Introduction
Thyroid cancer is the most common endocrine
malignancy and the fastest increasing cancer worldwide (1). Despite this, the mortality rate has
increased only slightly and has ranged from 0.4 to 0.5 per 100,000
people per year since 1980 (1).
Thyroid cancer is classified based on their cellular origin. Those
that originate from follicular cells comprise follicular thyroid
cancer, papillary thyroid cancer (PTC) and anaplastic thyroid
cancer (1). Medullary thyroid
cancer (MTC) develops from neuroendocrine parafollicular C cells
(1). PTC accounts for ~85% of the
total number of thyroid cancer cases (1). MTC is a rare type of cancer that
accounts for ~4% of all thyroid cancer. MTC and PTC can coexist in
thyroid tissues (2,3). Concomitant medullary and papillary
thyroid carcinoma is divided into two forms. The first form
includes ≥2 lesions in the same or different thyroid lobes, which
indicates coexistence of MTC and PTC (2,3).
However, PTC and MTC can also simultaneously exist in a mixed tumor
containing features of both types of cancer named mixed
medullary-follicular thyroid carcinoma (MMFTC) (4). To the best of our knowledge, MMFTC has
rarely been reported. The present study reports a rare case of
MMFTC and a literature review of MTC and PTC and MMFTC.
Case report
A 60-year-old female was referred to Weifang
People's Hospital (Weifang, China) with a small mass on the left
side of her neck in September 2020. The mass was discovered during
a routine health examination 2 days prior. The patient had no
history of radiation exposure or family history of any endocrine
disease. Physical examination revealed a 2 cm, well-defined,
non-tender nodule in the left thyroid lobe, which was moved with
deglutition. The patient underwent Doppler ultrasound (US) and
computed tomography (CT) examination. Ultrasonic examination was
performed using a GE Logiq E9 color Doppler US system (GE
Healthcare) equipped with a GE 3.5C convex array transducer probe
with a frequency of 6–9 MHz. The patient was placed in a supine
position with the neck fully exposed. The thyroid gland was scanned
for transverse, longitudinal and oblique views. When a nodule was
found, its size, morphology, parenchymal echo pattern and internal
echo were analyzed. US examination of the neck indicated the
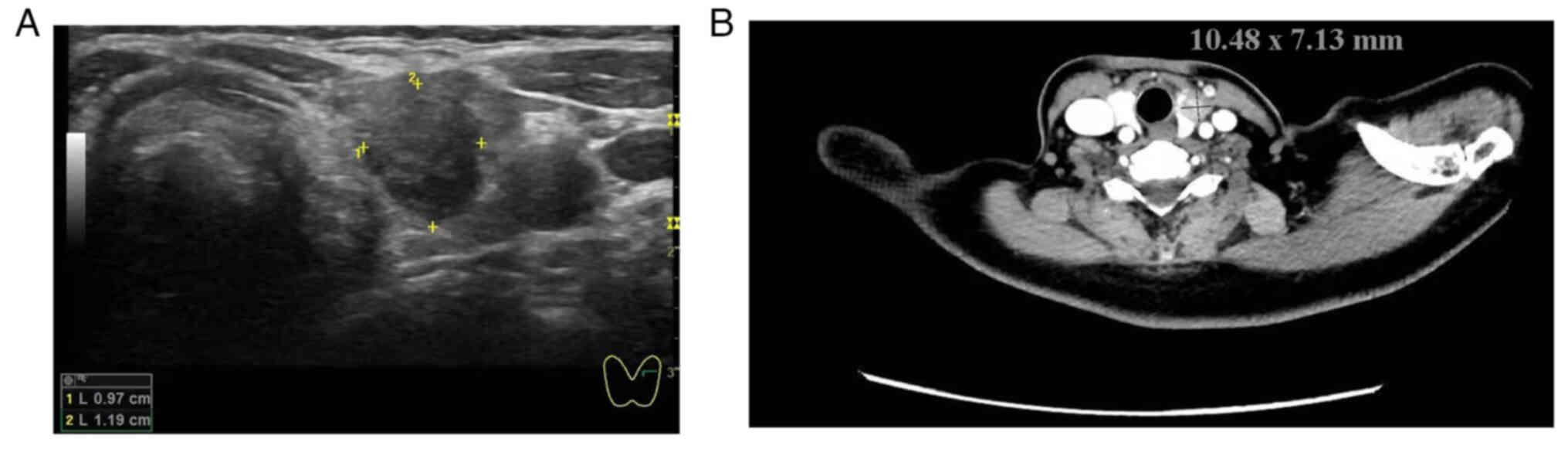
presence of a hyperechoic nodule measuring ~11.9×9.7 mm2
in the left lobe of the thyroid gland (Fig. 1A).
