Introduction
The occurrence and death rates of lung cancer (LC)
remain the highest among all malignant neoplasms (1,2), with
~6 million patients dying of LC each year in China (3). LC may be classified as non-small cell
LC (NSCLC) or SCLC based on its histological characteristics. Among
all LC cases, NSCLC accounts for 80–85% and its 5-year overall
survival is only 16% (4). Being an
insidious disease, LC is commonly diagnosed at an advanced
stage.
Overexpression of microRNA (miRNA)-27a is observed
in numerous types of cancer, such as pancreatic cancer (5), breast cancer (BC) (6), ovarian cancer (OC) (7), esophageal cancer (8) and renal cell carcinoma (RCC) (9), and it is associated with the
biological behaviors of tumors cells. High levels of miRNA-27a are
also related to the survival rates and clinical outcome of patients
with BC (10). miRNA-27a-3p
overexpression may contribute to the invasiveness and metastasis of
RCC cells and oral squamous cell carcinoma stem cells (11,12).
However, certain studies have demonstrated that miRNA-27a-3p is
downregulated in hepatocellular carcinoma (HCC) and esophageal
squamous cell carcinoma, and may exhibit inhibitory effects on
these tumor types (13,14). At present, the abnormal expression
and functional role of miRNA-27a-3p in tumor cells are still
controversial.
Chronic inflammation is one of the physiological
causes of LC and inflammatory response is one of the important
characteristics of early NSCLC (15). It has been indicated that
upregulation of COX-2 has a prominent role in NSCLC initiation
(16). In addition, there are
various cytokines participating in tumor processes (17). C-X-C motif chemokine ligand 2
(CXCL2) is involved in chronic inflammatory responses by
recruiting, maturating and activating immune cells and secretory
proteins with small molecular weight (18). Certain studies have confirmed that
CXCL2 exhibits strong chemotactic activity, promotes inflammatory
injury, induces angiogenesis and increases cancer cell
proliferation (19,20). Other studies have also indicated
that CXCL2 is abnormally expressed in numerous types of tumor and
immune cells (21–25). However, the regulatory relationship
between CXCL2 and miRNA-27a-3p remains to be clarified.
In the present study, both miRNA-27a-3p and CXCL2
levels were detected in the macrophages and peripheral blood of
patients with early NSCLC, and the regulatory relationship between
them was predicted and verified. In addition, the functional roles
of miRNA-27a-3p and CXCL2 in regulating the proliferation of human
pulmonary macrophage cell lines were studied and the underlying
molecular mechanisms were explored.
Materials and methods
Subjects
A total of 36 patients with NSCLC treated at the
Central Hospital Affiliated to Shandong First Medical University
(Jinan, China) between June 2016 and September 2017 were enrolled
into an observation group. Furthermore, 29 healthy subjects who
underwent medical examinations during the same period of time were
enrolled into a control group. Fasting peripheral blood was
withdrawn from all subjects and kept in EDTA anticoagulant tubes at
−20°C. Lung tumor tissues were freshly obtained from the patients
with NSCLC and their tumor-adjacent tissues were used as controls.
The tissues were frozen in liquid nitrogen prior to use. Pulmonary
macrophages were isolated from a proportion of the tissues using a
tissue macrophage extraction kit (cat. no. JH0217; Beijing Baiao
Laibo Technology Co., Ltd.). Among the patients with NSCLC, 21 were
males and 15 were females (age range, 38–62 years; mean age, 52.3
years). Among the healthy subjects, 18 were males and 11 were
females (age range, 36–65 years; mean age, 51.8 years) (Table I). All patients were having the
first onset and diagnosed with early NSCLC (stages 1–2), and had no
history of hormone therapy, chemotherapy, radiotherapy or
Traditional Chinese Medicine administration. Ethical approval for
the present study was obtained from the Ethics Committee of
Shandong University (no. H18026). All subjects or their family
members provided written informed consent.
 | Table I.Clinicopathological parameters of the
patients of the present study. |
Table I.
Clinicopathological parameters of the
patients of the present study.
|
|
| Sex |
|
| Number of cases at
stage |
|---|
|
|
|
|
|
|
|
|---|
| Group | N | Male | Female | Age, years | BMI,
kg/m2 | IIb | IIIa | IIIb |
|---|
| NSCLC | 36 | 21 | 15 | 52.30±9.23 | 21.43±3.82 | 5 | 20 | 11 |
| Control | 29 | 18 | 11 | 51.8±10.06 | 22.76±2.97 | 3 | 16 | 10 |
Isolation of pulmonary
macrophages
Pulmonary macrophages were isolated from a
proportion of the tissues using a tissue macrophage extraction kit
(JH0217; Beijing Baiao Laibo Technology Co., Ltd.). In brief, lung
tissues were ground into powder in frozen form and washed with cold
PBS prior to filtration with a mesh (50 µm). The resulting cell
suspension was mixed with the solution from the tissue macrophage
extraction kit (volume ratio, 1:1) prior to centrifugation at room
temperature and 4,000 × g for 20 min. The white cloudy cell layer
between the upper layer and middle layer was aspirated and then
dispensed into a 15-ml centrifuge tube. After washing the extracted
cells with 5 volumes of PBS for 3 times (centrifugation conditions:
37°C, 2,500 × g and 5 min), the pellet was resuspended with
F12/DMEM medium plus 10% FBS (Thermo Fisher Scientific, Inc.).
Pulmonary macrophages were thereby isolated, and their identity was
verified according to a previous report (26).
