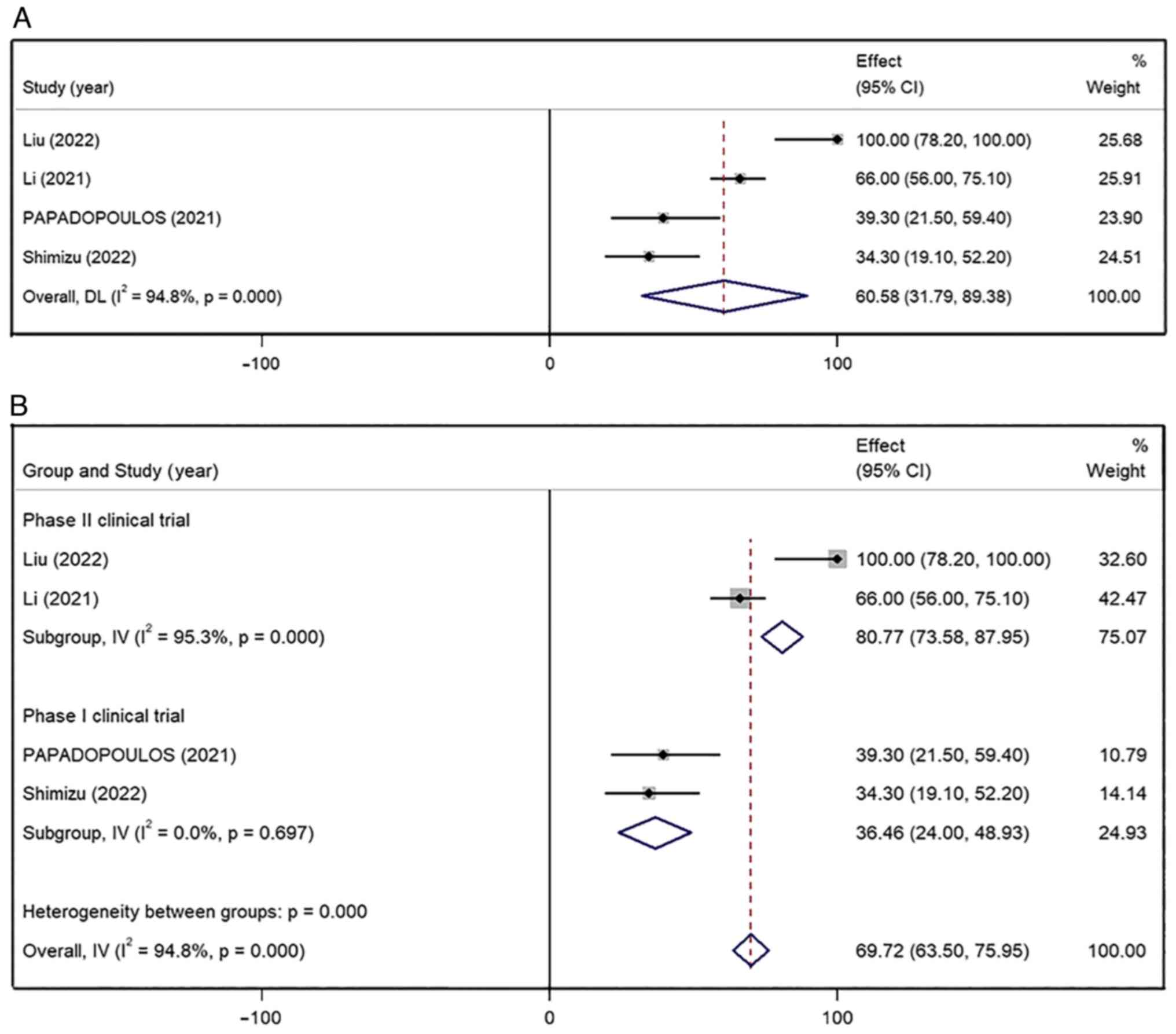
All four studies reported the DCR and the combined
result was 60.58% (95% CI, 31.79–89.38%), with high inter-study
heterogeneity (I2=94.8%; P<0.001) (Fig. 3A). The DCR of phase II clinical
trial group (80.77%; 95% CI, 73.58–87.95%; I2=95.3%;
P<0.001) was higher than that in phase I clinical trial group
(36.46%; 95% CI, 24.00–48.93%; I2=0.0%; P=0.697).