CT scanning was performed using the SOMATOM
Definition Flash (Siemens Healthcare) with the patient in a supine
position. The mandibular and shoulder positions were required to
avoid the influence of clavicle artifacts. The patient was
instructed to hold their breath and avoid swallowing to avoid
breathing and swallowing artifacts. The scanning parameters were as
follows: A tube voltage, 100 kV; reference current, 186 mAs; B tube
voltage, Sn140 kV; reference current, 125 mAs; fusion coefficient,
0.5; pitch, 0.65; open CARE Dose 4D, Q30 (SAFIRE strength 3) and
slice thickness/interval, 1.5/1.5 mm. Scanning was performed from
the skull base to the thoracic entrance. CT images demonstrated a
solid nodule ~10.5×7.7 mm2 in size in the left thyroid
lobe (Fig. 1B). US and CT
examination indicated no abnormalities in the right thyroid lobe
and isthmus.. The levels of serum calcitonin were elevated [143.20
pg/ml; normal levels (NL), 0.0–6.4 pg/ml], while carcinoembryonic
antigen (CEA; <0.5 ng/ml; NL, 0–4.99 pg/ml), thyroid stimulating
hormone (1.57 µg/ml; NL, 038–4.34 pg/ml), free thyroxine (19.45
pmol/l; NL, 10–22 pmol/l) and anti-thyroglobulin autoantibody serum
levels (2.23 IU/ml; NL, <75 IU/ml) were in the expected
ranges.
The case was discussed at the regional thyroid
multidisciplinary team meeting and surgery was proposed. The
patient underwent total thyroidectomy and bilateral central
compartment lymph node dissection in October 2020. During gross
examination, a poorly demarcated yellowish mass measuring 15.2×12.1
mm2 was observed in the left lobe of the thyroid gland
(Fig. 2A). Tissue specimens were
fixed with 4% formalin at room temperature for 12 h, embedded in
paraffin at 60°C for 15 min, cut into 4-µm sections, stained for 5
min at room temperature with hematoxylin and eosin and observed
under a light microscope (Nikon Corporation). Light microscopy
images demonstrated a large tumor ~18.2 mm composed of two distinct
parts that were joined (Fig. 2B).
The cells in one component exhibited PTC pathology with nuclear
clearing, grooving and occasional pseudoinclusion (Fig. 2B). The other component exhibited
features consistent with MTC (2,3), such
as irregular and solid nests of pleomorphic cells surrounded by a
fibrovascular stroma with an abundance of acidophilic homogenous
material, large and polygonal cells, prominent nucleoli and finely
granular cytoplasm (Fig. 2B).
Immunohistochemical analysis of the tissue was
performed. Tumor tissue was fixed with 4% neutral formalin at room
temperature for 12 h, embedded in paraffin at 60°C for 15 min, cut
into 4-µm sections and sealed with 3% hydrogen peroxide at room
temperature for 10 min. Antigen retrieval was performed with EDTA
at 100°C for 2.5 min followed by washing with PBS. Primary antibody
incubation was performed at 37°C for 60 min and secondary antibody
incubation at 37°C for 20 min. Anti-CEA was bought from Santa Cruz
Biotechnology. The other primary antibodies were purchased from
Beyotime Institute of Biotechnology. The following primary
antibodies were used: Calcitonin (cat. no. AG8159; 1:100), CEA
(sc-48364; 1:100), thyroid transcription factor 1 (TTF-1; cat. no.
