Cluster of differentiation 47 (CD47) expression
levels are influenced by an organism's physiological state and cell
type (1). Under normal
physiological conditions, the expression level of CD47 has a vital
role in maintaining homeostasis. For instance, young erythrocytes
have high CD47 expression on their surface. By contrast, senescent
erythrocytes have low CD47 expression on their surface, which
allows macrophages to eliminate CD47 for erythrocyte renewal
(2). As previously reported,
binding different ligands to CD47 also results in different
biological effects. For example, CD47 binds to signal regulatory
protein α (SIRPα) to activate a signaling pathway that inhibits
phagocytosis and the killing of tumor cells by macrophages in the
tumor microenvironment (TME) by modulating the immune response
(3). Under pathological conditions,
CD47 is highly expressed in hematological tumors, and by binding to
its ligand SIRPα, CD47 transmits a series of inhibitory signals to
macrophages; consequently, the phagocytosis of tumor cells by
macrophages is prevented (4). The
expression level of CD47 and blockade of the signaling pathway
activated by CD47 also significantly impact the fate of tumor
cells, and the upregulation or downregulation of CD47 expression
and blockade of CD47 signaling can determine the fate of tumor
cells. Blocking CD47 signaling can also determine the survival of
tumor cells. In recent years, blocking CD47 has emerged as a
potential therapeutic strategy for tumor immunotherapy (5), and immunotherapies targeting CD47 have
achieved significant success in certain cancer patients. However,
remission rates vary; not all individuals benefit from current
treatments (6). Various drugs
targeting CD47, such as monoclonal antibodies (mAbs), SIRPα fusion
proteins (SIRPα-Fc), bispecific antibodies (BsAbs), small-molecule
inhibitors and nanotechnology-based delivery systems, are being
developed (7,8). Preclinical studies and early clinical
trials have demonstrated that CD47-targeted therapies have a
promising future for application. In addition, CD47-targeted
therapies have potential limitations and challenges, including
adverse reactions such as anemia and thrombocytopenia, as well as
resistance to drugs (9).
CD47 has a molecular weight of 45–55 kDa and is a
member of the immunoglobulin (Ig) superfamily (IgSF) (4,13,14).
Its molecular structure includes one N-terminal extracellular
IgG-like domain, five highly hydrophobic transmembrane segments and
one hydrophilic cytoplasmic tail at the C-terminus. Hatherley et
al (15,16) investigated the crystal structure of
the IgG structural domain. They showed that the structure of CD47
has a typical IgV-like fold and is similar to that of myelin
oligodendrocyte glycoprotein. CD47 mediates vascular smooth muscle
cell (VSMC) proliferation and migration (17), as well as platelet activation and
spreading (18), and recruits
granulocytes and T cells to the site of infection (11).
The distribution and functions of different isoforms
within tissues vary. bladder, ovarian and breast cancer cells are
examples of keratinocytes and tumor cells expressing type 1 CD47
(22,23). The most extensively expressed form,
type 2 CD47, is mainly involved in signaling between astrocytes and
is primarily expressed in hematopoietic cells, epithelial cells and
vascular endothelial cells. Neuronal, testicular and intestinal
mucosal cells explicitly express type 3 and 4 CD47 (24). Types 3 and 4 are thought to be
closely related to memory mechanisms because their expression is
markedly elevated in the brains of rats with good memory (25,26).
Protein linking IAP with cytoskeleton 1 (PLIC-1) cytoplasmic
protein regulates cyclic adenosine monophosphate (cAMP) signaling
by CD47 by binding to the cytoplasmic tails of types 2 and 4,
recruiting heterotrimeric G proteins to CD47 (27), inhibiting chemotactic signaling
induced by the Gi-coupled receptor C-X-C motif chemokine receptor 4
(28) and activating the PI3K/Akt
pathway in astrocytomas (29). More
research is required to determine the functional distinctions
between the cytoplasmic tails of the various CD47 isoforms, as the
studies on this aspect of CD47 isoforms have been minimal in recent
years, resulting in a limited understanding of the regulatory
mechanisms and roles of the cytoplasmic tails of different isoforms
of CD47.