Nonetheless, the heterogeneity remained significantly higher in the
phase II clinical trial group after subgroup analysis
(I2=95.3%; P=0.00) (Fig.
3B). In the study by Liu et al (9), patients with gastric and esophageal
cancer were treated with a combination of envafolimab and mFOLFOX6
chemotherapy, whereas in Li et al (10), patients with common solid tumors
were treated with envafolimab alone. These two studies included
patients with different cancer types and this difference may
explain the significant heterogeneity. A sensitivity analysis was
also conducted by combining the other studies and removing one
study at a time to evaluate if the results were significantly
influenced by that specific study. The heterogeneity of the
combined DCR did not show significant fluctuation upon the removal
of one study at the time. The sensitivity analysis confirmed the
stability and statistical significance of the results.
mPFS
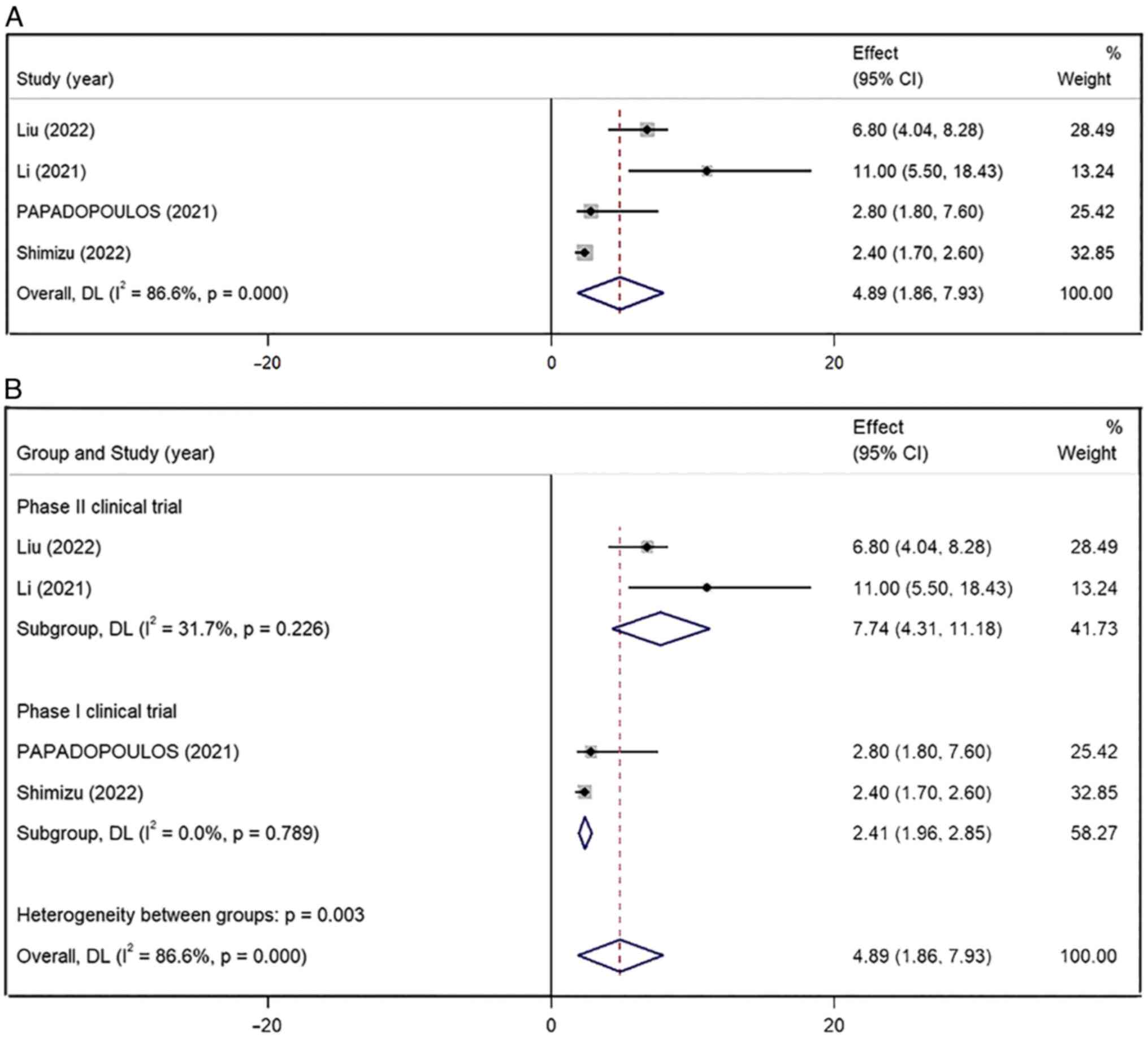
