Introduction
Gastric cancer (GC) is the most prevalent malignant
tumor of the digestive tract, with over 1 million new cases
identified annually and China accounts for ~50% of the global GC
incidence (1). Despite a decline in
incidence and mortality worldwide during the last five years, GC
remains a serious public health concern in China and across the
globe (2). Therefore, prevention
and new therapeutic strategies are urgently needed.
At present, it is widely believed that the
occurrence of GC results from a combination of cytogenetic
variation accumulation and tumor immune evasion (3). The programmed death receptor
1/programmed death receptor ligand 1 (PD-1/PD-L1) axis is crucial
for tumor immune escape and PD-L1 overexpression is predictive of
poor prognosis (4–6). Immune checkpoint inhibitors (ICIs)
targeting PD-1/PD-L1 are often used to treat patients with advanced
GC and PD-L1 overexpression. However, increasing evidence has
suggested contradictory findings (7–12).
Therefore, the usefulness of PD-L1 expression in GC patient
prognosis requires additional investigation.
PD-L1 is extensively expressed in several cell
types, including tumor cells and activated immune cells associated
with tumors, such as dendritic cells (DCs), macrophages and
monocytes. Studies have reported that the prognosis in patients
with tumors is not only related to PD-L1 expression but also to the
tumor microenvironment (TME) immune cells, especially in
tumor-infiltrating T cells (TILs) (13–15).
Increasing evidence demonstrates that tumor-associated macrophages
(TAMs) are one of the most important immune cells in TME and serve
crucial roles in tumor occurrence and progression. Macrophages in
TME can secret cytokines to regulate immune response against cancer
through the PD1/PD-L1 axis of tumor cells or immune cells.
Therefore, macrophages in combination with PD-L1 may be a potential
prognosis indicator of patients with GC. However, studies of GC in
this area are still limited and the results obtained are
inconsistent. This may be related to macrophage plasticity, spatial
distribution and the methods used to evaluate PD-L1 expression.
Based on this consideration, in the present study, mIHC combined
with an automated pathological analysis system was used to quantify
the expression and spatial distribution of PD-L1 and cluster of
differentiation (CD)68, a biomarker of pan-microphages, in primary
GC and paired adjacent normal tissues. In addition, to optimize
PD-L1-based prognostic biomarkers in patients with GC, two other
proteins, human epidermal growth factor receptor 2 (HER2) and
CD133, closely related to the regulation of PD-L1 expression, were
also evaluated in this study (16).
Materials and methods
Patient characteristics
The HStnA180Su17 tissue microarray (TMA; Xinchao
Company) consisted of paired gastric adenocarcinoma tissues and
neighboring normal tissues (≥5 cm from tumor tissues) derived from
94 patients with GC, of which a cohort of 83 cases with integral
information and 10 cases with censored data (gastric adenocarcinoma
tissues only, no adjacent normal tissues) was taken into final
analyses. One case lost with multi-cutting was excluded. Patients
had had surgery between March 2007 and February 2008 and follow-up
information was provided between March 2007 and July 2011. Prior to
the operation, patients did not receive routine radiotherapy or
chemotherapy treatments. The Institutional Ethics Committee of the
First People's Hospital of Yunnan Province approved the research
(approval no. KHLL2019-KY037). Every process was carried out under
regulations (17). Table I summarizes the clinicopathological
features of patients.
 | Table I.Clinicopathological characteristics
of a cohort of 93 patients with primary GC. |
Table I.
Clinicopathological characteristics
of a cohort of 93 patients with primary GC.
| Clinical
characteristics | Sex | Age | TNM stage | Lymph node |
Differentiation | Disease status |
|---|
|
|
|
|
|
|
|
|---|
| Marker | N (%) | χ2 | P-value | χ2 | P-value | χ2 | P-value | χ2 | P-value | χ2 | P-value | χ2 | P-value |
|---|
| Sex |
| 1.000 | - | 0.043 | 0.532 | 2.245 | 0.204 | 0.025 | 0.873 | 0.059 | 0.808 | 0.818 | 0.366 |
|
Male | 71 (76.3) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Female | 22 (23.7) |
|
|
|
|
|
|
|
|
|
|
|
|
| Age (years) |
| 0.043 | 0.532 | 1.000 | - | 0.788 | 0.375 | 0.012 | 0.914 | 0.527 | 0.468 | 0.075 | 0.784 |
|
≤60 | 27 (29) |
|
|
|
|
|
|
|
|
|
|
|
|
|
>60 | 66 (71) |
|
|
|
|
|
|
|
|
|
|
|
|
| TNM stage |
| 0.788 | 0.375 | 0.788 | 0.375 | 1.000 | - | 44.214 | <0.001 | 9.139 | 0.003 | 14.190 | <0.001 |
| Early
(I+II) | 34 (36.6) |
|
|
|
|
|
|
|
|
|
|
|
|
| Late
(III+IV) | 59 (63.4) |
|
|
|
|
|
|
|
|
|
|
|
|
| Lymph node |
| 0.025 | 0.873 | 0.012 | 0.914 | 44.214 | <0.001 | 1.000 | - | 7.423 | 0.006 | 13.705 | <0.001 |
|
Negative | 20 (21.5) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Positive | 73 (78.5) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Differentiation |
| 0.059 | 0.808 | 0.527 | 0.468 | 9.139 | 0.003 | 7.423 | 0.006 | 1.000 | - | 1.951 | 0.163 |
|
Well | 36 (38.7) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Poor | 57 (61.3) |
|
|
|
|
|
|
|
|
|
|
|
|
| Disease status |
| 0.818 | 0.366 | 0.075 | 0.784 | 14.190 | <0.001 | 13.705 | <0.001 | 1.951 | 0.163 | 1.000 | - |
|
Survival | 19 (20.4) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Succumbed | 74 (79.6) |
|
|
|
|
|
|
|
|
|
|
|
|
| Tumor size
(cm) |
| 0.536 | 0.595 | 0.316 | 0.627 | 10.078 | 0.002 | 7.503 | 0.012 | 0.746 | 0.439 | 3.381 | 0.092 |
| L<5
cm | 28 (30.1) |
|
|
|
|
|
|
|
|
|
|
|
|
| L≥5
cm | 65 (69.9) |
|
|
|
|
|
|
|
|
|
|
|
|
Samples and TMA preparation
Based on the pathological diagnosis of each tissue,
TMAs were produced. At least two independent pathologists examined
formalin-fixed, dehydrated, paraffin-embedded hematoxylin and eosin
(H&E) stained tumor and nearby normal tissue samples. For
fixation, cut tissue blocks were fixed in 4% paraformaldehyde (PFA)
at a volume ratio of 1:7 tissue block to 4% PFA for 24 h. The fixed
tissues were dehydrated with graded alcohol (30 min each in 85, 90
and 100% ethanol) and cleared with xylene for 20 min. Tissue was
placed in a mold with paraffin wax. Subsequently, the mold is moved
to a bench at −10°C. The paraffin wax solidified rapidly and the
tissue was fixed in it. The representative tumor regions of each
donor block were then identified. Next, 1 mm diameter core
cylinders were punched from each of these regions and placed in a
recipient paraffin block to create TMAs. Finally, 4 mm-thick TMA
sections were sliced and mounted on slides coated with
poly-L-Lysine for multiplexed immunohistochemistry (mIHC)
analysis.