Reverse transcription-quantitative PCR
(RT-qPCR)
An RNA extraction kit (Tiangen Biotech, Co., Ltd.)
was employed to isolate total RNA from tissues and macrophages. The
integrity of total RNA was detected by gel electrophoresis, while
the purity of total RNA was examined by calculating the RNA
absorbance at 260/280 nm ratio with a spectrophotometer (NanoDrop
OneC; Thermo Fisher Scientific, Inc.). Total RNA (1 µg) was used
for RT (cat. no. KR107; TIANScript II cDNA First Strand Synthesis
Kit; Tiangen Biotech, Co., Ltd.) according to the manufacturer's
protocol and the template cDNA was stored in a freezer (−20°C). The
primer sequences were as follows: MiRNA-27a-3p forward,
5′-CGCCGTTCACAGTGGCTAAG-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′;
and U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′. The RT-qPCR reaction mixture (20 µl)
consisted of qPCR mixture [10 µl; SuperReal PreMix (SYBR Green);
cat. no. FP204; Tiangen Biotech, Co., Ltd.], forward and reverse
primers (0.5 µl each), cDNA (2 µl) and ddH2O (7 µl).
According to the manufacturer's instructions, the reaction
conditions comprised an initial denaturation step for 5 min at
95°C, followed by 46 cycles of 10 sec at 95°C, 25 sec at 56°C and
30 sec at 72°C. The results were analyzed by the 2−ΔΔCq
method (27) and the ratio of
miRNA-27a-3p to U6 was calculated.
For the analysis of CXCL2, the primer sequences were
as follows: CXCL2 forward, 5′-CTGCTGCTCCTGCTCCTG-3′ and reverse,
5′-TGAGACAAGCTTTCTGCCCA-3′; and GAPDH forward,
5′-AGGAGCGAGACCCCACTAACAT-3′ and reverse,
5′-GTGATGGCATGGACTGTGGT-3′. The qPCR reaction mixture [20 µl;
SuperReal PreMix (SYBR Green); cat. no. FP204; Tiangen Biotech,
Co., Ltd.] consisted of qPCR mixture (10 µl), upstream and
downstream primers (0.5 µl each), cDNA (2 µl) and ddH2O
(7 µl). According to the manufacturer's instructions, the reaction
conditions comprised an initial denaturation for 5 min at 95°C,
followed by 46 cycles of 20 sec at 95°C, 20 sec at 55°C and 30 sec
at 72°C. The results were analyzed by the 2−ΔΔCq method
(27) and the ratio of CXCL2 vs.
GAPDH was calculated.
Cell transfection
Cells (3×105) were grown in 24-well
plates with F12/DMEM medium plus 10% FBS. Upon attaining 70%
confluency, the cells were subjected to transfection. In the first
and second vials, 0.5 µg plasmids/agomiR (designed/customized by
Sangon Biotech Co., Ltd.) and 1 µl Lipofectamine® 2000
(Thermo Fisher Scientific, Inc.) were added into 50 µl Opti Mem
medium (Thermo Fisher Scientific, Inc.) separately. Following a
5-min incubation, the two vials were combined and incubated again
at room temperature for 20 min. Subsequently, the cells were
exposed to the mixture at 37°C for 6 h and the medium was replaced
with 10% FBS-containing F12/DMEM. Following a 48-h incubation, the
cells were harvested for subsequent analyses. The sequence for
hsa-agomiRNA-27a-3p was
5′-UUCACAGUGGCUAAGUUCCGCGCGGAACUUAGCCACUGUGAA-3′ and that for
agomiR-negative control (NC) was
5′-UUUGUACUACACAAAAGUACUGCAGUACUUUUGUGUAGUACAAA-3′.
Bioinformatics analysis
The functional roles of miRNAs can be assessed by
bioinformatic prediction tools. In this experiment, miRwalk3.0
(http://mirwalk.umm.uni-heidelberg.de/) was employed to
identify the downstream target of miRNA-27a-3p.
Dual-luciferase reporter (DLR)
assay
Based on the bioinformatics prediction data obtained
using miRwalk3.0 (http://mirwalk.umm.uni-heidelberg.de/), the mutant and
wild-type (WT) miRNA-27a-3p seed regions in the 3′ untranslated
region of CXCL2 were identified and synthesized chemically by
Sangon Biotech Co., Ltd. Their two ends were first joined by
HindIII (D6389; Beyotime Institute of Biotechnology) and
SpeI (RK21113; ABclonal Technology Co., Ltd.) restriction
sites and subsequently cloned into the luciferase reporter vector
pMIR-REPORT (Ambion; Thermo Fisher Scientific, Inc.).
AgomiRNA-27a-3p (100 nM; Sangon Biotech Co., Ltd.) was
co-transfected with the plasmid containing mutant or WT 3′
untranslated region sequences (0.8 µg) into 293T cells (Cell Bank
of the Chinese Academy of Sciences). Furthermore, pMIR-REPORT empty
vector and agomiR-27a-3p were transfected into 293T cells as a
negative control group. Following a 24-h incubation, a DLR assay
kit (cat. no. E1980; Promega Corporation) was used to lyse the
transfected cells. The resulting luminescence intensities were
recorded using a GloMax 20/20 luminometer (Promega Corporation).
Renilla luciferase activity was employed as a standard
reference.
Western blot analysis
The tissue (100 mg) was homogenized and lysed with
600 µl ice-cold RIPA buffer (Beyotime Institute of Biotechnology)
for 30 min. After centrifugation (8,000 × g, 10 min, 4°C), the
total protein content was determined by a BCA assay kit (cat. no.