In addition, certain cells can adapt to various
physiological and pathological changes by switching their subtype,
e.g., Reinhold et al (23)
used PCR to detect mRNA expression and found that primary mouse
endothelial cells cultured in vitro predominantly expressed
CD47 type 2 mRNA, and endothelial cells transformed with
intermediate T antigen expressed all four types of mRNA. However,
certain researchers dispute this; for instance, Mateo et al
(30) observed no change in the
expression of CD47 isoforms. These studies indicate that the role
of the CD47 types in tumorigenesis and development and the
mechanism of interconversion require further investigation.
CD47 receptors include integrin, thrombospondin-1
(TSP-1) and SIRPα. Based on published reviews, it may be summarized
that CD47 affects multiple biological functions of target cells by
binding to these ligands (31,32).
In addition, the gene expression of the three ligands of CD47 under
physiological conditions and in tumors may be summarized through
the GEPIA database (http://gepia.cancer-pku.cn/index.html) and the Human
Protein Atlas (https://www.proteinatlas.org/), as elaborated
below.
Integrins are transmembrane ligands that bridge the
gap between cells and the extracellular matrix and regulate
signaling processes such as the cell cycle, morphology and motility
(26,32,33).
CD47 was initially found to interact with αvβ3 intergrin, hence the
designation IAP. Under normal physiological conditions, αvβ3
integrins are mainly expressed in cardiomyocytes, oligodendrocytes
and astrocytes, while under pathological conditions, they may be
widely expressed mainly in cancers, such as glioblastoma,
esophageal, thyroid and pancreatic cancers. The CD47-integrin
complex may activate multiple heterotrimeric G proteins by linking
IAP to PLIC-1, thereby inducing CD47 to activate cAMP signaling
(34). Lindberg et al
(35), through a study using a
CD47-deficient human cell line, showed that CD47 is required for
αvβ3 integrin-mediated binding of hyaluronan to encapsulated
microbeads. In addition to αvβ3 integrin, CD47 binds to αIIbβ3
integrin and induces platelet aggregation and increased adhesion
spot kinase tyrosine phosphorylation (18). In addition, CD47 binds to α4β1
integrin and mediates reticulocyte adhesion (36); CD47 binds to α5β1 integrin and is
involved in chondrocyte mechanotransduction (37); and CD47 binds to α6β1 integrin and
has a role in fibrillar β-amyloid-mediated activation and
phagocytosis of microglia (38).
TSP is an extracellular matrix calcium-binding
glycoprotein that is highly expressed on monocytes, mucus cells and
macrophages under normal physiological conditions and is widely
expressed in cancers, such as breast adenocarcinoma carcinoma, lung
adenocarcinoma, pancreatic adenocarcinoma and gastric
adenocarcinoma, mainly under pathological conditions. There are
currently five known isoforms of TSP, i.e., TSP-1-5 (39). TSP-1 is the first identified
endogenous ligand of CD47 and it has a variety of biological
functions, including the inhibition of angiogenesis, activation of
transforming growth factor-β and participation in tissue repair
(40). Protein-related studies have
shown that TSP-1 binds to the CD47 extracellular IgV structural
domain through its C-terminal structural domain peptide 4N1K and
has a role in several biological processes, including inflammation,
immune response, cell proliferation, apoptosis, adhesion and
migration (41). The mechanism of
CD47-TSP-1 interaction has not been studied in detail because the
crystal structure of the CD47-TSP-1 complex still needs to be
clarified. Early experiments have shown that CD47 affects signaling
through heterotrimeric Gi proteins in a pertussis toxin-sensitive
manner (28), thereby modulating
TSP-1-induced cell spreading and platelet activation. Isenberg
et al (42) measured cGMP
levels by immunoassay, indicating that binding of CD47 to TSP-1
inhibits nitric oxide signaling in endothelial and VSMCs, thereby
promoting platelet aggregation. To date, we have found that
CD47-TSP-1 expression serves as a marker for predicting patient
response to immune checkpoint blockade therapy, but there is no
targeted therapy for the CD47-TSP-1 axis. It is hypothesized that
this may be because CD47 has little effect on the adaptive immune
response through its interaction with TSP-1, and therefore,
blocking the CD47-TSP-1 axis has little clinical therapeutic
significance. However, a novel immunotherapeutic drug, TAX2
peptide, which acts as an orthosteric antagonist of the interaction
between TSP-1 and CD47, has shown a good safety profile in mouse
models of ovarian cancer and is effective in killing tumor cells
(43).