Fluorescent mIHC of TMA
For mIHC labeling, the antibody concentrations for
HER2 (1:200; cat. no. BX50015; PerkinElmer, Inc.), PD-L1 (1:200;
cat. no. BX00005; PerkinElmer, Inc.), CD133 (1:100; cat. no. 86781;
Cell Signaling Technology, Inc.) CD68 (1:1,500; cat. no. BX50031;
PerkinElmer, Inc.) were optimized and a spectrum library was
constructed using single-stained slides. Then, using the PANO
7-plex IHC kit (cat. no. 0004100100, Panovue), multiplex
multispectral imaging of the identified proteins and
immunofluorescence staining were performed on a single TMA slide.
The slides were heated for ≤1 h at 60°C in a dry oven and
deparaffinized three times for 10 min using xylene. The slide was
rehydrated in a sequence of 100, 95 and 85% alcohols to distilled
water. After washing for 5 min in distilled water, antigen
retrieval was conducted in citrate buffer (pH 6.0) for 15 min using
microwave treatment and cooled to room temperature. After washing
and blocking with a 3% H2O2 blocking solution
for 10 min, the slide was stained with the primary antibody. The
Opal Polymer HRP Ms+Rb Kit (PerkinElmer, Inc.) was used for
detection after overnight incubation at 4°C with each primary
antibody. The slide was then treated with a 1:100 dilution of
tyramide (TSA)-conjugated fluorophore (TSA Fluorescence Kit;
Panovue). The TSA was then vacuumed off and the slide was washed
twice with 1X TBST for 3 min before the subsequent staining step.
For each additional marker, the procedure was repeated by microwave
heat-treating the slide for retrieving antigen, followed directly
by one primary antibody staining in each cycle, ordered as HER2,
CD133, PD-L1 and CD68, respectively and then by the aforementioned
downstream procedures. After labeling all human antigens, nuclei
were counterstained with 4′-6′-diamidino-2-phenylindole (DAPI; cat.
no. D9542; MilliporeSigma).
Multispectral imaging and scoring
multispectral images
At ×20 magnification, ≤5 non-overlapping image
fields were collected for each slide using the Vectra system
(PerkinElmer, Inc.) and processed using inform softwarev2.3.0
(PerkinElmer, Inc.). Briefly, tissue autofluorescence and each
fluorescein spectra were extracted from unstained and
single-stained sections images, respectively. The extracted images
were then used to create a spectrum library for images of sections
with autofluorescence removed. For scoring, the expression of CD68,
CD133, HER2 and PD-L1 levels were assessed using H-score as
previously described (18).
Human protein atlas database (HPA) and
gene expression profiling interactive analysis database analysis
(GEPIA)
HPA and GEPIA are two online databases. The PD-L1
expression levels in cellular components of GC tissues and link
with 5-year survival were analyzed using the Human Protein Atlas
(HPA) website (http://www.proteinatlas.org). GEPIA (http://gepia.cancer-pku.cn) is an online database
making gene expression profiling and interactive analyses with
cancer and normal samples. In the present study, GEPIA was used to
verify the correlation between PD-L1 and CD68 protein levels and
the link with disease-free survival of primary GC patients. The
Spearman correlation statistical method was used to calculate the
correlation coefficient.
Statistical analysis
The Mann-Whitney U test determined the expression
differences of the four detected proteins in studied specimens.
Clinical correlation was calculated by Spearman analysis. The
Kaplan-Meier analysis assessed overall survival (OS) rates and the
log-rank test was performed to plot survival curves. Statistical
analyses were performed with GraphPad Prism 6 (GraphPad Software;
Dotmatics) and SPSS 22.0 (IBM Corp.).
Results
Demographics characteristics
There were 71 male patients with an average age of
66±10 years and 22 female patients with an average age of 64±11
years. Other basic information about patients, such as sex,
pathological differentiation, lymph nodes, TNM stages and survival
status, is listed in Table I.
Statistical analysis of the clinical variables showed no
significant correlation between either age or sex and lymph node
metastasis (LNMs), survival rate, pathological differentiation, or
TNM stage of patients with GC (P>0.05). Pathological
differentiation and tumor size were significantly correlated with
the LNMs and TNM stages of patients. However, there was no
statistically significant difference in the OS of the patients
(P>0.01).
