Introduction
Multiple primary intracranial tumors are
characterized by the simultaneous presence of two or more primary
intracranial tumors. These cases are exceptionally rare, with
limited instances documented in the medical literature and the
specific incidence remains undetermined (1). Of note, the coexistence of a
vestibular schwannoma and a dermoid cyst is an uncommon occurrence,
and no such case has hitherto been reported to our knowledge. It is
now understood that intracranial dermoid cysts constitute
approximately 0.3% of all intracranial tumors and typically
originate from residual mesodermal and ectodermal tissue within the
neural tube during embryonic development (2,3). By
contrast, vestibular schwannomas, arising from Schwann cells of the
vestibular branch of the vestibulocochlear nerve, represent a
relatively common type of intracranial tumors, accounting for ~8%
of all intracranial tumors (4).
Surgical resection remains the primary curative approach for both
of these tumor types. However, the surgical approach requires
meticulous consideration when they coexist within a patient. A
single-stage surgical approach should be carefully considered to
achieve gross total resection. The current study presented the case
of a patient afflicted by both a vestibular schwannoma and a
dermoid cyst who underwent one-stage surgery, resulting in the
successful achievement of gross total resection. This case report
aims to enhance awareness regarding the occurrence and management
of multiple intracranial tumors.
Case report
A 28-year-old male patient presented at Department
of Neurosurgery, Beijing Tiantan Hospital (Beijing, China) with the
chief complaint of ‘recurrent headaches for the past six years,
which had worsened over the last year’ in November 2021.
Approximately six years ago, the patient began experiencing
persistent occipital headaches that would occasionally subside on
their own. However, the severity and frequency of these headaches
had intensified over the course of the past year. Of note, there
were no records of intracranial tumors or neurological diseases
among the patient's family members. Upon physical examination, the
patient was determined to exhibit a head tilt to the right and
torticollis. The patient was alert and oriented, and neurological
examination revealed no focal deficits. Magnetic resonance imaging
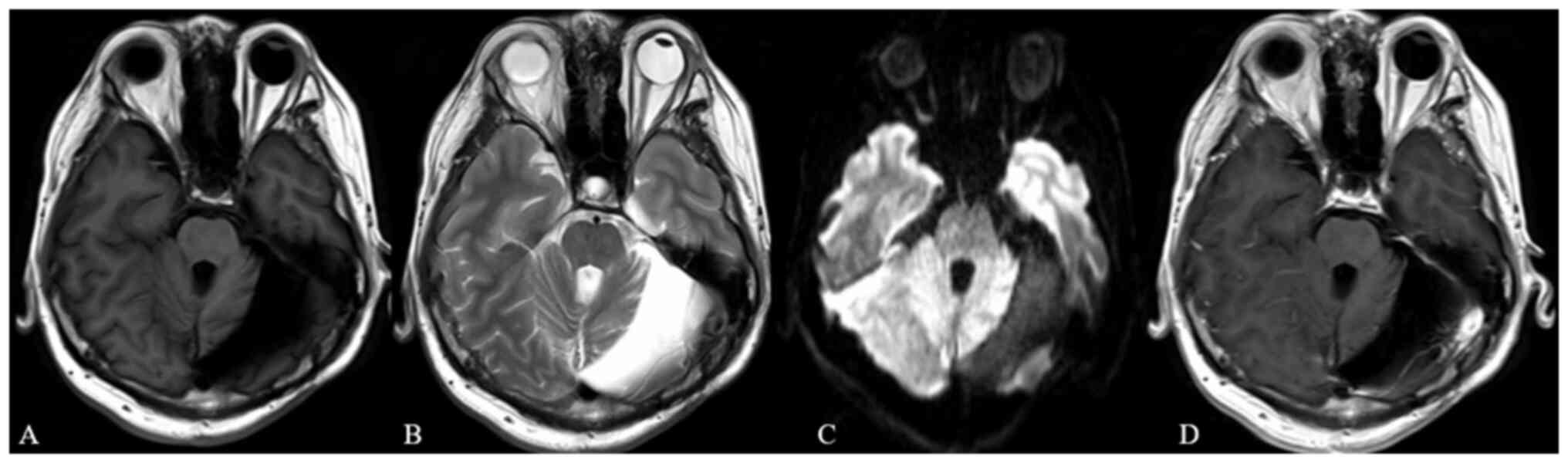
(Fig. 1A-E) revealed the presence
of two lesions. One was a 63×63×44 mm cystic-solid lesion centered
in the left cerebellar hemisphere, exhibiting well-demarcated
borders and causing compression of the fourth ventricle with
resultant supratentorial hydrocephalus. The second was a 14×19×17
mm solid, homogenously enhancing tumor located within the left
cerebellopontine angle and extending into the internal auditory
canal. Head CT revealed calcifications along the cyst wall and
enlargement of the left internal auditory canal (Fig 1F). Audiometry findings (Fig. 2) indicated bilateral hearing
consistent with Class B, as per the American Academy of
Otolaryngology-Head and Neck Surgery (AAO-HNS) criteria.