SIRPα, the ligand with the highest affinity for
CD47, is a member of the SIRP family and was first identified by
Kharitonenkov et al (44) in
the 1990s. Belonging to the IgSF, under normal physiological
conditions, SIRPα is extensively expressed on the surface of cells
such as monocytes, macrophages, neutrophils, dendritic cells (DCs)
and microglia. Under pathological conditions, it is widely
expressed in cancers such as glioblastoma, melanoma, renal cancer
and head and neck cancer (45,46).
The intracellular region of SIRPα contains four
tyrosine phosphorylation sites and two immunoreceptor tyrosine
inhibition motifs (ITIMs), and the extracellular region has three
IgSF structural domains, namely, one N-terminal IgV-like domain and
two C-like domains (47,48). The crystal structure of the
N-terminal IgV-like domain of SIRPα suggested an IgV-like fold and
four-loop structure (BC, CD, DE and FG loops) with an overall
structure similar to that of the T-cell receptor (16,49).
SIRPα binds to CD47 through its N-terminal FG and BC
loop, thus forming a highly entangled, well-fitted complex
structure (15). The long disulfide
bond between Cys33 of the IgV structural domain and Cys263 of the
transmembrane structural domain in CD47 is essential for enhancing
binding to SIRPα (50–52). According to X-ray computational
crystallography calculations and analysis, when CD47 interacts with
SIRPα, the total distance between the two cell types approximates
the entire distance of the immune synapse (~14 nm) (53). Therefore, the binding of SIRPα to
CD47 may occur via an antigen receptor rather than through the
usual cell-cell structural domain binding interaction (16).
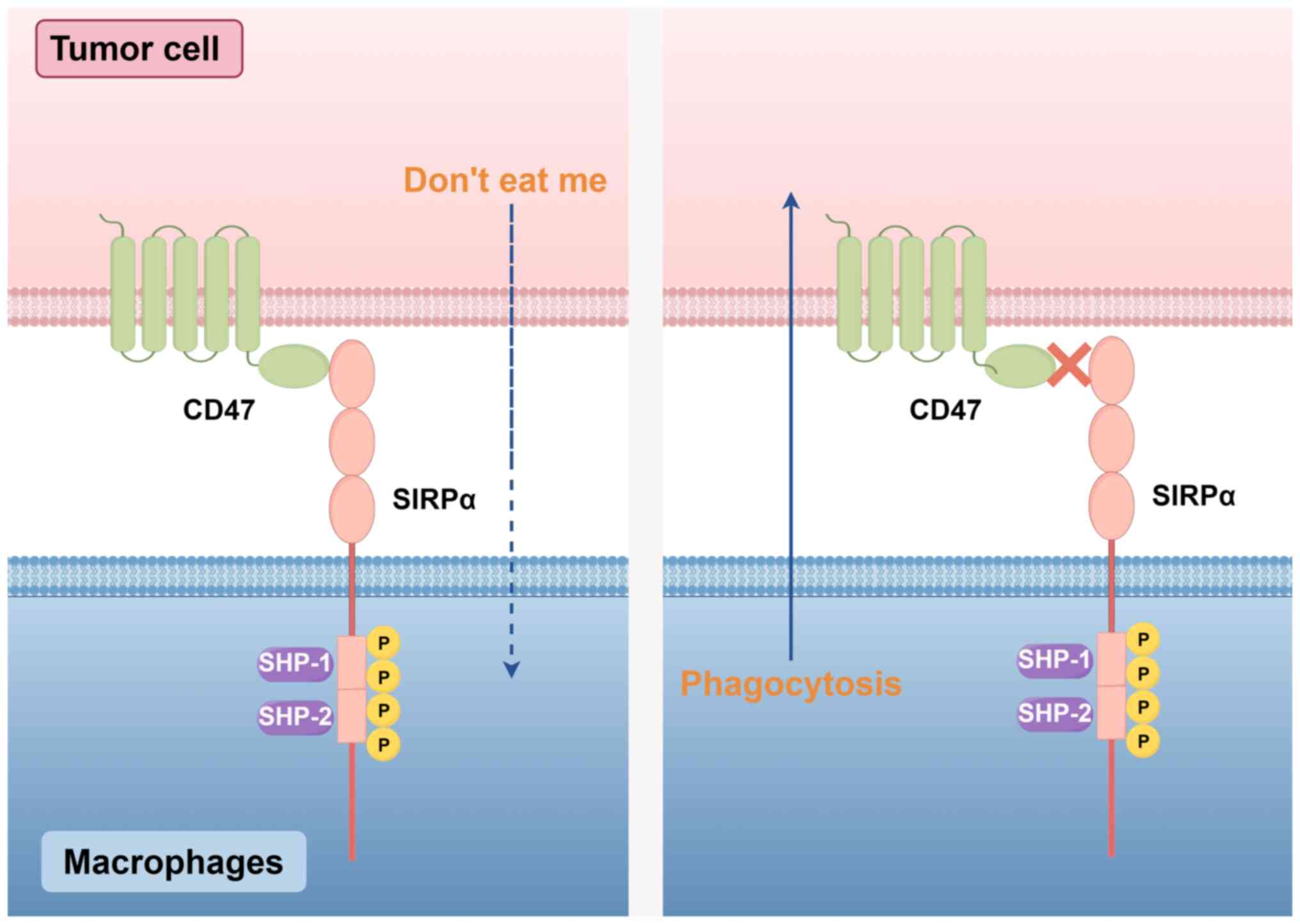
The binding of SIRPα to CD47 promotes the