Differential expression of the
potential proteins between the tumor tissues and adjacent normal
tissues
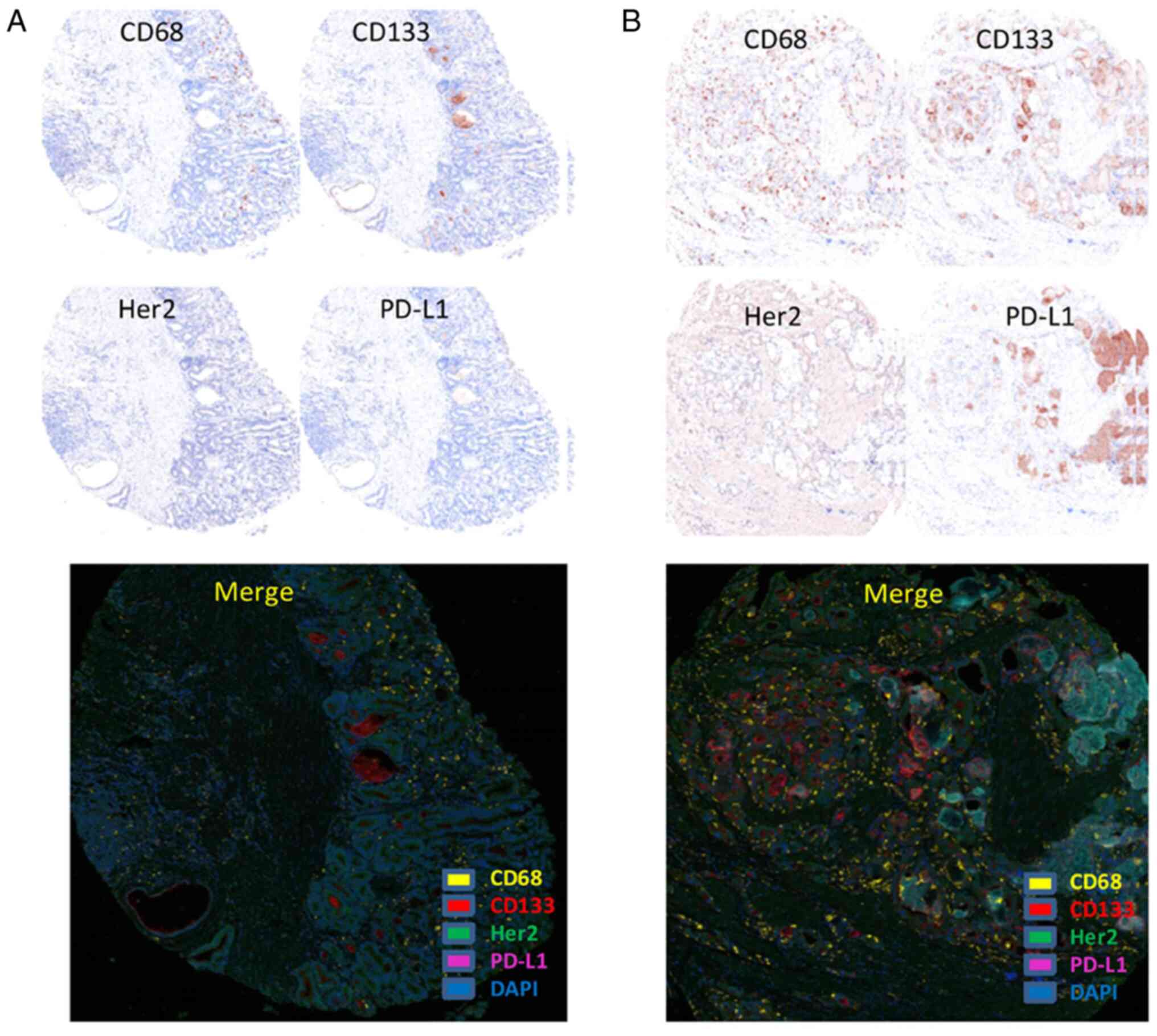
HER2, CD133 and PD-L1 antigens were mainly expressed
on the cell membrane, particularly CD133 expression on
glandular-luminal tumor cell membrane surface (luminal expression,
L-type), but CD68 is found in both the cell membrane and cytoplasm
(Fig. 1). Statistical analysis
revealed that the HER2, CD133 and CD68 expression levels were
considerably greater in tumor tissues than in surrounding normal
tissues (P<0.05), while no substantial link was observed for
PD-L1 expression (P=0.0560; Fig.
2A). The spatial distribution revealed that the CD133, HER2 and
CD68 expression levels were greater in TN and tumor stroma (TS)
than in the neighboring normal tissues (P<0.05). In contrast,
PD-L1 expression was considerably lower in TN of tumor tissues than
in adjacent areas of normal tissue, although no significant
difference was observed in TS. (Fig. 2B
and C). In addition, although the proportion of double-positive
and triple-positive antigens is low, there is also a significant
difference in the expression level between cancer and adjacent
normal tissues (Table II).
 | Table II.Spearman analysis for the correlation
of each protein distributed in tumor tissues. |
Table II.