Preoperative laboratory values were within normal limits.
The chosen surgical approach was the left far
lateral approach, as opposed to the retrosigmoid or posterior
median approach. The upper boundary extended to the transverse
sinus and the foramen magnum was opened inferiorly without grinding
the occipital condyle. The cystic-solid tumor located in the left
cerebellar hemisphere was found to have an irregular cystic wall.
Beneath the tumor, the contents had ruptured through the cystic
wall, containing grey-white material filled with hair and sebaceous
gland-like substances. After decompressing the tumor, careful
separation along the cystic wall ensured the integrity of the
remaining cystic wall, followed by complete tumor resection. The
cystic wall was found to be partially calcified. The other tumor
was exposed in the left cerebellopontine angle (CPA) region by
gently retracting the cerebellum medially. This tumor had a
grey-yellow appearance, firm texture and rich blood supply. The
arachnoid membrane overlying the tumor surface was dissected,
followed by the opening of the tumor capsule in an avascular plane
to achieve adequate intratumoral decompression. The posterior wall
of the internal auditory canal (IAC) was then drilled stepwise to
expose the IAC tumor, which was subsequently resected while
preserving the anatomical integrity of the ipsilateral facial and
cochlear nerves. The estimated intraoperative blood loss was 200 ml
over the 7-h procedure.
Postoperative histopathological examination revealed
that the cystic wall of the cerebellar hemisphere tumor (Fig. 3A) consisted of two layers: A
cornified layer and a stratified squamous epithelium layer. Within
the fibrous connective tissue inside the cyst, sebaceous glands and
other skin appendages were visible, along with focal lymphocytic
infiltration, supporting a diagnosis of dermoid cyst. The left CPA
tumor (Fig. 3B) displayed
spindle-shaped cells with elongated elliptical nuclei, closely and
parallelly arranged in a palisade pattern, indicating a diagnosis
of schwannoma (WHO I).
The patient demonstrated good postoperative
recovery. Facial symmetry was maintained with normal muscle tone at
rest. The motion of the upper forehead was basically normal and the
patient was able to close the eyelids totally with force, with only
mild asymmetry of the oral commissure (House-Brackmann grade II, as
presented in Fig. 4A) (5). Audiometry conducted three months after
surgery (Fig. 4B) revealed a left
ear pure-tone average (PTA) of 52 dB and a speech discrimination
score (SDS) of 100% (data not shown), while the right ear had a PTA
of 30 dB and an SDS of 100%. Postoperative imaging (Fig. 4C-F) confirmed complete tumor
resection with no residual lesions. Regular follow-up examinations
at 3, 12 and 24 months post-surgery revealed resolution of the
headache symptoms and stable bilateral hearing levels. However, the
patient did report episodic tinnitus. Head MRI at one year
post-surgery (Fig. 5) showed no
evidence of residual or recurrent tumors.
Discussion
The present study reported on a rare case of
multiple primary intracranial tumors, which may be classified into
homologous and heterologous types based on their histological
origin. The homologous type consists of multiple tumors with the
same origin, such as multiple meningiomas and multiple gliomas,
while the heterologous type comprises tumors with different
histological origins (6).