AG8751; 1:100) and thyroglobulin (TG; cat. no. AG3385; 1:100).
Biotinylated Goat anti-Mouse and Rabbit secondary antibodies were
obtained from OriGene Technologies, Inc. (cat. no. PV-6000; 1:500).
Finally, tissue sections were stained with 3,3′-diaminobenzidine at
room temperature for 5 min, counterstained with hematoxylin at room
temperature for 5 min and imaged using a light microscope (Nikon
Corporation). MTC cells were positive for calcitonin (Fig. 3A), CEA (Fig. 3B) and TTF-1 (Fig. 3C) but negative for TG (Fig. 3D). The PTC cells were negative for
calcitonin (Fig. 3E) and CEA
(Fig. 3F) but positive for TTF-1
(Fig. 3G) and TG (Fig. 3H). A final pathology diagnosis
identified MMFTC in the thyroid gland.
No lymph node metastasis was detected in the 15
lymph node samples harvested from the bilateral central compartment
lymph node.
Immediately following surgery, serum calcitonin
levels decreased to 5.3 pg/ml. After 2 years of follow-up, the
patient remained healthy with no symptoms of discomfort.
Literature review
MTC-PTC and MMFTC are rare thyroid tumor, and the
reported incidence rate is <0.1/100,000 individuals/year
(2,3), the data pertaining to this type of
thyroid cancer are mainly acquired from case reports (2–6). The
present review summarizes advances in the epidemiology, histology,
molecular techniques, presentation and therapeutic strategies of
MTC-PTC and MMFTC.
Epidemiology
The morbidity of thyroid cancer worldwide has
increased by 300% over the past 30 years (1). PTC, which originates from follicular
cells, accounts for ~85% of the total number of thyroid cancer
cases (1). MTC, which develops from
neuroendocrine parafollicular C cells, is a rare type of cancer
that accounts for ~4% of all thyroid cancer (1). Concomitant medullary and papillary
thyroid carcinoma is a rare phenomenon, which accounts for
2.7–12.3% of all medullary thyroid cancer and is divided into two
forms. The first form includes ≥2 lesions in the same or different
thyroid lobes, which indicates coexistence of MTC and PTC (2,3). The
other form comprises a tumor with dual differentiation, known as
MMFTC (4). The incidence of the two
forms vary and over the past two decades the incidence of MTC-PTC
has increased from 2.7% of all MTCs to 12.3% (2). This figure is consistent with that of
a previous study that indicated that 19% of MTC cases displayed
concurrent PTC (5). However, no
case of MMFTC was reported in the aforementioned study (5). The increased incidence of simultaneous
MTC-PTC may be due to enhanced resolution of medical imaging and
its widespread application, in addition to careful pathological
examinations diagnosing more thyroid abnormalities (2). Most patients with MTC-PTC are aged
>45 years (median age 53.5±6.5 y), which is older than patients
with MTC (median age 44.5±12.6 y) but younger than patients with
PTC (median age 57.3±9.8 y) (1–3).
MTC-PTC is more common in female patients, with previous studies
reporting a ~2:1 female-to-male ratio (2,3,5). The
risk factors for development of MTC-PTC have not been fully
elucidated but may be similar to those of MTC or PTC, which include
ionizing radiation exposure, sex, alcohol and tobacco use, obesity
and previous family history (1,6).
Clinical presentation
The clinical presentation of MTC-PTC exhibits no
additional symptoms compared with traditional MTC or PTC. Usually,
a thyroid nodule or neck mass is the initial sign (6). Flushing or diarrhea accompanied by
high levels of calcitonin may be present (6). However, if opportunistic screening is
combined with high-resolution US, thyroid cancer can be observed in
patients with a thyroid nodule, either as a single or multinodular
goiter without other specific symptoms (1).
Ultrasonography is currently the most common method
for detecting thyroid cancer and can detect ≥1 hyperechoic nodules
with or without capsular invasion and microcalcification (7). Neither CT nor magnetic resonance
imaging (MRI) can characterize thyroid nodules accurately, as these
technologies cannot depict the fine architectural features that
distinguish benign and malignant nodules (8). However, CT and MRI can illustrate the
association between thyroid tumors and surrounding tissues
pertaining to organs, such as the trachea and esophagus (8).