Spearman analysis for the correlation
of each protein distributed in tumor tissues.
|
|
| CD133 | HER2 | PD-L1 | CD68 |
|---|
|
|
|
|
|
|
|
|---|
| Measured
region | Marker | r | P-value | r | P-value | r | P-value | r | P-value |
|---|
| Total | CD133 | 1.000 | - | 0.055 | 0.598 | −0.161 | 0.122 | 0.065 | 0.534 |
|
| HER2 | 0.055 | 0.598 | 1.000 | - | 0.062 | 0.555 | 0.446 | <0.001 |
|
| PD-L1 | −0.161 | 0.122 | 0.062 | 0.555 | 1.000 | - | 0.345 | 0.001 |
|
| CD68 | 0.065 | 0.534 | 0.446 | <0.001 | 0.345 | 0.001 | 1.000 | - |
| TN | CD133 | 1.000 | - | 0.048 | 0.649 | −0.162 | 0.120 | −0.015 | 0.884 |
|
| HER2 | 0.048 | 0.649 | 1.000 | - | −0.065 | 0.537 | 0.377 | <0.001 |
|
| PD-L1 | −0.162 | 0.120 | 0.065 | 0.537 | 1.000 | - | 0.368 | <0.001 |
|
| CD68 | −0.015 | 0.884 | 0.377 | <0.001 | 0.368 | <0.001 | 1.000 | - |
| TS | CD133 | 1.000 | - | −0.009 | 0.932 | −0.136 | 0.193 | 0.177 | 0.090 |
|
| HER2 | −0.009 | 0.932 | 1.000 | - | 0.009 | 0.928 | 0.480 | <0.001 |
|
| PD-L1 | −0.136 | 0.193 | 0.009 | 0.928 | 1.000 | - | −0.024 | 0.821 |
|
| CD68 | 0.177 | 0.090 | 0.480 | <0.001 | −0.024 | 0.821 | 1.000 | - |
Correlation of the detected proteins
expressed in GC tissues
The correlation analysis showed that the PD-L1 and
CD68 and HER2 and CD68 expression were positively correlated in
both the whole tumor tissue and the TN (P<0.01). In the TS, only
the HER2expression was positively correlated with CD68 (P<0.01),
while no significant correlation was found between the expression
of PD-L1 and CD68 (P>0.05; Table
II).
The relationship between the detected
proteins and clinical features
Although the CD133 and HER2 proteins expression
levels were significantly elevated in GC tissues than in
neighboring normal tissues, there was no significant correlation
between the expression levels of these two proteins in tumor
tissues and clinical variables such as sex, age, survival status,
pathological differentiation, TNM stage and LNMs. The PD-L1
expression level had no significant correlation with sex and age
but had a significant correlation with TNM stage and pathological
differentiation no matter in whole tumor tissues or TN (P<0.05),
with a marginally significant association with survival status in
TN (P=0.056) and significantly associated with pathological
differentiation in TS (Fig. 3A-C).
Survival curve analysis revealed that patients with GC with high
PD-L1 expression survived longer than those with low expression;
however, the difference between the two groups was not
statistically significant (P=0.088; Fig. 4A and B). The degree of CD68
expression in TS was substantially associated with pathological
differentiation of patients with GC (P=0.0376), while there was no
significant association in TN (Fig.
3C). However, PD-L1+CD68+ macrophages in
TN were significantly related to tumor size, TNM stages,
pathological differentiation and survival status (P<0.05;
Fig. 3D). To obtain support for the
results of the present study from clinical samples, the correlation
of PD-L1 and CD68 expression in tumor tissues from patients with GC
was analyzed with clinical outcomes using two public online
databases (HPA and GEPIA). PD-L1 was expressed on fibroblasts,
endothelial cells, neutrophils and macrophages of GC tissues
(Fig. 5A). Moreover, PD-L1
expression was significantly correlated with CD68 (Fig. 5B). Although there was no statistical
difference between PD-L1 expression and disease-free survival (DFS)
or 5-year survival, patients with high PD-L1 expression had a
prolonged survival compared with patients with low PD-L1 expression
(Fig. 5C and D). These results are
consistent with the present study.
 | Figure 3.Correlation analysis of each protein
expression with single clinical variables. (A) In the whole tumor
tissues, PD-L1 expression was significantly associated with the TNM
stages and pathological tissue differentiation. (B) In the TNs,
PD-L1 expression was not only significantly correlated with
aforementioned two clinical parameters, but also with survival
status of patients. However, no significant correlation was
observed between the other proteins and clinical variables of
patients. (C) In the TS, both PD-L1 and CD68 expression was only
significantly correlated with pathological differentiation. (D) The
levels of PD-L1+CD68+ in the TNs (but not in
the TS) were significantly correlated with the patients' tumor
size, TNM stage, pathological differentiation and survival status.
No correlation was found between other protein combinations and
clinical variables. Spearman analysis was used to assess the
correlation between protein expression and clinical variables.
PD-L1, programmed death receptor ligand 1; TN, tumor nest; TS,
tumorstroma; CD, cluster of differentiation. |
Discussion
At present, PD-L1 expression is an important
molecular marker for the prognosis of GC and the selection of
targeted PD-1/PD-L1 ICIs (4–6).
However, the results reported in studies are often inconsistent
(7–12). The inconsistent results are related
to the choice of assays and assessment methods in different studies
on the one hand and suggest that the molecular markers for
prognosis of patients with GC based on PD-L1 still need to be
optimized on the other hand.
In the present study, mIHC and an automated
pathological analysis system were applied to detect the expression
level and spatial distribution of PD-L1, CD68, CD133 and HER2 in
tumor tissues of primary patients with GC arranged on TMA and its
potential prognostic value was explored.
In recent years, mIHC has emerged as an important
technique in the field of pathology research, allowing for the
detection of multi-targets in situ on cell or tissue
samples, as well as the quantitative pathological analysis of
spatial localization and quantification of each target and its
interactions in situ on tissue cells (19). In addition, all tissue samples in
the present study were prepared into TMA, which can further reduce
the influence of human factors on the results during the
experimental process.