The pooled mPFS of the four included studies was
4.89 months (95% CI, 1.86–7.93) and showed a high level of
inter-study heterogeneity (I2=86.6%; P<0.001)
(Fig. 4A). After classifying the
studies according to their design, the pooled mPFS of phase II
clinical trial group (7.74 months; 95% CI, 4.31–11.18 months;
I2=31.7%; P=0.226) was longer than that in the phase I
clinical trial group (2.41 months; 95% CI, 1.96–2.85 months;
I2=0.0%; P=0.789) (Fig.
4B). The subgroup analysis based on the research design
indicated a significantly lower heterogeneity.
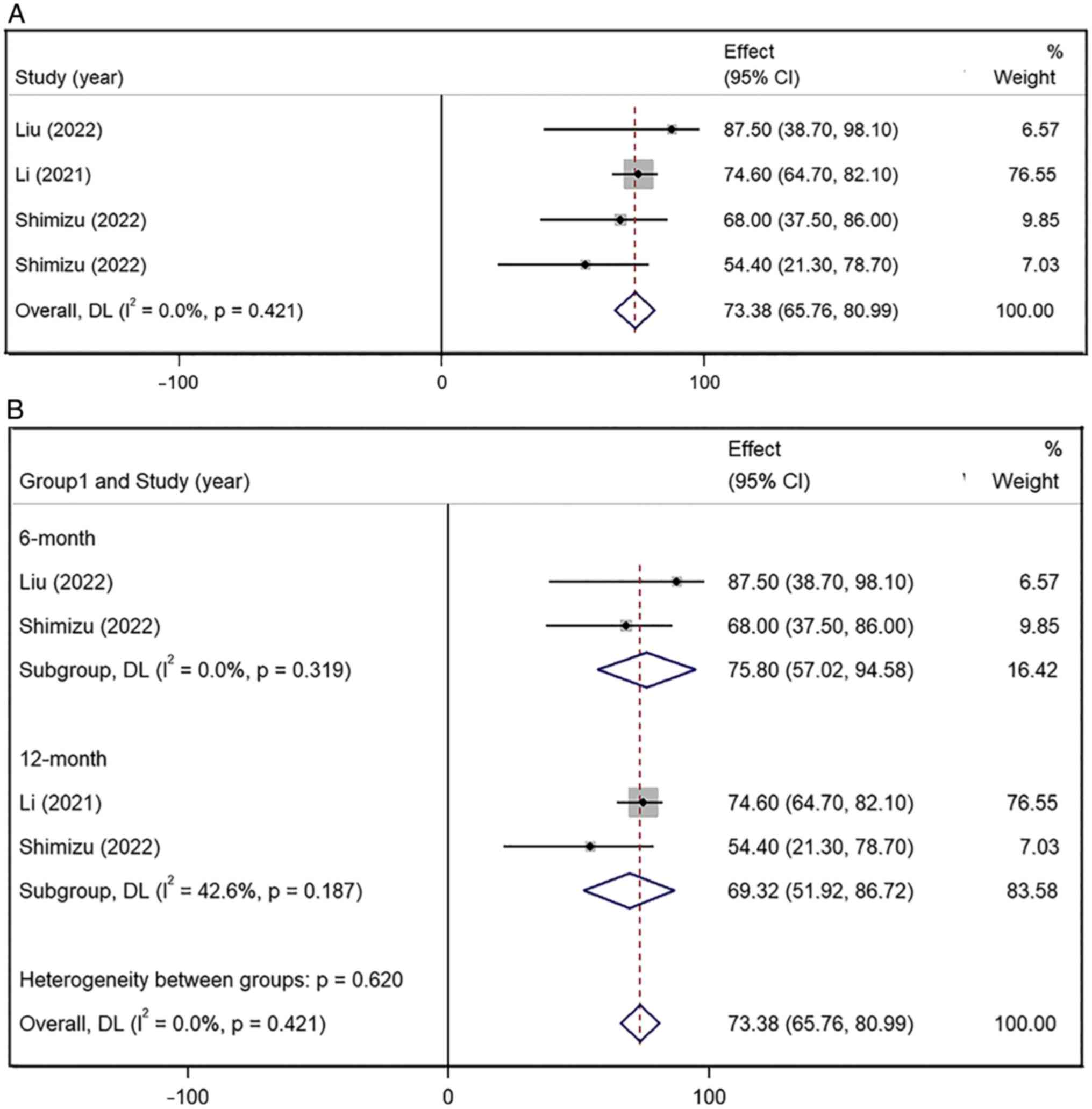
6- and 12-month OS rates
Only one of the four studies reported the mOS and
the other three studies only provided the 6- and 12-month OS rates
(9,10,12).
The pooled OS rate of the latter three studies was 73.38% (95% CI,
65.76–80.99%), with lower inter-study heterogeneity
(I2=0.0%; P=0.421) (Fig.
5A). The pooled 6-month OS rate (75.80%, 95% CI, 57.02–94.58%;
I2=0.0%; P=0.319) was higher than the 12-month OS rate