P0011; Beyotime Institute of Biotechnology). The protein samples
were mixed with 5X SDS loading buffer and then heated in a water
bath for 5 min. Following 10% SDS-PAGE, the separated protein
samples (20 µg) were transferred onto PVDF membranes
(MilliporeSigma) at 100 V for 2 h and then blocked with 5% non-fat
milk (cat. no. P0216; Beyotime Institute of Biotechnology) at room
temperature for 1 h. Subsequently, the membranes were exposed to
β-actin (cat. no. ab129348; 1:5,000 dilution; Abcam) or rabbit
anti-human CXCL2 (cat. no. ab9841; 1:1,000 dilution; Abcam)
polyclonal primary antibodies at 4°C for 24 h. After rinsing 3
times with PBS-Tween-20 (0.1%) for 5 min each, the membranes were
exposed to goat anti-rabbit HRP-conjugated secondary antibody (cat.
no. ab6721; 1:10,000 dilution; Abcam) at 37°C for 1 h and then
rinsed again with PBS-Tween-20 (3 times, 5 min each). Finally, the
protein blots were visualized with an ECL substrate kit (cat. no.
ab133406; Abcam) and quantified by Image lab V3.0 software (Bio-Rad
Laboratories, Inc.). The protein level of CXCL2 was normalized to
that of β-actin.
ELISA
A human MIP2 ELISA kit (CXCL2; Abcam) was employed
to detect the serum and extracellular levels of CXCL2. The samples
(10 µl liquid samples and 40 µl diluent) and standards (50 µl) were
loaded into the microplate wells, while for the blank group, wells
were left empty. HRP-labelled conjugate (100 µl) was added to the
sample and standard wells, followed by incubation at 37°C for 1 h.
After rinsing the plates 5 times, the substrates A and B (50 µl
each) were placed into all wells, which were then incubated at 37°C
for 15 min. Finally, stop solution (50 µl) was pipetted into each
well and the optical density (450 nm) was recorded using a
microplate reader (Thermo Fisher Scientific, Inc.) within 15
min.
MTT assay
The transfected cells (2×103 cells/well)
were grown in 96-well plates. After 1, 2 and 3 days of
transfection, MTT (5 g/l, 20 µl) was placed into the designated
wells, followed by incubation at 37°C for 4 h. The medium was
discarded and replaced with DMSO (150 µl) to solubilize the
formazan crystals. Finally, the optical density (490 nm) was
recorded using a microplate reader (Thermo Fisher Scientific, Inc.)
and a cell viability curve was constructed. This experiment was
performed in triplicate.
Transwell assay
Macrophages (cat. no. GNR 9; Cell Bank of the
Chinese Academy of Sciences) were precultured with serum-free DMEM
for 12 h to reduce the influence of serum and then seeded into
Transwell inserts (cat. no. CLS3412; Corning, Inc.) at a density of
2×105/well. The chambers were placed into 24-well plates
(bottom wells) containing 500 µl supernatants of cells transfected
with agomiRNA-27a-3p or agomiR-NC and then incubated at 37°C for 24
h. Finally, the Transwell inserts containing fixed cells were
soaked in 0.1% crystal violet solution at room temperature for
staining for 15 min (the cells on the lower side of the membrane