In the present study, the PD-L1 expression level in
tumor tissues was not statistically different from that in adjacent
normal tissues; nevertheless, PD-L1 expression was considerably
downregulated in TN relative to normal tissues. PD-L1 expression
was also associated with a favorable prognosis in GC, including
early TNM stage, excellent tumor differentiation and prolonged
overall survival. This conclusion contradicts earlier evidence that
the PD-L1 expression in patients with GC is associated with a worse
prognosis (20–23). Indeed, the PD-L1 expression-based
prognosis in GC remains controversial (8,9,22,24).
Böger et al (9) revealed
that a high PD-1/PD-L1 expression was strongly related to an
improved prognosis for patients with GC and PD-L1 became an
independent survivor prognosticator in Western patients. Although
Asian and non-Asian GCs have unique tumor immunity patterns
associated with T-cell activity, this may affect the association
between PD-1/PD-L1 expression and patient survival. Rha et
al (8) found that PD-L1
expression was not linked with OS in patients with GC, regardless
of whether they originated from Asia or the West.
It was hypothesized that those controversial results
might relate to the means of PD-L1 detection and assessment
methods. Not only do tumor cells express PD-L1, but so do immune
cells in the TME. Moreover, over the last several years, an
increasing number of studies have found that PD-L1 expressed on
immune cells in the TME is closely related to patient prognosis
(13,25). The present study showed that high
PD-L1 expression in TN of tumor tissues and a high number of
PD-L1+CD68+ macrophages were significantly associated with good
prognosis of primary patients with GC. The results appear that
PD-L1 expression in cancer cells may not be a critical factor in GC
and that immune cell deprivation in the TME may be a more critical
factor in GC occurrence. This is also consistent with the study
that the combined positive score of PD-L1 scoring systems in GC is
more prognostically valuable than tumor proportion score (26). However, as CD68 is an pan-macrophage
marker, which separates at least MI and M2 subtypes, which serve
different roles in immunity. Therefore, more research will be
conducted to further explore the prognostic potential of macrophage
subtypes co-expressed with PD-L1 in primary patients with GC.
HER2 is an epithelial cell-expressed
ligand-independent receptor tyrosine kinase involved in cell
differentiation. HER2 has been documented to be amplified and
overexpressed in a variety of human cancers, which is correlated
with a worse prognosis, higher recurrence rates and shorter OS
(27). A number of studies
established that HER2 controls the abnormal expression of PD-L1 in
the stomach and the combination of anti-HER2 and anti-PD-1 has
proven synergistic anticancer effects in animal models (16,28,29).
Further studies disclosed that dimerization of the HER2 receptor
activates two major intracellular signaling pathways: the
mitogen-activated protein kinase (MAPK) (Ras/Raf/MEK/ERK) and
phosphatidylinositol 3′-kinase (PI3K)/Akt, inducing cell-cycle
progression, proliferation and survival (30–32).
PD-L1 is a dynamic marker that can be expressed constitutively
(driven by endogenous oncogenic pathways) or in an inducible
fashion (motivated by exogenous signals) (33). Constitutive PD-L1 is mainly
regulated via MAPK (Ras/Raf/MEK/ERK) and PI3K/Akt pathways.
Therefore, both molecular routes are involved in the HER2
intracellular signaling pathway. Inhibiting these routes can
regulate PD-L1 expression, which can modify the efficacy of HER2
target treatments (34). Inducible
PD-L1 is regulated by extracellular signals such as cytokines,
epidermal growth factors and hypoxic conditions. Interferon-γ
(IFN-γ) is a cytokine which is known to regulate PD-L1 expression
(35). Yamashita et al
(26) showed that trastuzumab can
upregulate PD-L1 in HER2-amplified GC cells by interacting with NK
cells through the secretion of IFN-γ. Altogether, there is a
complex regulatory network between HER2 and PD-L1 expression, which
is not only affected by the tumor cells themselves, but also
closely related to the TME (36).
The relationship between molecular subtypes of GC and PD-L1, HER2
and combined HER2 and PD-L1 expression, requires further
investigation.
According to the present study, HER2 expression was
substantially greater in GC tissues than in nearby normal tissues.
However, HER2 expression did not correlate significantly with PD-L1
nor with clinical prognostic parameters in the TN. Notably, HER2
expression was significantly linked with CD68 expression in the TS
and poor tumor differentiation. This result is in accordance with
Chen et al (37) on breast
ductal carcinoma in situ. The spatial distribution of CD68
in GC tissues appeared quite different and associated with
clinicopathological parameters. For instance, CD68 expression in TS
was markedly linked with poor tumor differentiation, but
co-expression of CD68 with PD-L1 in TN was significantly associated
with excellent tumor differentiation. Similar results have been
observed for a number of tumor types, including non-small cell lung
cancer and breast cancer (38,39).
Several growth factors and proteases important in angiogenesis,
invasiveness and migration of cancer cells are secreted by cancer
stromal macrophages, hence supporting cancer development (40). By contrast, cancer-associated
macrophages release cytotoxic cytokines, such as IL-1a, IL-1b, IL-6
and TNF-a, which may inhibit tumor development (41–43).
Therefore, TN macrophages and TS macrophages may have distinct
biological activities in relation to tumor growth. Moreover, the
predictive significance of CD68 in the clinic must take into
account not only its expression level but also its spatial
localization. Although the western blotting assay can reflect the
relative expression of PD-L1 proteins in the whole tumor tissue, it
is not able to show spatial differences in TN or TS. Therefore, the
western blotting method was not used in the present study to
further validate its results. To obtain support for the results of
this study from clinical samples, the present study analyzed the
correlation of PD-L1 and CD68 expression in tumor tissues from
patients with GC with clinical outcomes using two public databases.
The results were consistent with the present study.
CD133 is one of the most often used markers for
cancer stem cells. Mounting evidence has shown that over-expression
of CD133 is strongly related to tumor progression, treatment
resistance and tumor recurrence (44–48).