(69.32%; 95% CI, 51.92–86.72%; I2=42.6%; P=0.187)
(Fig. 5B). The results were
statistically significant.
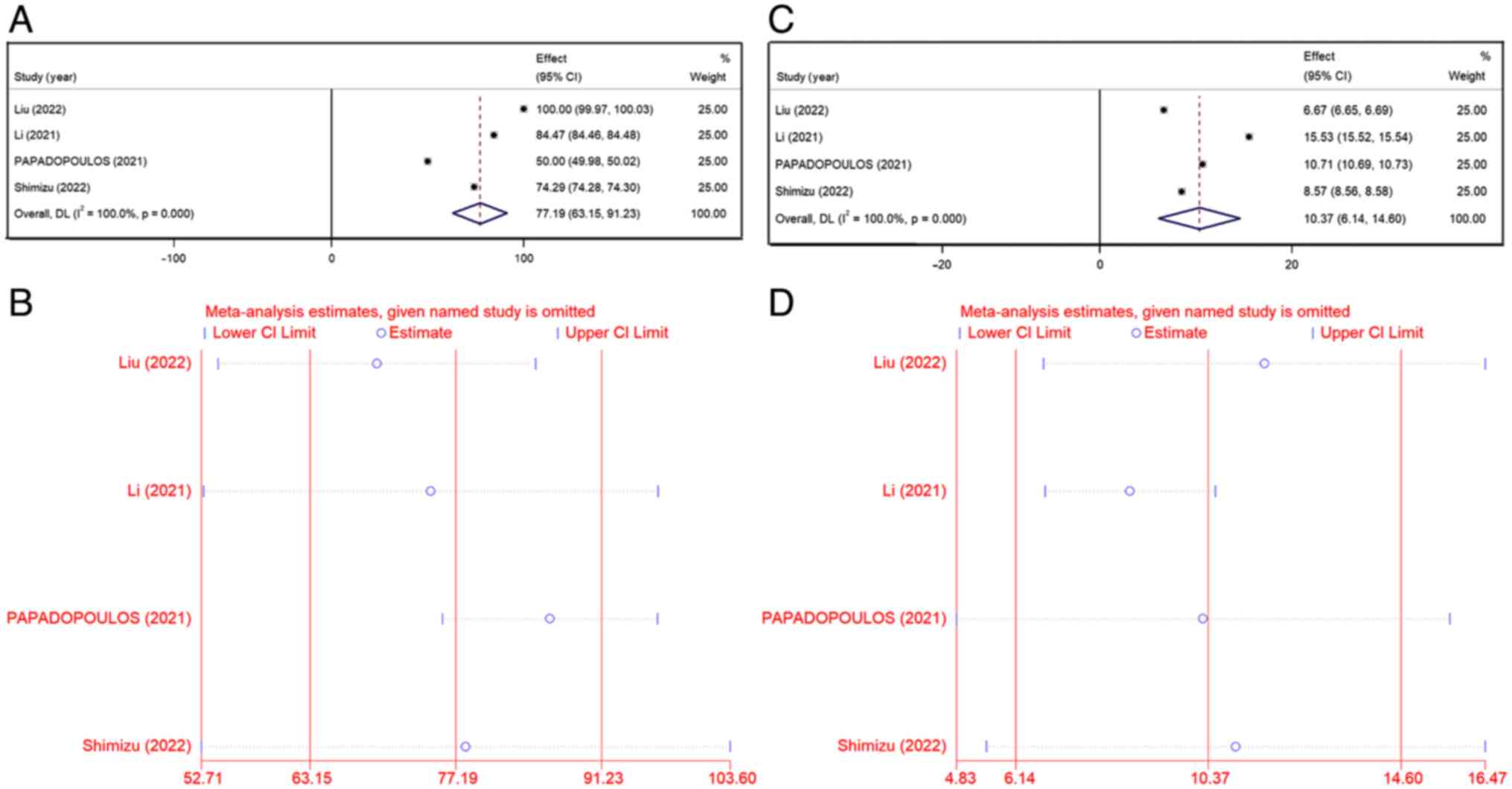
TEAEs
All the studies reported TEAEs in patients with
advanced dMMR/MSI-H solid tumors treated with envafolimab, mostly
grade 1/2 with a few 3/4 grades. Common adverse reactions were
decreased white blood cell count, decreased neutrophil count,
hypothyroidism, anemia, alanine aminotransferase, weakness,
diarrhea and injection site reactions. The combined data from all
studies on the rate of any grade TEAEs was 77.19% (95% CI,
63.15–91.23%), with higher inter-study heterogeneity
(I2=100%; P<0.001) (Fig.
6A). The heterogeneity did not show any significant fluctuation
by removing one study at a time (Fig.
6B) and the results were stable. The grade 3/4 TEAEs rate was
10.37% (95% CI, 6.14–14.60%; I2=100%; P<0.001)
(Fig. 6C). Sensitivity analysis
showed that these values decreased significantly after the
exclusion of the study by Li et al (10). The rate of grade 3/4 TEAEs for the
other studies was 8.65% (95% CI, 6.78–10.52%) (Fig. 6D). The study by Li et al
(10) was a phase II clinical trial
in which a uniform drug dose of 150 mg envafolimab once weekly was
administered and patients showed significantly improved mPFS (11
months) and 12-month OS rate (74.6%) compared with those reported
in the other studies. However, patients were treated continuously
with a higher dose of envafolimab for a longer time than in other
studies and this might have contributed to the higher drug
toxicity.
Discussion
All four eligible articles included in the present
meta-analysis were single-arm studies, including two phase I and
two phase II clinical trials. The results of the present study
demonstrated good outcomes and manageable adverse effects of
envafolimab treatment in patients with dMMR/MSI-H advanced solid
tumors. The rate of TEAEs was similar to that expected for other
anti-PD-L1 monoclonal antibodies (13–15).