phosphorylation of the intracellular region of the ITIM (15,47).
Phosphorylated ITIM recruits and activates Src homology region 2
(SH2)-containing tyrosine phosphatase-1 (SHP-1) and SHP-2 (54), which affects cytoskeletal function
by inactivating motor myosin IIA (55), thereby blocking tyrosine
phosphorylation-dependent signaling pathways and limiting
phagocytosis by macrophages and others (47). Although SHP-1 and SHP-2 are
typically inactive, phosphorylated ITIM recruits the SH2 structural
domain to the cell membrane, and a change in its conformation
activates SHP-1 and SHP-2. SHP-1 is present mainly in hematopoietic
and epithelial cells and is selectively expressed in myeloid cells,
which function as a negative regulator of phagocytosis. By
contrast, SHP-2 is widely expressed and promotes cell
proliferation, growth and migration mainly by regulating the
GTP-binding proteins RAS and Rho (26).
CD47-SIRPα interactions not only regulate the
maintenance of lymphocyte homeostasis (56), DC maturation and activation
(57), the correct localization of
DC subpopulations in sub-lymphoid organs and cell migration
(58) but also have an essential
role during remodeling of the nervous system and bone tissues
(59). The cellular responses
regulated by CD47-SIRPα interactions depend upon bidirectional
signaling between CD47 and SIRPα: CD47 on host cells acts as a
‘self-tag’ (60) and regulates
phagocytosis by binding to SIRPα. How this regulates phagocytosis
will be further discussed in a later section.
Complex cellular communication systems in
multicellular organisms have evolved to ensure adequate
intercellular communication, which is crucial for cell
differentiation, tissue and organ formation, individual development
in multicellular organisms and immune function regulation (61).