However, the clinical and prognostic value of CD133 in GC remains
debatable (44,49,50).
CD133 expression in tumor tissues was much greater than in nearby
normal tissues, as shown by the present study. However, no link
between CD133 expression and clinical outcome was discovered.
Moreover, two types of CD133 expression were detected in tumor
cells: glandular-luminal cell membrane surface expression (L-type
luminal expression) and cytoplasmic expression (C-type). CD133
expression can be broadly divided into two types that have been
reported in several tumors (50–52).
Hashimoto et al (50) noted
that the expression of CD133in C-type GC cells had a higher
malignant potential than that in L-type GC cells. Hashimoto et
al (50) reported that the
expression of CD133 in C-type GC cells predicted a higher malignant
potential than in L-type GC cells. Similar results were also
observed in hepatocellular carcinoma and rectal cancer (53,54).
Based on these studies, it was hypothesized that no significant
association was observed between CD133 and clinical relevance
because the overall CD133 expression in gastric cancer was
evaluated without dividing the cases into expression types. As the
automated pathological analysis system used in the present study
could not distinguish the two types of CD133 expression in cancer
cells. Therefore, the prognostic value of CD133 protein in GC needs
to be further investigated by distinguishing these two cell types
and correlating them with clinical parameters in future
studies.
In conclusion, the present study investigated the
prognostic value of PD-L1 in combination with CD68, CD133 andHER2
in primary GC. It was found that PD-L1+CD68+ macrophage
infiltration in TN might be a potential indicator of prognosis in
patients with primary GC which deserves further exploration.
Acknowledgements
Not applicable.
Funding
The present study was founded by the National Natural Science
Foundation of China (grant nos. 82260483 and 81460463); Yunnan
Provincial Department of Science and Technology Foundation for
Applied Basic Research (grant no. 202301AS070015); Yunnan
Provincial Department of Science and Technology-Kunming Medical
University Joint Foundation for Applied Basic Research (grant no.
202101AY070001-27); Yunnan Digestive Endoscopy Clinical Medical
Center Foundation for Health Commission of Yunnan Province (grant
nos. 2019LCZXKF-XH04 and 2020LCZXKF-XH09 of 2X2019-01-02) and
Yunnan Hematology Clinical Medical Center Foundation for Health
Commission of Yunnan Province (grant no. 2020LCZXKF-XY08).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZS, QG and HT designed the project; YZ analysed and
interpreted the data and wrote the manuscript; JW analyzed survival
data; JZ performed the experiments and collected the data; HT and
QG confirmed the authenticity of all the raw data. All authors
participated in critical revision of the manuscript and all authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The patients with GC for TMA (HStnA180Su17; Xinchao
Company) gave informed consent. The present study was conducted
under the approval of the Institutional Ethics Committee (approval
no. KHLL2019-KY037). All procedures were performed in accordance
with the relevant guidelines and regulations (17).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xie W, Yang T, Zuo J, Ma Z, Yu W, Hu Z and
Song Z: Chinese and global burdens of gastrointestinal cancers from
1990 to 2019. Front Public Health. 10:9412842022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iwasaki A, Shinozaki-Ushiku A, Kunita A,
Yamazawa S, Sato Y, Yamashita H, Fukayama M, Seto Y and Ushiku T:
Human leukocyte antigen class I deficiency in gastric carcinoma: An
adaptive immune evasion strategy most common in microsatellite
instable tumors. Am J Surg Pathol. 45:1213–1220. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qing Y, Li Q, Ren T, Xia W, Peng Y, Liu
GL, Luo H, Yang YX, Dai XY, Zhou SF and Wang D: Upregulation of
PD-L1 and APE1 is associated with tumorigenesis and poor prognosis
of gastric cancer. Drug Des Devel Ther. 9:901–909. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saito H, Kono Y, Murakami Y, Shishido Y,
Kuroda H, Matsunaga T, Fukumoto Y, Osaki T, Ashida K and Fujiwara
Y: Highly activated PD-1/PD-L1 pathway in gastric cancer with PD-L1
expression. Anticancer Res. 38:107–112. 2018.PubMed/NCBI
|
|
6
|
Zhang M, Dong Y, Liu H, Wang Y, Zhao S,
Xuan Q, Wang Y and Zhang Q: The clinicopathological and prognostic
significance of PD-L1 expression in gastric cancer: A meta-analysis
of 10 studies with 1,901 patients. Sci Rep. 6:379332016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pereira MA, Ramos MFKP, Dias AR, Ribeiro
R, Cardili L, Zilberstein B, Cecconello I, Ribeiro U Jr, de Mello
ES and de Castria TB: Scoring systems for PD-L1 expression and
their prognostic impact in patients with resectable gastric cancer.
Virchows Arch. 478:1039–1048. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rha SY, Ku GY, Kim HS, Chung HC, Amlashi
FG, Maru DM, Fein CA, Tang LH, Zhou W, Wu T, et al: PD-L1
expression and overall survival in Asian and western patients with
gastric cancer. Future Oncol. 18:2623–2634. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Böger C, Behrens HM, Mathiak M, Krüger S,
Kalthoff H and Röcken C: PD-L1 is an independent prognostic
predictor in gastric cancer of western patients. Oncotarget.