Diagnosis
Owing to the two different types of cancer cells in
MTC-PTC, the diagnosis of MTC-PTC or MMPTC should occur when tumors
exhibit characteristics of both MTC and PTC. MTC-PTC in different
lesions may be observed in surgery treatment for MTC or PTC
(9). In this condition, MTC and PTC
should be diagnosed (9). In
addition, 8.3% of cases of MTC-PTC are reported to be familial MTC
(10). Therefore, familial and
sporadic MTC should be distinguished based on germline mutation of
the RET protooncogene.
A cytological smear of MTC is characterized by
isolated, oval or round, large polygonal or spindled cells.
Although the MTC cytological pattern is generally typical,
diagnosis of a high percentage of cases of MTC remains difficult
(11). In ambiguous cases, elevated
basal levels of serum calcitonin enable the diagnosis of MTC,
especially at levels >100 pg/ml (11). For patients with calcitonin levels
in the expected range, a diagnosis can be ascertained through the
measurement of calcitonin in the washout fluid of the needle
puncture derived from a suspected thyroid nodule (11,12).
Serum CEA is an additional reliable MTC tumor marker and high CEA
levels predict distant metastases (12). In a previous study using
immunohistochemistry of calcitonin, CEA, synaptophysin and
chromogranin A levels, positive results were obtained for TTF-1 and
paired box 8 (PAX8), whereas those for TG were negative (13).
The cytological PTC specimen consists of cells
arranged in papillae and/or monolayer sheets with a syncytial
appearance (14). They exhibit
intranuclear cytoplasmic pseudoinclusions, nuclear crowding,
intranuclear grooves and pale nuclei with powdery chromatin
(14). Immunohistochemistry of
these sample types are positive for TTF-1, PAX8 and TG and negative
for calcitonin, synaptophysin and chromogranin A (13).
For diagnosis of MMFC, MTC and PTC cells should be
present in the same lesion (4). To
the best of our knowledge, familial MTC has not yet been reported
in this type of cancer. In previous microscopic analysis (15–17),
the investigated tumor was composed of two different parts that
were joined. Immunohistochemistry revealed that the PTC cells were
positive for TG, whereas the MTC cells were positive for calcitonin
(15–17).
Therapy
Given the presence of both MTC and PTC components,
treatment strategy should consist of treatment methods for both
types of cancer.
Surgical management
Owing to poor prognosis of MTC, surgery should be
performed to manage this condition (2,3,5). Total
or near total thyroidectomy is considered a standard treatment for
MTC or PTC (2,3,5). The
extent of lymph node dissection depends on levels of preoperative
calcitonin. If the levels are <20 pg/ml, prophylactic central
compartment lymph node dissection is inappropriate (2,3,5).
Calcitonin values >20, 50, 200 and 500 pg/ml are associated with
the presence of lymph node metastases in the ipsilateral central
and lateral neck, contralateral central neck, contralateral lateral
neck and upper mediastinum, respectively (18–20).
Therefore, the corresponding range of lymph nodes should be
dissected.
Radioactive iodine (RAI) therapy
RAI therapy is dependent on the sodium iodide
symporter (NIS) principle of differentiating thyroid cancer cells
that trap RAI, which facilitates remnant ablation, adjuvant therapy
and treatment of known residual or recurrent disease (10).
Patients with PTC who have high-risk factors
including tumor invading the normal tissue, are recommended to
undergo RAI therapy (10). However,
10% of patients with PTC develop advanced disease, which can become
resistant to RAI therapy (10). For
reversal of the sensitivity of RAI therapy, several drugs, such as
lithium carbonate and retinoic acid, have been utilized (10). Furthermore, various oncogenes,
including RAS, BRAF and RET, enhance activation of the MAP kinase
signaling pathway and subsequently inhibit expression of NIS
(21). Therefore, the use of the
MEK1 and MEK2 inhibitor selumetinib enhances RAI uptake and
achieves therapeutic effects (22).