Heterologous primary tumors are infrequent and predominantly affect
women aged 30 to 60 years. The most common combination includes
meningioma and pituitary adenoma, with meningiomas often coexisting
with gliomas, primary intracranial lymphoma, schwannoma and
craniopharyngioma (7). Another
intriguing phenomenon is ‘collision tumors’, where different
pathological tumor types occur in adjacent locations (1). While sporadic vestibular schwannoma
coexisting with other tumors has been reported in cases of the
neurofibromatosis type 2 (8–11),
there are no documented cases of concurrent intracranial dermoid
cysts. Of note, dermoid cysts have a lower incidence rate and
rarely coexist with other intracranial tumors. There has been one
documented case of a dermoid cyst in the frontal lobe coexisting
with craniopharyngioma (12).
For heterologous primary tumors, certain studies
have suggested that the lesion or surrounding edema may stimulate
the transformation of surrounding astrocytes or arachnoid granule
cells into tumor cells (7,13). A case report documented a
combination of dermoid cyst, intracranial aneurysm and glioblastoma
(GBM), where chronic inflammatory stimulation caused by the dermoid
cyst and repetitive epilepsy may have contributed to the
development of GBM (14,15). However, this theory did not explain
the occurrence of another tumor in a distant site. Certain studies
have proposed that both tumors may share the same genetic pathway,
promoting the occurrence of both tumors, but at present, there is
insufficient evidence to support this perspective (13). Dermoid cysts are widely thought to
result from embryonic developmental abnormalities, whereas sporadic
vestibular schwannoma appears unrelated to such developmental
issues (12,16). Further investigation is warranted to
understand the etiology of tumors in such cases.
Surgery for multiple intracranial tumors should be
determined based on their location and clinical characteristics. If
the tumors are close in proximity, a surgical approach covering
both should be chosen for one-stage resection. In cases where the
tumors are located far apart, there is debate regarding which tumor
should be resected first. Certain clinicians suggest that the more
malignant tumor should be removed initially before addressing the
relatively benign tumor in the second stage (7). However, the approach pursued in the
present study prioritizes the tumor with a more significant mass
effect or a greater impact on function. Careful consideration was
given to the selection of the surgical approach to achieve this
objective.
In the case of the present study, the patient had a
dermoid cyst in the left cerebellar hemisphere and a vestibular
schwannoma in the left CPA region. The dermoid cyst had compressed
the fourth ventricle, leading to hydrocephalus. Following our
surgical principle, the dermoid cyst was resected as the primary
procedure. Despite the vestibular schwannoma appearing asymptomatic
and the patient not experiencing hearing loss, it is important to
note that hearing loss associated with vestibular schwannoma tends
to deteriorate over time. It has been reported that the average
rate of deterioration in PTA for patients with vestibular
schwannoma is ~4.4 dB hearing degeneration per year. Furthermore,
hearing loss is irreversible, and preserving postoperative hearing
is the optimal outcome (17,18).
Given the mass effect caused by the dermoid cyst, the vestibular
schwannoma had contacted the brainstem, reaching Grade II according
to the Koos classification criteria. Currently, there is no
evidence to suggest that Grade II tumors are more suitable for
stereotactic radiosurgery treatment, whereas surgery is applicable
for tumors of all sizes (19).
Based on our experience and the necessity of resecting the dermoid
cyst, the present approach sought to achieve total tumor resection
and attempt to preserve hearing through a single-stage
procedure.
In the present case, the far-lateral approach was
innovatively selected for tumor resection. The far-lateral approach
offers extensive exposure for posterior fossa operations, providing
access to the entire CPA, foramen magnum and upper cervical region.
It allows for better visualization of the ventral or ventrolateral
brainstem compared to the retrosigmoid approach (20). However, the far-lateral approach is
more complex and time-consuming, with a risk of vascular injury,
particularly when dealing with the occipital condyle (21). The far-lateral approach is rarely
used for routine resection of CPA tumors, except when the tumor
extends inferiorly into the foramen magnum region (22). Sanai and McDermott (21) had proposed a modified far-lateral
approach for the resection of larger posterior fossa or CPA tumors.