were stained and those on the upper side were removed) prior to
observation and counting under a microscope.
Statistical analysis
Statistical analyses were performed using SPSS 18.0
(SPSS, Inc.). All values were expressed as the mean ± standard
deviation. After performing normality tests, differences between
two groups were compared using an unpaired Student's t-test.
Comparison among multiple groups was performed using one-way ANOVA.
In case of heterogeneity of variance, Dunnett's T3 or Tamhane's T2
method was employed, while for homogeneity of variance,
Student-Newman-Keuls and least-significant difference tests were
performed. P<0.05 was considered to indicate a statistically
significant difference.
Results
Differential expression of
miRNA-27a-3p in NSCLC tissues, pulmonary macrophages and blood
specimens
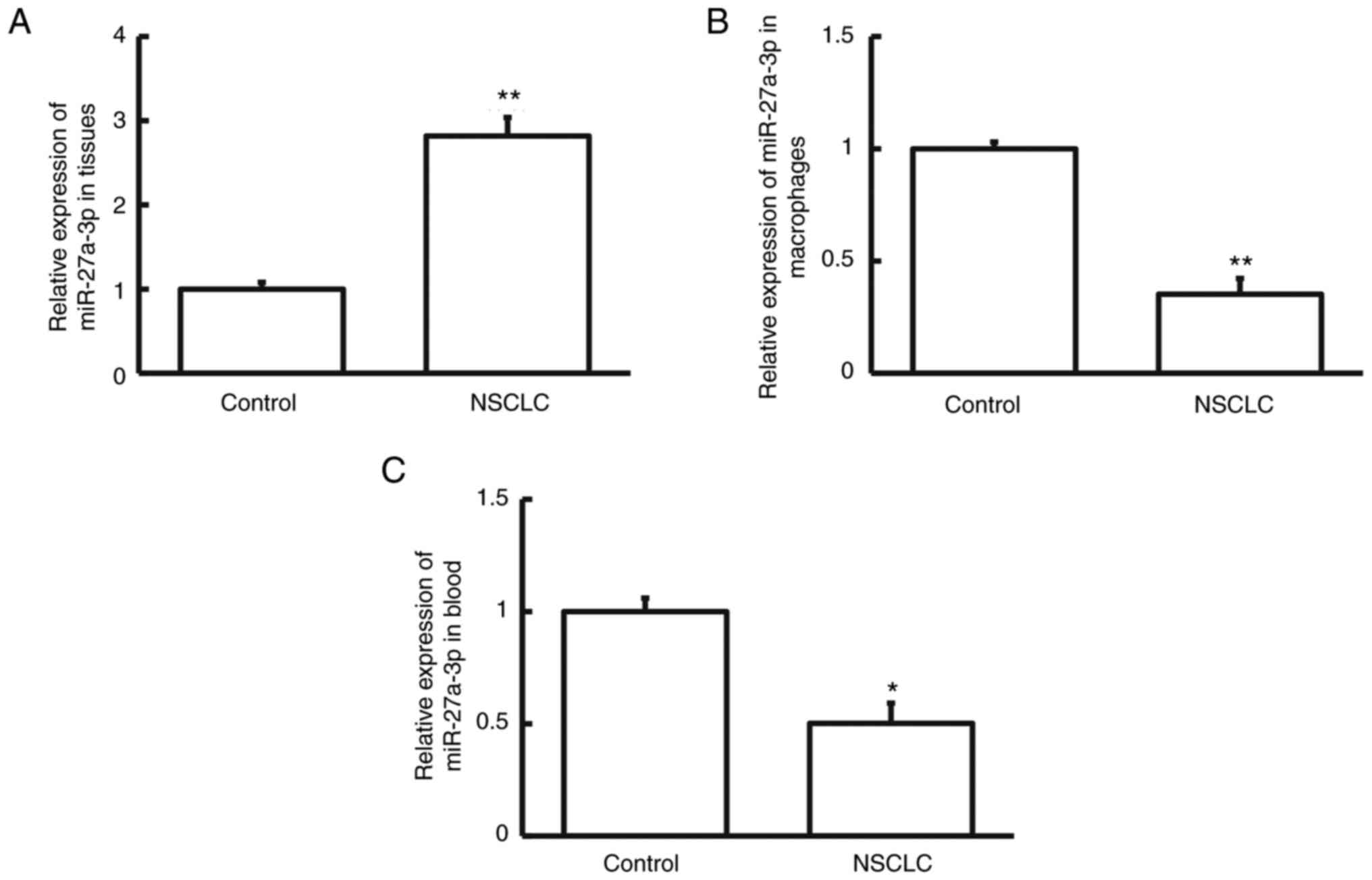
To measure the levels of miRNA-27a-3p, RT-qPCR was
performed. The results indicated that miRNA-27a-3p levels were
markedly higher in NSCLC tissue than in cancer-adjacent tissue
(Fig. 1A; P<0.01). However,
miRNA-27a-3p levels in the pulmonary macrophages isolated from
NSCLC tumor tissues were significantly lower than in those isolated
from cancer-adjacent tissues (Fig.
1B; P<0.01). Similarly, the peripheral blood level of
miRNA-27a-3p in patients with NSCLC was decreased compared to that
in healthy subjects (Fig. 1C;
P<0.05). The differential expression of miRNA-27a-3p in NSCLC
tissues, pulmonary macrophages and peripheral blood suggests that
miRNA-27a-3p exerts different roles in these specimens.
CXCL2 is upregulated in NSCLC tissues
and peripheral blood at both transcriptional and translational
levels
To examine the levels of CXCL2, RT-qPCR and western
blot analyses were performed. As presented in Fig. 2A and B, CXCL2 expression levels in
the pulmonary macrophages isolated from NSCLC tissue were markedly
higher than in those isolated from cancer-adjacent tissue
(P<0.05). As indicated in Fig. 2C
and D, the peripheral blood levels of CXCL2 in NSCLC were
significantly higher in patients with NSCLC than in control
subjects (P<0.05). These findings indicate that CXCL2 is
upregulated in NSCLC tissues and peripheral blood at both
transcriptional and translational levels.
The untranslated region of CXCL2 is
targeted by miRNA-27a-3p
On the basis of a bioinformatics prediction using
miRwalk3.0, CXCL2 was identified as a downstream target of
miRNA-27a-3p. The sequences of the agomiRNA-27a-3p seed region on
CXCL2 were synthesized (Fig. 3A).
The results of the DLR assay indicated that the fluorescence
intensity of pMIR-REPORT-WT and agomiRNA-27a-3p co-transfected
cells was markedly reduced compared to that of the NC group
(P<0.05; Fig. 3B). However, the
fluorescence intensity of pMIR-REPORT-mutant and agomiRNA-27a-3p
co-transfected cells was relatively similar compared to that of the
NC group (P>0.05; Fig. 3B). This
observation suggests that the untranslated region of CXCL2 is
targeted by miRNA-27a-3p prior to its transcriptional
activation.