The interaction between CD47 and SIRPα constitutes
an intercellular communication system whose role in regulating
immune system function is bidirectional (62). The CD47-SIRPα signaling pathway
negatively regulates DC activation. The fusion protein of CD47,
when bound to SIRPα, inhibits the phenotype and function of
immature DCs and the production of cytokines by mature DCs
(63). However, considering its
role in antigen presentation, SIRPα has a positive regulatory
effect. SIRPα is abundantly expressed on the surface of mature DCs.
When the immune system responds to pathogens, SIRPα helps DCs
present relevant antigens to T cells and costimulatory molecules
associated with initiating T cells, thus promoting T-cell
activation and proliferation (1,19).
Phagocytosis is the process by which tissue cell
debris and apoptotic cells are engulfed and digested, and this
process helps maintain a stable balance in the body's internal
environment. CD47 has a vital role in regulating phagocytosis. This
regulatory function is mediated by binding to the inhibitory
receptor SIRPα on phagocytes to activate the CD47-SIRPα signaling
pathway. CD47 binds to SIRPα and sends an inhibitory ‘do not eat
me’ signal to phagocytes, thus limiting phagocytosis (3,64)
(Fig. 1).
CD47-SIRPα signaling also has an essential
regulatory role in hematopoietic stem cell (HSC) transplantation.
HSCs upregulate CD47 expression to protect themselves from
phagocytosis by macrophages, thus achieving successful implantation
(68,69). In general, the CD47 of one species
has little interaction with the SIRPα of another species. However,
higher-polymorphism SIRPα on macrophages was observed in a nonobese
diabetic (NOD)-severe combined immunodeficiency xenograft mouse
model when compared with other mouse lines. These cells have an
exceptionally high affinity for human CD47, even higher than the
mouse-mouse or human-human affinity of CD47 and SIRPα (70,71).
Theocharides et al (72)
demonstrated that implantation of normal human HSCs in NOD mice was
also dependent on the interaction of human CD47 with SIRPa in NOD
mice by implanting HSCs into a NOD mouse model. These studies
demonstrated that the interaction between CD47 on human HSCs and
SIRPα on macrophages is critical for the successful implantation of
HSCs. In addition, human SIRPα is polymorphic and each polymorphic
variant has a different affinity for human CD47 in vitro.
This finding suggested that the human SIRPα polymorphism is
critical for successfully implanting HSCs (73,74).
The ‘do not eat me’ signal from the CD47-SIRPα
signaling pathway is also used to maintain homeostasis in body. The
body must remove various cells, including those that are
overproduced, damaged or aged. One removal mechanism is apoptosis,
through which macrophages clear apoptotic cells precisely and
efficiently. This mechanism is a key ‘do not eat me’ signal from
CD47 that occurs on the surface of healthy cells, and binds to
SIRPα inhibitory receptors on macrophages to prevent them from
being eaten by macrophages. CD47 expression on the surface of
apoptotic cells is downregulated, thereby attenuating the
inhibitory signal generated by CD47 binding to SIRPα. By contrast,
low IgG or C3b opsonization levels can cause the phagocytosis of
apoptotic cells by macrophages (75).
Malignant tumors like glioblastoma, acute
lymphoblastic leukemia, as well as ovarian, breast, gastric and
lung cancers express high levels of CD47 (76–78).
Liu et al (79) used flow
cytometry to detect the expression of CD47 in isolated primary lung
cancer cells and adjacent normal cells and the results showed that
the expression level of CD47 in tumor cells was higher than that in
normal cells. There were apparent differences between subtypes of
lung cancer, with the highest expression of CD47 in small-cell lung
cancer, followed by lung adenocarcinoma, and the lowest in lung
squamous carcinoma (79).