However, in this case, the vestibular schwannoma exhibited a
minimal mass effect and the far-lateral approach did not provide
sufficient surgical space to expose the lesion in the absence of a
dermoid cyst. With the presence of a dermoid cyst, the far-lateral
approach allows for exposure of the vestibular schwannoma by gently
pulling the cerebellum following excision of the dermoid cyst. In
addition, the far-lateral approach offers a more oblique view of
the fundus of the IAC compared to the widely used retrosigmoid
approach, necessitating less removal of the posterior wall of the
IAC. In contrast to the conventional far-lateral approach, the
primary focus in the present study was on exposing the left
cerebellar hemisphere and left cerebellopontine angle region, thus
preserving the occipital condyle. However, the bone window was
extended superiorly to optimize cerebellar exposure. Resecting the
dermoid cyst along the tumor boundary aimed to maintain the
integrity of the cyst wall and reduce the risk of postoperative
aseptic inflammatory response. Two key surgical techniques were
employed to achieve postoperative hearing preservation in patients
with vestibular schwannoma: Complete separation of the tumor
capsule and exposure of the tumor within the IAC. The far-lateral
approach facilitates safer and more effective tumor resection
through improved exposure.
Numerous studies have suggested a potential complex
relationship between intracranial dermoid cysts and craniocervical
junction (CVJ) malformations (23,24).
The need for surgical treatment of CVJ malformations during dermoid
cyst surgery remains controversial. A case report documented a
patient with a dermoid cyst accompanied by CVJ anomalies, where
occipitocervical fusion was not performed during surgery. Instead,
a neck collar was prescribed for one month, which yielded positive
therapeutic effects (1). However,
certain studies have advocated for occipitocervical fusion during
the surgery for resecting dermoid or epidermoid cysts, with the
preferred treatment involving surgical resection of the cysts along
with posterior fixation (24).
Given that the CVJ malformation was asymptomatic in this case,
performing occipitocervical fusion during the resection of both
intracranial tumors posed a high surgical difficulty and risk.
Surgery may be postponed until symptoms arise.
In conclusion, the coexistence of a vestibular
schwannoma and dermoid cyst is a rare condition within the realm of
multiple primary intracranial tumors. The pathogenesis of their
simultaneous occurrence remains elusive. The far-lateral approach
was selected to achieve gross total resection and preserve
neurological function in a one-stage surgery. The surgical
principle for multiple primary intracranial tumors aims to achieve
one-stage excision; if this is not feasible, the tumor with a more
significant mass effect or greater functional impact should be
addressed first. The surgical approach should also be adjusted to
suit the specific characteristics of the lesion.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WW provided medical records and designed the outline
of this case report. RZ wrote the manuscript, prepared the figures
and completed the follow-up. RF collected patient information and
medical records, and wrote the ‘case report’ section of the
manuscript. All authors were involved in the revision of the
manuscript. All authors confirm the authenticity of all the raw
data, and have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Beijing Tiantan Hospital (Beijing, China). Studies involving human
participants followed ethical guidelines established by the
institutional and/or national research committee, and complied with
the 1964 Helsinki Declaration and its subsequent amendments.
Patient consent for publication
The patient provided written consent for the
publication of his general information about his gender and age,
case information including chief complaint and medical history, as
well as clinical images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang Z, Yang Y, Zhang K, Zhuang J, Shao
F, Liu H, Xing Y and Xu S: Collision Tumor of glioblastoma and
meningioma: Case report and literature review. World Neurosurg.
117:137–141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adib SD and Tatagiba M: Surgical
management of collision-tumors between vestibular schwannoma and
meningioma in the cerebellopontine angle in patients with
neurofibromatosis type 2. Acta Neurochir (Wien). 161:1157–1163.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Velho VL, Khan SW, Agarwal V and Sharma M:
Intra-axial CNS dermoid cyst. Asian J Neurosurg. 7:42–44. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carlson ML and Link MJ: Vestibular
Schwannomas. N Engl J Med. 384:1335–1348. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
House JW and Brackmann DE: Facial nerve
grading system. Otolaryngol Head Neck Surg. 93:146–147. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu H and Shi X: CT diagnosis of
heterogeneous multiple primary intracranial tumors. Modern Med J
China. 10:104–105. 2008.(In Japanese).
|
|
7
|
Tunthanathip T, Kanjanapradit K,
Ratanalert S, Phuenpathom N, Oearsakul T and Kaewborisutsakul A:
Multiple, primary brain tumors with diverse origins and different
localizations: Case series and review of the literature. J Neurosci
Rural Pract. 9:593–607. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao LY, Jiang YN, Wang YB, Bai Y, Sun Y
and Li YQ: Coexistent vestibular schwannoma and meningioma in a
patient without neurofibromatosis: A case report and review of
literature. World J Clin Cases. 9:7251–7260. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Anazi AH, Al-Luwimi IM, Shawarby MA and
Mertol T: Mixed vestibular schwannoma and meningioma without
neurofibromatosis. Neurosciences (Riyadh). 14:371–373.