Hormone therapy
It has previously been recommended that hormone
therapy using L-thyroxine should commence immediately following
thyroidectomy (23). Given the
enhancement of thyrotropin in the metastasis and proliferation of
PTC, hormone suppressive therapy is conducted as per the PTC risk
stratification (23).
Prognosis and follow-up
Previous studies have reported that the size of
MTC-PTC tumors is significantly smaller compared with MTC tumors
(2,3). The 10-year disease-free survival rate
of MTC-PTC is 87%, which is notably higher than that of MTC which
is ~50% (2). All deaths pertaining
to MTC-PTC are reported to be due to MTC metastasis (24). Rigorous surveillance is required to
detect early metastasis. For patients who undergo surgery, serum
levels of TG, TG antibodies, calcitonin and CEA should be monitored
3 months after surgery (24). If
basal TG, TG antibodies and calcitonin levels are undetectable, the
patient can be considered in clinical remission with low risk of
recurrence (10%) (24). The
recurrence rate decreases to 3% if calcium stimulation test for
calcitonin remains negative (25).
Subsequently, patients can be followed up with neck US, TG serum,
TG antibody, calcitonin and CEA testing and physical examination
every 6 months (24,25). If calcitonin or TG is detectable,
further tests should be based on levels of circulating calcitonin
or TG (24,25). In the case of undetectable TG or
calcitonin <150 pg/ml, residual cervical disease is the most
likely hypothesis and cervical US should be performed. Furthermore,
the TG serum (>10 pg/ml) or calcitonin (>150 pg/ml) levels
indicate the presence of extra cervical metastases if total
thyroidectomy has been performed (24,25).
Therefore, other auxiliary examination methods, such as chest CT,
liver MRI and bone scintigraphy should be performed (26).
Advanced disease treatment
If recurrent disease is limited to the neck,
revision surgery is the most appropriate method of disease
treatment and a biochemical cure is often attainable (27). However, in cases of extensive
disease or distant metastasis, the disease may be incurable and
systemic therapy must be administered. Biopsy should be performed,
and MTC or PTC metastasis should be distinguished when feasible
(28). For patients with
locoregional disease, which is invasive of visceral structures,
postoperative radiation therapy may be an effective treatment
(28). Traditional cytotoxic drugs
are reported to have no significant effect on either MTC or PTC.
Regardless, targeted therapy has been used for advanced MTC and PTC
in recent decades (28). The Food
and Drug Administration (FDA) of the United States has approved
multitargeted kinase inhibitors for advanced MTC (cabozantinib) and
PTC (sorafenib and lenvatinib) (18). In addition, the FDA has approved two
RET-specific inhibitors (selpercatinib and pralsetinib) for
treatment of RET-mutant MTC (28).
Origin of MTC-PTC
The origin of MTC-PTC has not been
well-demonstrated. The leading theories include the collision
effect, stem cell and hostage theory. The hostage theory
hypothesizes that carcinoma is attributable to adenomatous areas
becoming sequestered by a different type of tumor (29). The stem cell theory hypothesizes
that the tumor originates from the same stem cell and
differentiates into two distinct types of carcinoma cells (30). The collision theory postulates that
the tumor originates from different polyclonal neoplasms that
manifest simultaneously (31).
Notably, collision theory exhibits the highest rational robustness.
In combination with the development of molecular diagnosis, Ciampi
et al (32) reported that
medullary tumors and PTC occurring simultaneously in the same gland
show different oncogene mutations, including RET, BRAF and RAS. In
addition, two population-level analyses reported that MTC-PTC
likely represents a primary tumor with an incidental pathological
finding of a second malignancy (2,5).