The main TEAEs were decreased white blood cell count, decreased
neutrophil count, hypothyroidism, anemia, alanine aminotransferase
and weakness. The combined TEAEs incidence was 97.60% (95% CI,
95.14–100.07%). The majority of TEAEs were grade 1/2 adverse
reactions, with a small number of TEAEs being grade 3/4 (10.37%;
95% CI, 6.14–14.60%). The number of patients requiring treatment
discontinuation due to TEAEs was 20 (19%) (10), 1 (3.6%) (3) and 4 (11.4%) (12). In addition, immune-related adverse
reactions of all grades were reported by Li et al (10) and Shimizu et al (12). The rates of adverse reactions to the
injection site specific for envafolimab were 9% (9/103) and 14.3%
(5/35), respectively, all of which were grade 1–2. However, the
vast majority of these TEAEs were correctable and no cases of
immune-associated pneumonia were reported in the aforementioned
studies.
The objective remission rates in the present
meta-analysis (29.53%; 95% CI, 8.61–50.45%) were similar to other
anti-PD-1/PD-L1 antibodies in patients with previously treated
advanced dMMR/MSI-H solid tumors (13–15).
The pooled mPFS was 4.89 months (95% CI, 1.86–7.93 months). In the
subgroup analysis, the pooled mPFS data from the phase II clinical
trials were improved, reaching 7.74 months (95% CI, 4.31–11.18
months). In the phase II clinical trial KEYNOTE-158 (14), the ORR for pembrolizumab in
noncolorectal cancer was 34.3% (95% CI, 28.3–40.8%) and mPFS was
4.1 months (95% CI, 2.4–4.9 months). In the phase III clinical
trial KEYNOTE-177 (13), which
included only Asian patients, the ORR for pembrolizumab in
metastatic colorectal cancer was 50% (95% CI, 28–72%) and mPFS was
not reached (NR) (95% CI, 1.9-NR). In a multi-country, multi-center
phase II trial (15), the ORR for
patients with dMMR/MSI-H colorectal cancer treated with nivolumab
was 31.1% (95% CI, 20.8–42.9%). In the latter study, the 12-month
PFS rate was 50.4% (95% CI, 38.1–61.4%) and the mPFS was not
reached. It can be concluded that envafolimab achieved therapeutic
effects similar to pembrolizumab and nivolumab.
Although PD-L1 inhibitors were shown to extend the
patient's survival, their cost-effectiveness should also be
considered. In the United States, the use of pembrolizumab as
first-line treatment for MSI-H/dMMR advanced colorectal cancer
strategy generated an incremental cost of $50,613.7 compared with
that associated with chemotherapy, resulting in an incremental
cost-benefit ratio (ICER) of $13,441 per quality life adjustment
year (QALY) (16). In China, the
pembrolizumab strategy yielded an incremental cost of $16,032.57,
resulting in an ICER of $8,285 per QALY (17). Due to the high costs of nivolumab,
nivolumab plus chemotherapy was not a cost-effective treatment
strategy. The incremental effectiveness and cost of nivolumab plus
chemotherapy vs. chemotherapy first-line therapy in patients with
advanced gastric cancer/gastroesophageal junction cancer/esophageal
adenocarcinoma were 0.28 QALYs and $78,626.53, resulting in an ICER
of $278,658.71/QALY (18). At
present, the price of 200 mg of envafolimab in China is $865.74.
Currently, there is a charity drug donation project (Beijing Health
Alliance Charitable Foundation) and the total cost for 2 years
after charity drug donation is $10,359.96, while the average annual
cost is $5,179.98. In terms of cost-effectiveness, envafolimab has
more advantages than pembrolizumab and nivolumab.
Although the SC injection of envafolimab showed good
therapeutic efficacy and was safe and controlled, there is a lack
of randomized controlled trials as only four papers were retrieved
and included in the present meta-analysis, all of which were
single-arm trials. Of these studies, two were phase I clinical
studies, resulting in a higher heterogeneity and biased data in the
combined results. The small number of patients enrolled in the
studies included in the present meta-analysis [excluding the study
by Li et al (10)] and the
fact that the majority of cases were colorectal, gastric and
esophageal cancers, limited the present results; therefore the
current conclusions have to be interpreted with caution.