miRNA-27a-3p regulates CXCL2
expression that affects the proliferation of human pulmonary
macrophages
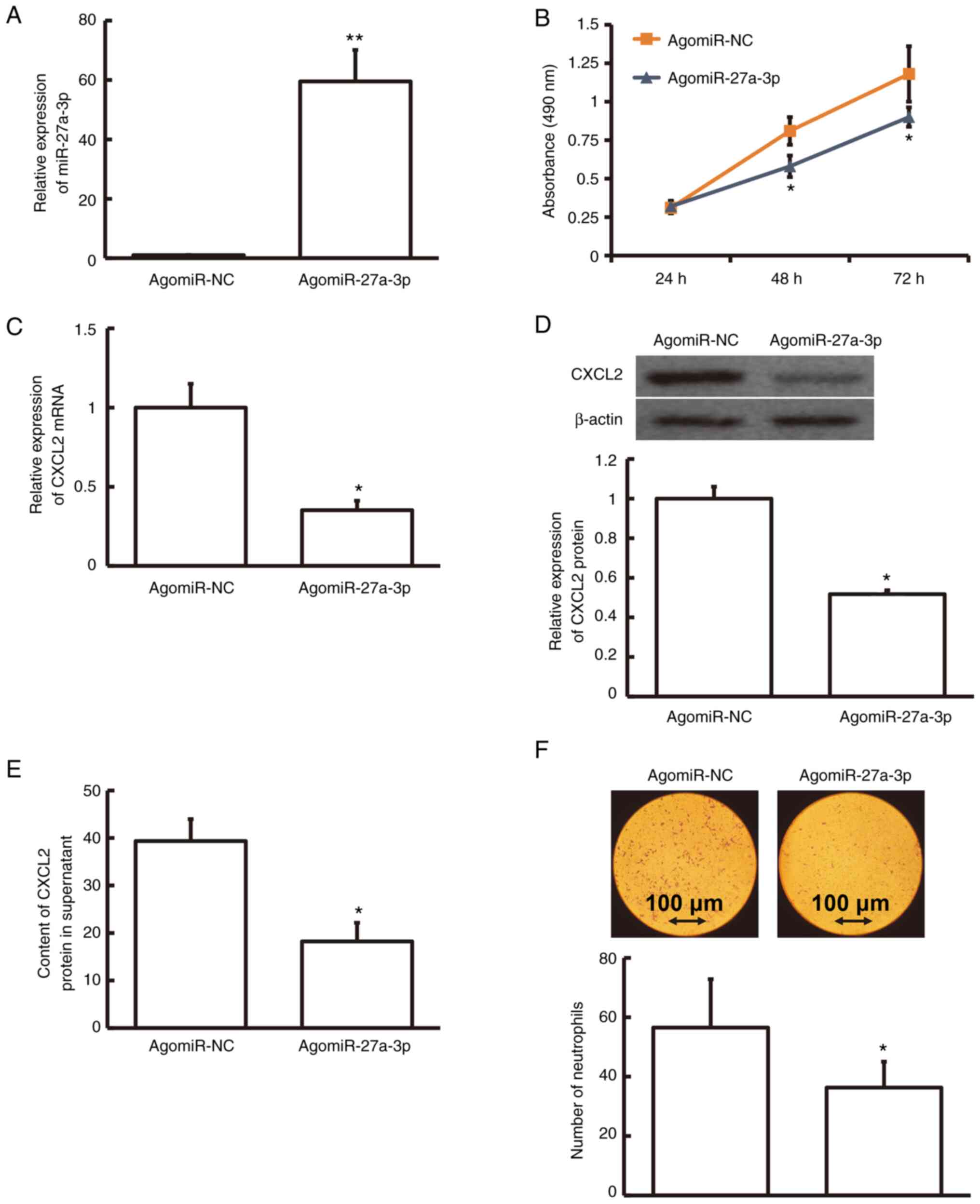
The level of miRNA-27a-3p was much higher in
agomiRNA-27a-3p-transfected human pulmonary macrophages than in
agomiR-NC-transfected macrophages (P<0.01; Fig. 4A). To test the effect on the
viability of macrophages, an MTT assay was performed. The result
demonstrated that agomiRNA-27a-3p-transfected human pulmonary
macrophages were less viable than agomiR-NC-transfected macrophages
at 48 and 72 h (P<0.05; Fig.
4B). The protein and mRNA levels of CXCL2 in
agomiRNA-27a-3p-transfected human pulmonary macrophages were
relatively lower compared to those in agomiR-NC-transfected
macrophages (P<0.05; Fig. 4C and
D). Furthermore, the CXCL2 content in the supernatant of
agomiRNA-27a-3p-transfected human pulmonary macrophages was
markedly decreased compared with that in agomiR-NC-transfected
macrophages (P<0.05; Fig. 4E).
After treatment of human neutrophils with agomiRNA-27a-3p- or
agomiR-NC-transfected human pulmonary macrophage supernatants,
Transwell data revealed that the number of neutrophils with
chemotaxis was markedly lower among the agomiRNA-27a-3p-transfected
macrophages than among agomiR-NC-transfected macrophages
(P<0.05; Fig. 4F). These
findings demonstrate that miRNA-27a-3p regulates CXCL2 expression
that affects the proliferation of human pulmonary macrophages.
Discussion
Approximately 80–85% of all LC cases are NSCLC and
the five-year overall survival rate is only 16% (28). It is particularly crucial to explore
the molecular mechanisms of LC occurrence and to find novel
specific diagnostic and therapeutic targets. The mechanisms of
miRNA-27a-3p action may be complex and several downstream targets
have been identified. It has been reported that miRNA-27a-3p
overexpression may downregulate the protein level of BTG2 and
subsequently activate c-myc via the ERK/MEK/Ras pathway, thus
inhibiting the apoptosis of gastric cancer cells (29). In addition, miRNA-27a-3p is
downregulated in HCC tissue and reduces the expression of dual
specificity phosphatase 16, thus inhibiting the growth, invasion
and migration of HCC cells (13).
miRNA-27a-3p is upregulated in colorectal cancer and promotes
colorectal cancer proliferation by modulating β-catenin/Wnt signal
transduction (30). miRNA-27a is
also overexpressed in cervical cancer and promotes the malignant
behavior of cervical tumor cells by downregulating the levels of
sprouty2, prohibitin and forkhead box O1 (31). However, miRNA-27a has an antitumor
effect in certain other tumor types. For instance, the C allele in
rs895819, a genetic polymorphism of miRNA-27a, was indicated to
reduce the risk of BC in Iranians, thereby protecting against this
disease (32). miRNA-27a exerts a
tumor-suppressor effect on acute leukemia by regulating the
expression of 14–3-θ, a family member of anti-apoptotic proteins
(33).