2009.PubMed/NCBI
|
|
10
|
Carlson ML, Patel NS, Glasgow AE,
Habermann EB, Grossardt BR and Link MJ: Vestibular schwannoma and
pituitary adenoma in the same patient: Coincidence or novel
clinical association? J Neurooncol. 128:101–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iacoangeli M, Di Rienzo A, Colasanti R,
Alvaro L, Nocchi N, Polonara G, Di Somma LG, Zizzi A, Scarpelli M
and Scerrati M: Rare synchronous association of vestibular
schwannoma and indolent insular oligodendroglioma in a patient
without neurofibromatosis: Controversial issue of timing for
surgical treatment of asymptomatic low-grade gliomas. Onco Targets
Ther. 5:357–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abou-Al-Shaar H, Abd-El-Barr MM, Zaidi HA,
Russell-Goldman E, Folkerth RD, Laws ER Jr and Chiocca EA: Frontal
dermoid cyst coexisting with suprasellar craniopharyngioma: A
spectrum of ectodermally derived epithelial-lined cystic lesions?
Neurosurg Focus. 41:E162016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Suzuki K, Momota H, Tonooka A, Noguchi H,
Yamamoto K, Wanibuchi M, Minamida Y, Hasegawa T and Houkin K:
Glioblastoma simultaneously present with adjacent meningioma: Case
report and review of the literature. J Neurooncol. 99:147–153.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alam Y, Mugge LA, Purdy J, Mrak RE and
Schroeder J: Long-term seizure disorder caused by a dermoid cyst
with catastrophic developments. Cureus. 10:e32722018.PubMed/NCBI
|
|
15
|
Kim KH and Cho JH: Ruptured intracranial
dermoid cyst associated with rupture of cerebral aneurysm. J Korean
Neurosurg Soc. 50:453–456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hosoya M, Wakabayashi T, Wasano K,
Nishiyama T, Tsuzuki N and Oishi N: Understanding the molecular
mechanism of vestibular schwannoma for hearing preservation
surgery: Otologists' perspective from bedside to bench. Diagnostics
(Basel). 12:10442022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scheller C, Wienke A, Tatagiba M,
Gharabaghi A, Ramina KF, Ganslandt O, Bischoff B, Matthies C,
Westermaier T, Antoniadis G, et al: Stability of hearing
preservation and regeneration capacity of the cochlear nerve
following vestibular schwannoma surgery via a retrosigmoid
approach. J Neurosurg. 125:1277–1282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Quaranta N, Baguley DM and Moffat DA:
Change in hearing and tinnitus in conservatively managed vestibular
schwannomas. Skull Base. 17:223–228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goldbrunner R, Weller M, Regis J,
Lund-Johansen M, Stavrinou P, Reuss D, Evans DG, Lefranc F,
Sallabanda K and Falini A: EANO guideline on the diagnosis and
treatment of vestibular schwannoma. Neuro Oncol. 22:31–45. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Graffeo CS, Perry A, Carlstrom LP, Leonel
L, Nguyen BT, Morris JM, Driscoll CLW, Link MJ and Peris-Celda M:
Anatomical Step-by-Step dissection of complex skull base approaches
for trainees: Surgical anatomy of the far lateral approach. J
Neurol Surg B Skull Base. 84:170–182. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sanai N and McDermott MW: A modified
far-lateral approach for large or giant meningiomas of the
posterior fossa. J Neurosurg. 112:907–912. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo W, Liu H, Li J, Yang J and Xu Y:
Choroid plexus papillomas of the cerebellopontine angle. World
Neurosurg. 95:117–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kennedy PT and McAuley DJ: Association of
posterior fossa dermoid cyst and Klippel-Feil syndrome. AJNR Am J
Neuroradiol. 19:195–196. 1998.PubMed/NCBI
|
|
24
|
Chandra PS, Gupta A, Mishra NK and Mehta
VS: Association of craniovertebral and upper cervical anomalies
with dermoid and epidermoid cysts: Report of four cases.
Neurosurgery. 56:E11552005.PubMed/NCBI
|