In conclusion, knowledge of this rare type of
thyroid cancer and immunohistochemical markers are key to make a
correct diagnosis., Owing to the coexistence of MTC and PTC,
MTC-PTC or MMPTC should be treated based on its respective stage
and currently available treatment guidelines. Owing to low
morbidity of MTC-PTC or MMPTC and the availability of only
retrospective data from case reports, extensive research is needed
on the condition. First, the molecular mechanism underlying MTC-PTC
or MMPTC development needs to be studied to identify novel
signaling pathways. Novel target therapies may lead to high
clinical effectiveness. To develop the most effective treatment
strategy, prospective, randomized and double-blinded studies may be
performed.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW and FK conceived the study and confirm the
authenticity of all the raw data. YW performed the surgery. DY, GR,
ZW and FK collected and analyzed the data. YW and GR wrote the
manuscript. GR and ZW revised the manuscript. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Weifang People's Hospital (Weifang, China; approval
no. WF2023011302). The patient provided written consent to
participate in the study.
Patient consent for publication
Written informed consent was given by the patient
for publication of data and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fagin JA and Wells SA Jr: Biologic and
clinical perspectives on thyroid cancer. N Engl J Med.
375:1054–1067. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Appetecchia M, Lauretta R, Barnabei A,
Pieruzzi L, Terrenato I, Cavedon E, Mian C, Castagna MG and Elisei
R; SIE (Italian Society of Endocrinology) Working Group, :
Epidemiology of simultaneous medullary and papillary thyroid
carcinomas (MTC/PTC): An Italian multicenter study. Cancers
(Basel). 11:15162019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wong RL, Kazaure HS, Roman SA and Sosa JA:
Simultaneous medullary and differentiated thyroid cancer: A
population-level analysis of an increasingly common entity. Ann
Surg Oncol. 19:2635–2642. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hales M, Rosenau W, Okerlund MD and
Galante M: Carcinoma of the thyroid with a mixed medullary and
follicular pattern: morphologic, immunohistochemical, and clinical
laboratory studies. Cancer. 50:1352–1359. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim WG, Gong G, Kim EY, Kim TY, Hong SJ,
Kim WB and Shong YK: Concurrent occurrence of medullary thyroid
carcinoma and papillary thyroid carcinoma in the same thyroid
should be considered as coincidental. Clin Endocrinol (Oxf).
72:256–263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seib CD and Sosa JA: Evolving
understanding of the epidemiology of thyroid cancer. Endocrinol
Metab Clin North Am. 48:23–35. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sharbidre KG, Lockhart ME and Tessler FN:
Incidental thyroid nodules on imaging: Relevance and management.
Radiol Clin North Am. 59:525–533. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shetty SK, Maher MM, Hahn PF, Halpern EF
and Aquino SL: Significance of incidental thyroid lesions detected
on CT: Correlation among CT, sonography, and pathology. AJR Am J
Roentgenol. 187:1349–1356. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Poller DN and Glaysher S: Molecular
pathology and thyroid FNA. Cytopathology. 28:475–481. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Biscolla RP, Ugolini C, Sculli M, Bottici
V, Castagna MG, Romei C, Cosci B, Molinaro E, Faviana P, Basolo F,
et al: Medullary and papillary tumors are frequently associated in
the same thyroid gland without evidence of reciprocal influence in
their biologic behavior. Thyroid. 14:946–952. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Costante G and Filetti S: Early diagnosis
of medullary thyroid carcinoma: Is systematic calcitonin screening
appropriate in patients with nodular thyroid disease? Oncologist.
16:49–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wells SA Jr, Asa SL, Dralle H, Elisei R,
Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, et al:
Revised American thyroid association guidelines for the management
of medullary thyroid carcinoma. Thyroid. 25:567–610. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Filetti S, Durante C, Hartl D, Leboulleux
S, Locati LD, Newbold K, Papotti MG and Berruti A; ESMO Guidelines
Committee, : Electronic address: simpleClinicalguidelines@esmo.org:
Thyroid cancer: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up†. Ann Oncol. 30:1856–1883. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vance J and Gilani SM: Thyroid
cytopathology: updates and molecular testing. Pathologica.