In the present study, it was observed that
miRNA-27a-3p was upregulated in NSCLC tissues and downregulated in
pulmonary macrophages and peripheral blood. Thus, it may be
speculated that differential miRNA-27a-3p expression exerted
different roles in patients with early-stage NSCLC. Considering the
key roles of inflammation in early NSCLC, the expression of
miRNA-27a-3p and CXCL2 in macrophages was examined. Bioinformatics
prediction suggested that CXCL2 was directly targeted by
miRNA-27a-3p; thus, the expression of CXCL2 was measured in the
corresponding samples. The present findings highlighted that the
expression trend of miRNA-27a-3p was contrary to that of CXCL2,
which was in good compliance with the negative regulation of
microRNA on mRNA. Furthermore, the DLR assay demonstrated that
miRNA-27a-3p directly targeted CXCL2 mRNA. Due to the low
expression of miRNA-27a-3p in macrophages, miRNA-27a-3p was
overexpressed in macrophages by plasmid transfection. It was
observed that the proliferation rate of macrophages decreased
significantly, suggesting that miRNA-27a-3p exerts a regulatory
effect on macrophage immune responses in patients with early
NSCLC.
CXCL2 is a member of a chemokine family that
regulates inflammatory response and injury repair and also
participates in important physiological functions, such as
cytoskeleton reconstruction, cell migration and immune response
(34). CXCL2 is a proto-oncogene
that may involve in the interaction between tumor and immune cells,
and its overexpression has non-negligible roles in angiogenesis,
tumor formation and metastasis. In esophageal cancer, CXCL2 gene
knockout may significantly inhibit cisplatin-induced apoptosis,
mainly by delaying the activation of caspase. In addition, the
serum level of CXCL2 in patients with esophageal cancer is
noticeably increased and the degree of increment is positively
related to tumor size, TNM staging and the degree of lymph node
diffusion (35). CXCL2
overexpression is also observed in other tumor types, such as OC,
endometrial cancer, bladder cancer, HCC and gastrointestinal
stromal tumor (36–39). Numerous chemokines are also involved
in tumorigenesis and malignant transformation. For instance,
chemokine-mediated angiogenesis is involved in the malignant
transformation of tumors (40). The
expression levels of CXCL1, CXCL2 and CXCL3 are significantly
increased in human melanoma; knockout of these three genes in mouse
cells significantly reduced the angiogenesis and growth rate of
melanoma (41). In a tumorigenic
experiment of esophageal cancer cells in mice, temozolomide
effectively inhibited the growth rate of the tumor and improved the
survival rate of mice. In vitro experiments indicated that
temozolomide significantly reduced the expression of CXCL2
(42), suggesting that CXCL2 has an
essential role in tumor progression. CXCL2 is overexpressed in OC
tissues and overall survival analysis revealed that CXCL2 is
related to metastasis and unfavorable survival of patients with OC
(43). Another study suggested that
the serum level of CXCL2 in patients with HCC is higher than that
in patients with benign liver tumor or that in healthy subjects,
and its overexpression is associated with TNM stage, tumor size,
vascular embolism and Edmondson grade (44).
In the present study, it was first observed that the
expression of miR-27a-3p was upregulated in tumor tissues and
downregulated in pulmonary macrophages and blood of patients with
early NSCLC. It may be speculated that these differences have a
role in the processes of early NSCLC. Considering the key role of
inflammation in early NSCLC, the study then focused on macrophages.
After overexpression of miR-27a-3p, not only a significant
downregulation of CXCL2 expression in macrophages was observed, but
also a significant reduction of CXCL2 released into the cell
supernatant. As CXCL2 is a chemokine, the supernatant of cells
transfected with agomiR-27a-3p, or agomiR-NC as the control, was
used to induce the chemotaxis of human neutrophils. The results
were consistent with the speculations. It was indicated that in
early NSCLC, the expression of miR-27a-3p in macrophages is
decreased, resulting in increased release of CXCL2, so as to
recruit more immune cells in the early stage of LC and participate
in early tumor immunity.
In the present study, miRNA-27a-3p overexpression
not only significantly decreased CXCL2 expression in macrophages,
but also significantly reduced the content of CXCL2 released into
the cell supernatant. As CXCL2 is a chemokine, the supernatants of
cells transfected by agomiRNA-27a-3p or agomiR-NC were used to
induce human neutrophil chemotaxis and the results were consistent
with the expected outcomes. Macrophages transfected with
agomiRNA-27a-3p had significantly inhibited chemotaxis of
neutrophils. It may be speculated that in early NSCLC, the
downregulation of miRNA-27a-3p in macrophages leads to an increase
in CXCL2 release in order to recruit more immune cells in patients
with early-stage LC to activate early tumor immunity. However, the
present study has certain limitations. It was only speculated that
miRNA-27a has the potential to become a biomarker. Due to
individual differences and the common characteristics of miRNA, it
is difficult for a single miRNA to become an independent marker. In
the present study, the clinicopathological data and miRNA-27a
levels exhibited no significant correlation. Therefore, no receiver
operating characteristic curve was drawn.
In conclusion, the present study demonstrated that
the abnormal expression patterns of miRNA-27a-3p and CXCL2 in the
peripheral blood and macrophages of patients with early NSCLC may
have a prospect for clinical diagnosis and may be utilized as an
outstanding auxiliary for determining the prognosis of patients
with NSCLC. However, direct evidence for the interaction between
miRNA-27a-3p and CXCL2 in patients with NSCLC is required in order
to provide a solid foundation for the diagnosis, treatment and
prevention of this disease.