Furthermore, compared to normal myeloid cells from healthy
individuals, acute myeloid leukemia (AML) and chronic myeloid
leukemia cells expressed higher levels of CD47. Furthermore, a
positive association was found between high levels of CD47
expression and poor treatment response and patient prognosis
(80). A study confirmed that CD47
mRNA and protein levels were higher in leukemic stem cells of
patients with AML than in normal healthy stem cells (81). In a study on Epstein-Barr virus
(EBV)-associated gastric cancer (EBVaGC), the expression of CD47 in
EBVaGC was higher than that in EBV-negative gastric cancer tissue
samples, which also indicated that high expression of CD47 was
associated with poor prognosis in EBVaGC (82). Yu et al (83) detected the expression of CD47 in
ovarian cancer tissues by immunohistochemistry, which showed that
the prognosis of patients with low expression of CD47 was better
than that of patients with high CD47 expression. The above studies
indicate that CD47 expression levels are closely related to the
prognosis of patients with cancer.
Recent research has demonstrated that controlling
the expression of CD47 in tumor cells and inhibiting the signaling
pathway that CD47 activates have crucial regulatory roles in
determining the fate of tumor cells. The mechanisms of action
include the following: i) Upregulation of CD47 expression, which
binds to the macrophage surface receptor SIRPα and transmits the
‘do not eat me’ signal to promote phagocytosis of tumors by
macrophages; ii) blockade of CD47 enhances the phagocytosis of
tumor cells by DCs and promotes antigen delivery from DCs to T
lymphocytes, initiating an antitumor adaptive immune response; iii)
blockade of CD47 is capable of clearing tumor cells through natural
killer cell-mediated antibody-dependent cell-mediated cytotoxicity
(ADCC) and complement-dependent cytotoxicity (CDC) to clear tumor