MSI-H and tumor mutational burden (TMB) are
predictive biomarkers for immune-checkpoint inhibitors. Among
tumors assessed by immunohistochemistry, loss of co-expression of
MLH1/PMS2 was more common than loss of MSH2/MSH6, and was
associated with lower mean TMB (19). Moreover, the four included articles
did not mention the four MMR mutations and their effect on the
efficacy of envafolimab. Further literature searches did not find
other relevant studies. Therefore, the lack of data regarding the
four MMR mutations is a factor limiting the result and envafolimab
should still be used with caution. The data for the mOS were not
available for the present meta-analysis and further refinement is
needed when more studies are published. The following ongoing
clinical trials can currently be accessed: i) Envafolimab And
Envafolimab With Ipilimumab In Patients With Undifferentiated
Pleomorphic Sarcoma Or Myxofibrosarcoma (NCT04480502); ii)
Multicenter Phase 2 Study of Envafolimab in Biliary Tract Cancers
(NCT04910386); iii) Effect and Safety of Envafolimab Combined With
Endostar/S-1 in Second-line of Advanced Non-small Cell Lung Cancer
(NCT05529355). More refined experimental data will become available
when the results of these studies will. be published.
The current meta-analysis provided a pioneering
systematic review of the efficacy and safety of envafolimab for the
treatment of advanced solid tumors. Compared with the pembrolizumab
and nivolumab interventions, envafolimab showed competitive
efficacy with similar mPFS, objective remission rates and incidence
of TEAEs. It is also worth mentioning that envafolimab is the first
single-domain PD-L1 targeting antibody to be administered
subcutaneously for the treatment of advanced solid tumors, making
it more convenient and facilitating patient compliance. Therefore,
envafolimab has a promising application in clinical practice.
Acknowledgements
The authors would like to thank Dr Longmei Tang
(Hebei Medical University, Shijiazhuang, China) for providing
guidance on statistical methods and Dr Liang Ce (Hebei Medical
University) for providing pharmacological guidance and medical
writing support.
Funding
Funding: No funding was received.
Availability of data and materials
The data used to support the findings of this study
are available from the corresponding author upon request.
Authors' contributions
SF and GW conceived and designed the study. SF and
GW prepared the manuscript with comments from all authors. SF and
CG searched, screened and extracted the data from the literature.
SF and CG were responsible for data analysis and manuscript
writing. BL performed the statistical analysis, supervised the
study and edited the manuscript. All authors have read and approved
the final version of the manuscript. SF and GW confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Das S and Johnson DB: Immune-related
adverse events and anti-tumor efficacy of immune checkpoint
inhibitors. J Immunother Cancer. 7:3062019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Le DT, Durham JN, Smith KN, Wang H,
Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et
al: Mismatch repair deficiency predicts response of solid tumors to
PD-1 blockade. Science. 357:409–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Papadopoulos KP, Harb W, Peer CJ, Hua Q,
Xu S, Lu H, Lu N, He Y, Xu T, Dong R, et al: First-in-human phase I
study of envafolimab, a novel subcutaneous single-domain anti-PD-L1
antibody, in patients with advanced solid tumors. Oncologist.
26:e1514–e1525. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Markham A: Envafolimab: First approval.
Drugs. 82:235–240. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Machiels JP, Tao Y, Burtness B, Tahara M,
Licitra L, Rischin D, Waldron J, Simon C, Gregoire V, Harrington K,
et al: Pembrolizumab given concomitantly with chemoradiation and as
maintenance therapy for locally advanced head and neck squamous
cell carcinoma: KEYNOTE-412. Future Oncol. 16:1235–1243. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Galsky MD, Mortazavi A, Milowsky MI,
George S, Gupta S, Fleming MT, Dang LH, Geynisman DM, Walling R,
Alter RS, et al: Randomized double-blind phase II study of
maintenance pembrolizumab versus placebo after first-line
chemotherapy in patients with metastatic urothelial cancer. J Clin
Oncol. 38:1797–1806. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ömeroğlu Şimşek G: Lung cancer management
in COVID-19 pandemic. Turk Thorac J. 21:340–344. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
National Medical Products Administration
(NMPA), . Envafolimab Injection. Full prescribing information.