111:51–57. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gurkan E, Gurbuz Y, Tarkun I, Canturk Z
and Cetinarslan B: Mixed medullary-papillary carcinoma of the
thyroid: report of two cases and review of the literature. Indian J
Pathol Microbiol. 57:598–602. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jain M, Verma D, Thomas S and Chauhan R:
Mixed medullary-papillary carcinoma thyroid: An uncommon variant of
thyroid carcinoma. J Lab Physicians. 6:133–135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nangue C, Bron L, Portmann L, Volante M,
Ris HB, Monnier P and Andrejevic-Blant S: Mixed medullary-papillary
carcinoma of the thyroid: Report of a case and review of the
literature. Head Neck. 31:968–974. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Araque KA, Gubbi S and Klubo-Gwiezdzinska
J: Updates on the management of thyroid cancer. Horm Metab Res.
52:562–577. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim M and Kim BH: Current guidelines for
management of medullary thyroid carcinoma. Endocrinol Metab
(Seoul). 36:514–524. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Viola D and Elisei R: Management of
medullary thyroid cancer. Endocrinol Metab Clin North Am.
48:285–301. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Durante C, Puxeddu E, Ferretti E, Morisi
R, Moretti S, Bruno R, Barbi F, Avenia N, Scipioni A, Verrienti A,
et al: BRAF mutations in papillary thyroid carcinomas inhibit genes
involved in iodine metabolism. J Clin Endocrinol Metab.
92:2840–2843. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu D, Hu S, Hou P, Jiang D, Condouris S
and Xing M: Suppression of BRAF/MEK/MAP kinase pathway restores
expression of iodide-metabolizing genes in thyroid cells expressing
the V600E BRAF mutant. Clin Cancer Res. 13:1341–1349. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stramazzo I, Capriello S, Antonelli A,
Fallahi P, Centanni M and Virili C: Seeking optimization of LT4
treatment in patients with differentiated thyroid cancer. Hormones
(Athens). 21:537–543. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pellegriti G, Leboulleux S, Baudin E,
Bellon N, Scollo C, Travagli JP and Schlumberger M: Long-term
outcome of medullary thyroid carcinoma in patients with normal
postoperative medical imaging. Br J Cancer. 88:1537–1542. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Franc S, Niccoli-Sire P, Cohen R, Bardet
S, Maes B, Murat A, Krivitzky A and Modigliani E; French Medullary
Study Group (GETC), : Complete surgical lymph node resection does
not prevent authentic recurrences of medullary thyroid carcinoma.
Clin Endocrinol (Oxf). 55:403–409. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Giraudet AL, Vanel D, Leboulleux S,
Aupérin A, Dromain C, Chami L, Ny Tovo N, Lumbroso J, Lassau N,
Bonniaud G, et al: Imaging medullary thyroid carcinoma with
persistent elevated calcitonin levels. J Clin Endocrinol Metab.
92:4185–4190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Machens A, Lorenz K and Dralle H:
Prediction of biochemical cure in patients with medullary thyroid
cancer. Br J Surg. 107:695–704. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Walgama E, Busaidy N and Zafereo M: Novel
therapeutics and treatment strategies for medullary thyroid cancer.
Endocrinol Metab Clin North Am. 51:379–389. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bangaraiahgari R, Panchangam RB,
Puthenveetil P, Mayilvaganan S, Bangaraiahgari R, Banala RR,
Karunakaran P and Md R: Is there adenoma-carcinoma sequence between
benign adenoma and papillary cancer of thyroid: A genomic linkage
study. Ann Med Surg (Lond). 60:695–700. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoo MH and Hatfield DL: The cancer stem
cell theory: Is it correct? Mol Cells. 26:514–516. 2008.PubMed/NCBI
|
|
31
|
Pishdad R, Cespedes L, Boutin R, Jaloudi M
and Raghuwanshi M: Coexistence of two different thyroid
malignancies: A collision phenomenon. Cureus.
12:e75392020.PubMed/NCBI
|
|
32
|
Ciampi R, Romei C, Pieruzzi L, Tacito A,
Molinaro E, Agate L, Bottici V, Casella F, Ugolini C, Materazzi G,
et al: Classical point mutations of RET, BRAF and RAS oncogenes are
not shared in papillary and medullary thyroid cancer occurring
simultaneously in the same gland. J Endocrinol Invest. 40:55–62.
2017. View Article : Google Scholar : PubMed/NCBI
|