cells; iv) blockade of CD47 also activates the apoptotic pathway
and directly induces apoptosis in tumor cells.
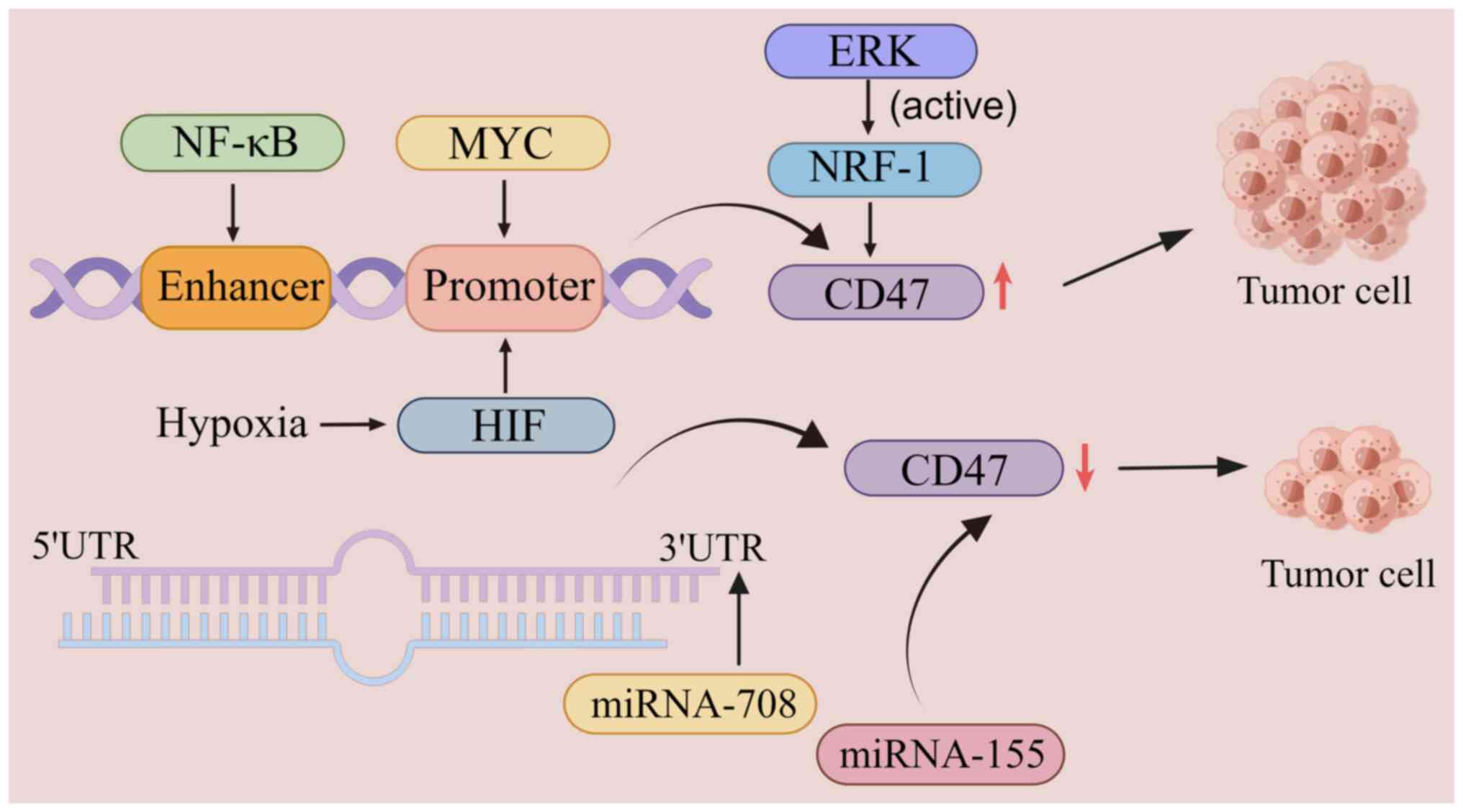
The expression level of CD47 is regulated by
transcription factors such as nuclear factor κB (NF-κB), the MYC
oncogene and hypoxia-inducible factor-1 (HIF-1), which regulate the
phagocytosis of tumor cells by upregulating or downregulating the
expression of CD47 (4). In a T-cell
acute lymphoblastic leukemia xenograft model, MYC directly binds to
the CD47 promoter and upregulates its expression, thus promoting
the growth of tumor cells. By contrast, inactivation of MYC
downregulates the expression of CD47 and enhances macrophage
infiltration and phagocytosis, thereby inhibiting tumor cell growth
(84). In addition, activated NF-κB
directly binds to specific enhancer components of CD47 and
upregulates CD47 expression in breast cancer cells, thereby
promoting tumor growth (85). In a
clinical analysis of thousands of patients with breast cancer,
Zhang et al (78) reported a
strong correlation between CD47 and HIF-1. Under hypoxic
conditions, HIF-1 binds to the CD47 promoter and upregulates its
expression, thereby inhibiting the phagocytosis of breast cancer
cells (84). In addition, ERK
signaling inhibits tumor-cell phagocytosis by activating nuclear
respiratory factor-1 and upregulating CD47 expression in melanoma
cells. Conversely, microRNA (miRNA)-mediated downregulation of CD47
expression promotes tumor-cell phagocytosis. MiR-708 is inversely
associated with CD47 expression and its binding to the
3′-untranslated region of CD47 induces tumor-cell phagocytosis by
suppressing CD47 expression (86).
In multiple myeloma, CD47 expression on the surface of myeloma
cells can be inhibited by upregulating the expression of the tumor
suppressor gene miRNA-155, thereby inducing phagocytosis of tumor
cells by macrophages (87)
(Fig. 2).