Acknowledgements
The authors would like to thank Dr Bing Luo
(Department of Microbiology, School of Basic Medicine, Qingdao
University, Qingdao, China) for his help with the preparation and
modification of this manuscript.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CZ and RZ contributed to the design of the study.
CZ, BL, FK and SZ performed the experiments. CZ and BL analyzed the
data. CZ, BL and RZ interpreted results and prepared the
manuscript. FK and SZ confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in the current study were
approved by the Ethics Committee of Shandong University (no.
H18026). Written informed consent was obtained from all patients or
their families.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kang CG, Lee HJ, Kim SH and Lee EO:
Zerumbone suppresses osteopontin-induced cell invasion through
inhibiting the FAK/AKT/ROCK pathway in human non-small cell lung
cancer A549 Cells. J Nat Prod. 79:156–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
She J, Yang P, Hong Q and Bai C: Lung
cancer in China: challenges and interventions. Chest.
143:1117–1126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen G, Umelo IA, Lv S, Teugels E, Fostier
K, Kronenberger P, Dewaele A, Sadones J, Geers C and De Grève J:
miR-146a inhibits cell growth, cell migration and induces apoptosis
in non-small cell lung cancer cells. PLoS One. 8:e603172013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma Y, Yu S, Zhao W, Lu Z and Chen J:
miR-27a regulates the growth, colony formation and migration of
pancreatic cancer cells by targeting Sprouty2. Cancer Lett.
298:150–158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guttilla IK and White BA: Coordinate
regulation of FOXO1 by miR-27a, miR-96, and miR-182 in breast
cancer cells. J Biol Chem. 284:23204–23216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Z, Hu S, Wang J, Cai J, Xiao L, Yu L
and Wang Z: MiR-27a modulates MDR1/P-glycoprotein expression by
targeting HIPK2 in human ovarian cancer cells. Gynecol Oncol.
119:125–130. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu XZ, Wang KP, Song HJ, Xia JH, Jiang Y
and Wang YL: MiR-27a-3p promotes esophageal cancer cell
proliferation via F-box and WD repeat domain-containing 7 (FBXW7)
suppression. Int J Clin Exp Med. 8:15556–15562. 2015.PubMed/NCBI
|
|
9
|
Peng H, Wang X, Zhang P, Sun T, Ren X and
Xia Z: miR-27a promotes cell proliferation and metastasis in renal
cell carcinoma. Int J Clin Exp Pathol. 8:2259–2266. 2015.PubMed/NCBI
|
|
10
|
Tang W, Zhu J, Su S, Wu W, Liu Q, Su F and
Yu F: MiR-27 as a prognostic marker for breast cancer progression
and patient survival. PLoS One. 7:e517022012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qiao B, He BX, Cai JH, Tao Q and King-Yin
Lam A: MicroRNA-27a-3p Modulates the Wnt/β-Catenin signaling
pathway to promote epithelial-mesenchymal transition in oral
squamous carcinoma stem cells by targeting SFRP1. Sci Rep.
7:446882017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakata W, Uemura M, Sato M, Fujita K,
Jingushi K, Ueda Y, Kitae K, Tsujikawa K and Nonomura N: Expression
of miR-27a-3p is an independent predictive factor for recurrence in
clear cell renal cell carcinoma. Oncotarget. 6:21645–21654. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li JM, Zhou J, Xu Z, Huang HJ, Chen MJ and
Ji JS: MicroRNA-27a-3p inhibits cell viability and migration
through down-regulating DUSP16 in hepatocellular carcinoma. J Cell
Biochem. 119:5143–5152. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang Y, Duan Y and Zhou H: MicroRNA-27a
directly targets KRAS to inhibit cell proliferation in esophageal
squamous cell carcinoma. Oncol Lett. 9:471–477. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eiró N and Vizoso FJ: Inflammation and
cancer. World J Gastrointest Surg. 4:62–72. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Butkiewicz D, Krześniak M, Drosik A,
Giglok M, Gdowicz-Kłosok A, Kosarewicz A, Rusin M, Masłyk B,
Gawkowska-Suwińska M and Suwiński R: The VEGFR2, COX-2 and MMP-2
polymorphisms are associated with clinical outcome of patients with
inoperable non-small cell lung cancer. Int J Cancer. 137:2332–2342.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boutsikou E, Domvri K, Hardavella G,
Tsiouda D, Zarogoulidis K and Kontakiotis T: Tumour necrosis
factor, interferon-gamma and interleukins as predictive markers of
antiprogrammed cell-death protein-1 treatment in advanced non-small
cell lung cancer: A pragmatic approach in clinical practice. Ther
Adv Med Oncol. 10:17588359187682382018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chistiakov DA, Orekhov AN and Bobryshev
YV: Chemokines and Relevant microRNAs in the Atherogenic Process.