NMPA; Beijing: 2021
|
|
9
|
Liu R, Yin X, Deng Y, Xu N, Xiang S, Zhang
Y, Gong Y, Liu D and Xu J: Safety and efficacy of envafolimab
combined with FOLFOX as first-line treatment in patients with
locally advanced or metastatic gastric/gastroesophageal junction
adenocarcinoma in a phase II clinical trial. Chin J New Drugs.
031:1502–1508. 2022.(In Chinese).
|
|
10
|
Li J, Deng Y, Zhang W, Zhou AP, Guo W,
Yang J, Yuan Y, Zhu L, Qin S, Xiang S, et al: Subcutaneous
envafolimab monotherapy in patients with advanced defective
mismatch repair/microsatellite instability high solid tumors. J
Hematol Oncol. 14:952021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moga C, Guo B, Schopflocher D and Harstall
C: Development of a quality appraisal tool for case series studies
using a modified delphi technique. 2012.
|
|
12
|
Shimizu T, Nakajima TE, Yamamoto N,
Yonemori K, Koyama T, Kondo S, Sunakawa Y, Izawa N, Horie Y, Xiang
S, et al: Phase I study of envafolimab (KN035), a novel
subcutaneous single-domain anti-PD-L1 monoclonal antibody, in
Japanese patients with advanced solid tumors. Invest New Drugs.
40:1021–1031. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Diaz LA Jr, Shiu KK, Kim TW, Jensen BV,
Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs
P, et al: Pembrolizumab versus chemotherapy for microsatellite
instability-high or mismatch repair-deficient metastatic colorectal
cancer (KEYNOTE-177): Final analysis of a randomised, open-label,
phase 3 study. Lancet Oncol. 23:659–670. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marabelle A, Le DT, Ascierto PA, Di
Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M,
Penel N, Hansen AR, et al: Efficacy of pembrolizumab in patients
with noncolorectal high microsatellite instability/mismatch
repair-deficient cancer: Results from the phase II KEYNOTE-158
study. J Clin Oncol. 38:1–10. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Overman MJ, McDermott R, Leach JL, Lonardi
S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, et al:
Nivolumab in patients with metastatic DNA mismatch repair-deficient
or microsatellite instability-high colorectal cancer (CheckMate
142): An open-label, multicentre, phase 2 study. Lancet Oncol.
18:1182–1191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chongqing T, Sini L, Xiaohui Z, Liubao P,
Ye P, Shuxia Q, Liting W, Meiyu W and Xiaomin W: Cost-effectiveness
of first-line versus second-line pembrolizumab or chemotherapy in
patients with microsatellite-instability-high/mismatch
repair-deficient advanced colorectal cancer. Front Pharmacol.
12:8029422021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu T, Liu S, Guan S, Tai Y, Jin Y and
Dong M: Cost-effectiveness analysis of pembrolizumab versus
chemotherapy for microsatellite instability-high or mismatch
repair-deficient metastatic colorectal cancer. J Chemother. Jan
2–2023.(Epub ahead of print). View Article : Google Scholar
|
|
18
|
Shu Y, Ding Y and Zhang Q:
Cost-effectiveness of nivolumab plus chemotherapy vs chemotherapy
as first-line treatment for advanced gastric
cancer/gastroesophageal junction cancer/esophagel adenocarcinoma in
China. Front Oncol. 12:8515222022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salem ME, Bodor JN, Puccini A, Xiu J,
Goldberg RM, Grothey A, Korn WM, Shields AF, Worrilow WM, Kim ES,
et al: Relationship between MLH1, PMS2, MSH2 and MSH6 gene-specific
alterations and tumor mutational burden in 1057 microsatellite
instability-high solid tumors. Int J Cancer. 147:2948–2956. 2020.
View Article : Google Scholar : PubMed/NCBI
|