7:24269–24283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang F, Zhang J, Zhao L, Zhai M, Zhang T
and Yu D: A PD-L1 negative advanced gastric cancer patient with a
long response to PD-1 blockade after failure of systematic
treatment: A case report. Front Immunol. 12:7592502021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ribeiro HSC, Menezes JN, da Costa WL Jr, F
de Jesus VH, Diniz AL, Godoy AL, de Farias IC, Torres SM, Neotti T,
Mello CAL, et al: PD-L1 expression in gastric and gastroesophageal
junction cancer patients treated with perioperative chemotherapy. J
Surg Oncol. 126:150–160. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu Y, Cao D, Qu L, Cao X, Jia Z, Zhao T,
Wang Q and Jiang J: PD-1 and PD-L1 co-expression predicts favorable
prognosis in gastric cancer. Oncotarget. 8:64066–64082. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ju X, Shen R, Huang P, Zhai J, Qian X,
Wang Q and Chen M: Predictive relevance of PD-L1 expression with
pre-existing TILs in gastric cancer. Oncotarget. 8:99372–99381.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park IH, Kong SY, Ro JY, Kwon Y, Kang JH,
Mo HJ, Jung SY, Lee S, Lee KS, Kang HS, et al: Prognostic
implications of tumor-infiltrating lymphocytes in association with
programmed death ligand 1 expression in early-stage breast cancer.
Clin Breast Cancer. 16:51–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Q, Lou W, Di W and Wu X: Prognostic
value of tumor PD-L1 expression combined with CD8+ tumor
infiltrating lymphocytes in high grade serous ovarian cancer. Int
Immunopharmacol. 52:7–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chakrabarti J, Koh V, Steele N, Hawkins J,
Ito Y, Merchant JL, Wang J, Helmrath MA, Ahmad SA, So JBY, et al:
Disruption of Her2-induced PD-L1 inhibits tumor cell immune evasion
in patient-derived gastric cancer organoids. Cancers (Basel).
13:61582021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
China Medical Biotechnology Association
Biobank (Trial), . Chin Med Biotechnol. 6:71–79. 2011.(In
Chinese).
|
|
18
|
Zou Y, Zhang J, Zhang L and Yan X:
Interferon-induced protein 16 expression in colorectal cancer and
its correlation with proliferation and immune signature markers.
Onco Lett. 22:6872021. View Article : Google Scholar
|
|
19
|
Taube JM, Akturk G, Angelo M, Engle EL,
Gnjatic S, Greenbaum S, Greenwald NF, Hedvat CV, Hollmann TJ, Juco
J, et al: The society for immunotherapy of cancer statement on best
practices for multiplex immunohistochemistry (IHC) and
immunofluorescence (IF) staining and validation. J Immunother
Cancer. 8:e0001552020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG and
Xu N: Immunohistochemical localization of programmed death-1
ligand-1 (PD-L1) in gastric carcinoma and its clinical
significance. Acta Histochem. 108:19–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hassen G, Kasar A, Jain N, Berry S, Dave
J, Zouetr M, Priyanka Ganapathiraju VLN, Kurapati T, Oshai S, Saad
M, et al: Programmed death-ligand 1 (PD-L1) positivity and factors
associated with poor prognosis in patients with gastric cancer: An
umbrella meta-analysis. Cureus. 14:e238452022.PubMed/NCBI
|
|
22
|
Chen L, Wang L, Li X, Zhang G, Li Z and
Wang Y: Clinic-pathological characteristics and prognostic value of
PD-L1 and HER2 in gastric cancer. DNA Cell Biol. 40:405–413. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Amatatsu M, Arigami T, Uenosono Y,
Yanagita S, Uchikado Y, Kijima Y, Kurahara H, Kita Y, Mori S,
Sasaki K, et al: Programmed death-ligand 1 is a promising blood
marker for predicting tumor progression and prognosis in patients
with gastric cancer. Cancer Sci. 109:814–820. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He PX, Ma ZL, Han H, Zhang XY, Niu SH, Du
LN, Zheng YC and Liu HM: Expression of programmed death ligand 1
(PD-L1) is associated with metastasis and differentiation in
gastric cancer. Life Sci. 242:1172472020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zeng D, Li M, Zhou R, Zhang J, Sun H, Shi
M, Bin J, Liao Y, Rao J and Liao W: Tumor microenvironment
characterization in gastric cancer identifies prognostic and
immunotherapeutically relevant gene signatures. Cancer Immunol Res.
7:737–750. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamashita K, Iwatsuki M, Harada K, Eto K,
Hiyoshi Y, Ishimoto T, Nagai Y, Iwagami S, Miyamoto Y, Yoshida N,
et al: Prognostic impacts of the combined positive score and the
tumor proportion score for programmed death ligand-1 expression by
double immunohistochemical staining in patients with advanced
gastric cancer. Gastric Cancer. 23:95–104. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fong C and Chau I: HER2 inhibition in
gastric cancer-novel therapeutic approaches for an established
target. Cancers (Basel). 14:38242022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oki E, Okano S, Saeki H, Umemoto Y,
Teraishi K, Nakaji Y, Ando K, Zaitsu Y, Yamashita N, Sugiyama M, et
al: Protein expression of programmed death 1 ligand 1 and HER2 in
gastric carcinoma. Oncology. 93:387–394. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Attia S, Abd El Hafez A, Abdel-Aziz A,
Elmetwaly S and Mokhtar N: Prognostic value of PD-L1
immunohistochemical marker in gastric carcinoma and its correlation
with HER2 status. Asian Pac J Cancer Prev. 23:1466–1444. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pous A, Notario L, Hierro C, Layos L and
Bugés C: HER2-positive gastric cancer: The role of immunotherapy
and novel therapeutic strategies. Int J Mol Sci. 24:114032023.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoon SO, Shin S, Lee HJ, Chun HK and Chung
AS: Isoginkgetin inhibits tumor cell invasion by regulating
phosphatidylinositol 3-kinase/Akt-dependent matrix
metalloproteinase-9 expression. Mol Cancer Ther. 5:2666–2675. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fink MY and Chipuk JE: Survival of
HER2-positive breast cancer cells: Receptor signaling to apoptotic
control centers. Genes Cancer. 4:187–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang X, Zhou J, Giobbie-Hurder A, Wargo J
and Hodi FS: The activation of MAPK in melanoma cells resistant to
BRAF inhibition promotes PD-L1 expression that is reversible by MEK
and PI3K inhibition. Clin Cancer Res. 19:598–609. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen J, Jiang CC, Jin L and Zhang XD:
Regulation of PD-L1: A novel role of pro-survival signalling in
cancer. Ann Oncol. 27:409–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang D, Chen X, Du Y, Li X, Ying L, Lu Y,
Shen B, Gao X, Yi X, Xia X, et al: Associations of HER2 mutation
with immune-related features and immunotherapy outcomes in solid
tumors. Front Immunol. 13:7999882022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen XY, Thike AA, Md Nasir ND, Koh VCY,
Bay BH and Tan PH: Higher density of stromal M2 macrophages in
breast ductal carcinoma in situ predicts recurrence. Virchows Arch.