Mini Rev Med Chem. 18:597–608. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kollmar O, Scheuer C, Menger MD and
Schilling MK: Macrophage inflammatory protein-2 promotes
angiogenesis, cell migration, and tumor growth in hepatic
metastasis. Ann Surg Oncol. 13:263–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schramm R and Thorlacius H: Neutrophil
recruitment in mast cell-dependent inflammation: Inhibitory
mechanisms of glucocorticoids. Inflamm Res. 53:644–652. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eide HA, Halvorsen AR, Sandhu V, Fåne A,
Berg J, Haakensen VD, Kure EH, Brustugun OT, Kiserud CE, Kyte JA
and Helland Å: Non-small cell lung cancer is characterised by a
distinct inflammatory signature in serum compared with chronic
obstructive pulmonary disease. Clin Transl Immunology. 5:e1092016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Burgess M, Cheung C, Chambers L,
Ravindranath K, Minhas G, Knop L, Mollee P, McMillan NA and Gill D:
CCL2 and CXCL2 enhance survival of primary chronic lymphocytic
leukemia cells in vitro. Leuk Lymphoma. 53:1988–1998. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Doll D, Keller L, Maak M, Boulesteix AL,
Siewert JR, Holzmann B and Janssen KP: Differential expression of
the chemokines GRO-2, GRO-3, and interleukin-8 in colon cancer and
their impact on metastatic disease and survival. Int J Colorectal
Dis. 25:573–581. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Armstrong DA, Major JA, Chudyk A and
Hamilton TA: Neutrophil chemoattractant genes KC and MIP-2 are
expressed in different cell populations at sites of surgical
injury. J Leukoc Biol. 75:641–648. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang N, Zhu S, Lv X, Qiao Y, Liu YJ and
Chen J: MicroRNAs: Pleiotropic regulators in the tumor
microenvironment. Front Immunol. 9:24912018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song Q, Li H, Shao H, Li C and Lu X:
MicroRNA-365 in macrophages regulates Mycobacterium
tuberculosis-induced active pulmonary tuberculosis via
interleukin-6. Int J Clin Exp Med. 8:15458–15465. 2015.PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Y, Tsai YH and Tseng SH: The
potential of tetrandrine as a protective agent for ischemic stroke.
Molecules. 16:8020–8032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou L, Liang X, Zhang L, Yang L, Nagao N,
Wu H, Liu C, Lin S, Cai G and Liu J: MiR-27a-3p functions as an
oncogene in gastric cancer by targeting BTG2. Oncotarget.
7:51943–51954. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang J, Tang J, Shi H, Li H, Zhen T, Duan
J, Kang L, Zhang F, Dong Y and Han A: miR-27a-3p targeting RXRα
promotes colorectal cancer progression by activating Wnt/β-catenin
pathway. Oncotarget. 8:82991–83008. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun Y, Yang X, Liu M and Tang H: B4GALT3
up-regulation by miR-27a contributes to the oncogenic activity in
human cervical cancer cells. Cancer Lett. 375:284–292. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Parchami Barjui S, Reiisi S, Ebrahimi SO
and Shekari B: Study of correlation between genetic variants in
three microRNA genes (hsa-miR-146a, hsa-miR-502 binding site,
hsa-miR-27a) and breast cancer risk. Curr Res Transl Med.
65:141–147. 2017.PubMed/NCBI
|
|
33
|
Scheibner KA, Teaboldt B, Hauer MC, Chen
X, Cherukuri S, Guo Y, Kelley SM, Liu Z, Baer MR, Heimfeld S and
Civin CI: MiR-27a functions as a tumor suppressor in acute leukemia
by regulating 14-3-3θ. PLoS One. 7:e508952012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Haskill S, Peace A, Morris J, Sporn SA,
Anisowicz A, Lee SW, Smith T, Martin G, Ralph P and Sager R:
Identification of three related human GRO genes encoding cytokine
functions. Proc Natl Acad Sci USA. 87:7732–7736. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dong QM, Zhang JQ, Li Q, Bracher JC,
Hendricks DT and Zhao XH: Clinical significance of serum expression
of GROβ in esophageal squamous cell carcinoma. World J
Gastroenterol. 17:2658–2662. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang H, Ye YL, Li MX, Ye SB, Huang WR,
Cai TT, He J, Peng JY, Duan TH, Cui J, et al: CXCL2/MIF-CXCR2
signaling promotes the recruitment of myeloid-derived suppressor
cells and is correlated with prognosis in bladder cancer. Oncogene.
36:2095–2104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu Y, Li S, Ma L, Li Y, Zhang X, Peng Q,
Mo C, Huang L, Qin X and Liu Y: Type conversion of secretomes in a
3D TAM2 and HCC cell co-culture system and functional importance of
CXCL2 in HCC. Sci Rep. 6:245582016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao H, Zhu H, Jin Q, Zhang S, Wang W,
Wang D and Huang J: Association of high expression of Groβ with
clinical and pathological characteristics of unfavorable prognosis
in gastrointestinal stromal tumors. Dis Markers. 2015:1710352015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kavandi L, Collier MA, Nguyen H and Syed
V: Progesterone and calcitriol attenuate inflammatory cytokines
CXCL1 and CXCL2 in ovarian and endometrial cancer cells. J Cell
Biochem. 113:3143–3152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Keeley EC, Mehrad B and Strieter RM: CXC
chemokines in cancer angiogenesis and metastases. Adv Cancer Res.
106:91–111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Luan J, Shattuck-Brandt R, Haghnegahdar H,
Owen JD, Strieter R, Burdick M, Nirodi C, Beauchamp D, Johnson KN
and Richmond A: Mechanism and biological significance of
constitutive expression of MGSA/GRO chemokines in malignant
melanoma tumor progression. J Leukoc Biol. 62:588–597. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bruyère C, Lonez C, Duray A, Cludts S,
Ruysschaert JM, Saussez S, Yeaton P, Kiss R and Mijatovic T:
Considering temozolomide as a novel potential treatment for
esophageal cancer. Cancer. 117:2004–2016. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ye Q, Zhai X, Wang W, Zhang S, Zhu H, Wang
D and Wang C: Overexpression of growth-related oncogene-β is
associated with tumorigenesis, metastasis, and poor prognosis in
ovarian cancer. Dis Markers. 2015:3873822015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li Y, Wang Y and Zhang P: Clinical
significance of serum expression of GROβ in hepatocellular
carcinoma. Tumour Biol. 36:6445–6449. 2015. View Article : Google Scholar : PubMed/NCBI
|