476:825–833. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Y, Zugazagoitia J, Ahmed FS, Henick
BS, Gettinger SN, Herbst RS, Schalper KA and Rimm DL: Immune Cell
PD-L1 colocalizes with macrophages and is associated with outcome
in PD-1 pathway blockade therapy. Clin Cancer Res. 26:970–977.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang ZQ, Milne K, Derocher H, Webb JR,
Nelson BH and Watson PH: PD-L1 and intratumoral immune response in
breast cancer. Oncotarget. 8:51641–51651. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tan HY, Wang N, Zhang C, Chan YT, Yuen MF
and Feng Y: Lysyl oxidase-like 4 fosters an immunosuppressive
microenvironment during hepatocarcinogenesis. Hepatology.
73:2326–2341. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kawai O, Ishii G, Kubota K, Murata Y,
Naito Y, Mizuno T, Aokage K, Saijo N, Nishiwaki Y, Gemma A, et al:
Predominant infiltration of macrophages and CD8(+) T Cells in
cancer nests is a significant predictor of survival in stage IV
nonsmall cell lung cancer. Cancer. 113:1387–1395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ohno S, Inagawa H, Dhar DK, Fujii T, Ueda
S, Tachibana M, Suzuki N, Inoue M, Soma G and Nagasue N: The degree
of macrophage infiltration into the cancer cell nest is a
significant predictor of survival in gastric cancer patients.
Anticancer Res. 23:5015–5022. 2003.PubMed/NCBI
|
|
43
|
Medrek C, Pontén F, Jirström K and
Leandersson K: The presence of tumor associated macrophages in
tumor stroma as a prognostic marker for breast cancer patients. BMC
Cancer. 12:3062012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Attia S, Atwan N, Arafa M and Shahin RA:
Expression of CD133 as a cancer stem cell marker in invasive
gastric carcinoma. Pathologica. 111:18–23. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jiang Y, He Y, Li H, Li HN, Zhang L, Hu W,
Sun YM, Chen FL and Jin XM: Expressions of putative cancer stem
cell markers ABCB1, ABCG2, and CD133 are correlated with the degree
of differentiation of gastric cancer. Gastric Cancer. 15:440–450.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu Y, Yu J, Wang S, Lu R, Wu J and Jiang
B: Overexpression of CD133 enhances chemoresistance to
5-fluorouracil by activating the PI3K/Akt/p70S6K pathway in gastric
cancer cells. Oncol Rep. 32:2437–2444. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen XL, Chen XZ, Wang YG, He D, Lu ZH,
Liu K, Zhang WH, Wang W, Li CC, Xue L, et al: Clinical significance
of putative markers of cancer stem cells in gastric cancer: A
retrospective cohort study. Oncotarget. 7:62049–62069. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Razmi M, Ghods R, Vafaei S, Sahlolbei M,
Saeednejad Zanjani L and Madjd Z: Clinical and prognostic
significances of cancer stem cell markers in gastric cancer
patients: A systematic review and meta-analysis. Cancer Cell Int.
21:1392021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yiming L, Yunshan G, Bo M, Yu Z, Tao W,
Gengfang L, Dexian F, Shiqian C, Jianli J, Juan T and Zhinan C:
CD133 overexpression correlates with clinicopathological features
of gastric cancer patients and its impact on survival: A systematic
review and meta-analysis. Oncotarget. 6:42019–42027. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hashimoto K, Aoyagi K, Isobe T, Kouhuji K
and Shirouzu K: Expression of CD133 in the cytoplasm is associated
with cancer progression and poor prognosis in gastric cancer.
Gastric Cancer. 17:97–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ishigami S, Ueno S, Arigami T, Uchikado Y,
Setoyama T, Arima H, Kita Y, Kurahara H, Okumura H, Matsumoto M, et
al: Prognostic impact of CD133 expression in gastric carcinoma.
Anticancer Res. 30:2453–2457. 2010.PubMed/NCBI
|
|
52
|
Zhao P, Li Y and Lu Y: Aberrant expression
of CD133 protein correlates with Ki-67 expression and is a
prognostic marker in gastric adenocarcinoma. BMC Cancer.
10:2182010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sasaki A, Kamiyama T, Yokoo H, Nakanishi
K, Kubota K, Haga H, Matsushita M, Ozaki M, Matsuno Y and Todo S:
Cytoplasmic expression of CD133 is an important risk factor for
overall survival in hepatocellular carcinoma. Oncol Rep.
24:537–546. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jao SW, Chen SF, Lin YS, Chang YC, Lee TY,
Wu CC, Jin JS and Nieh S: Cytoplasmic CD133 expression is a
reliable prognostic indicator of tumor regression after neoadjuvant
concurrent chemoradiotherapy in patients with rectal cancer. Ann
Surg Oncol. 19:3432–3440. 2012. View Article : Google Scholar : PubMed/NCBI
|