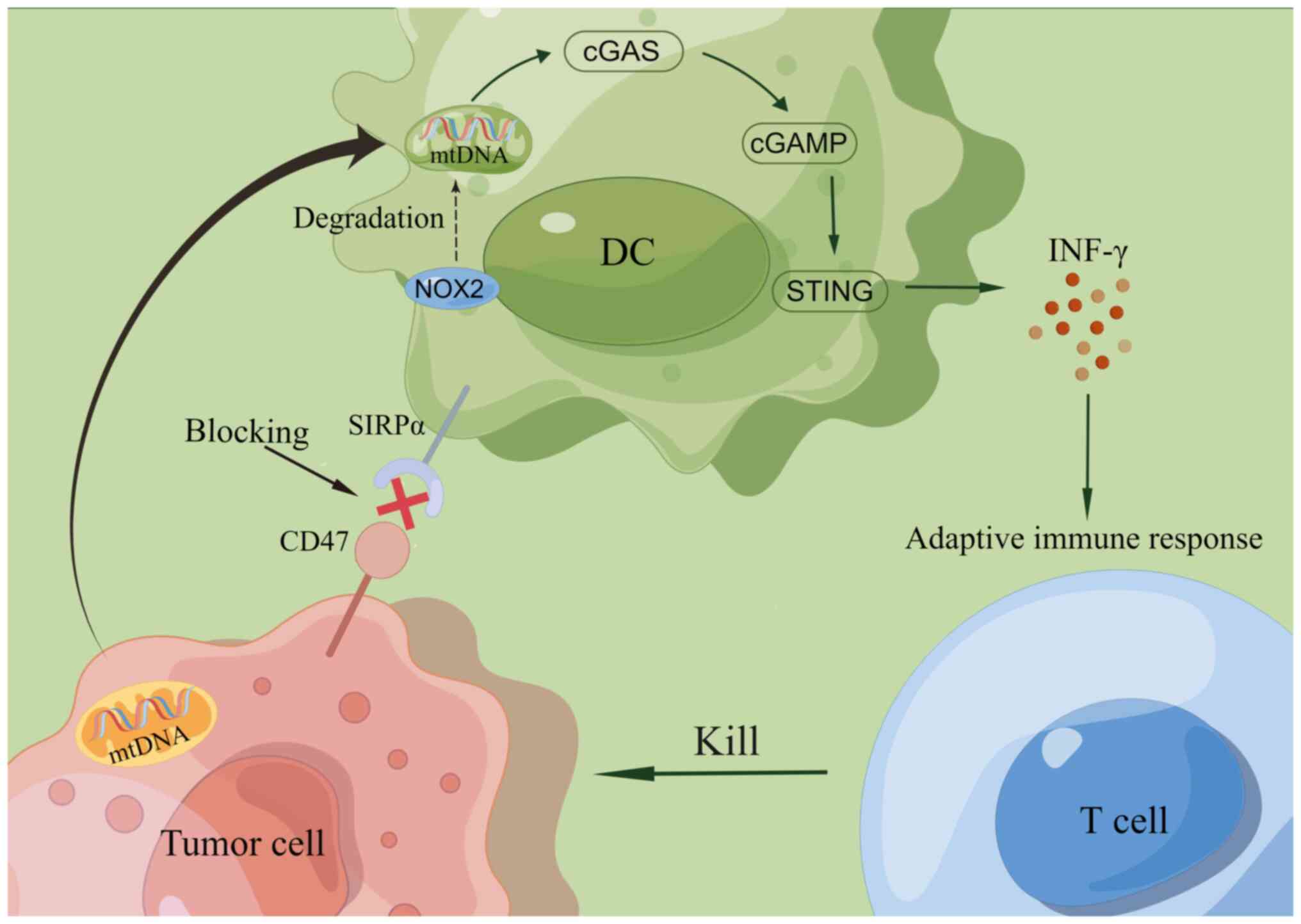
In addition, blocking CD47 induces tumor cell death
only when endogenous activation signals are present (64). A study by Chen et al
(92) revealed the presence of an
endogenous activation signal on the surface of tumor cells called
SLAM family member 7 (SLAMF7), a prophagocytic signal of SLAMF7,
which is a prophagocytic ‘eat-me’ signal and usually interacts with
the macrophage-1 antigen, promoting the phagocytosis of tumor cells
by macrophages. Furthermore, they contended that CD47-mediated
phagocytosis requires SLAMF7. However, He et al (93) refuted this view by finding that
phagocytosis was also effectively induced in SLAMF7-negative
diffuse large B-cell lymphomas cells after they blocked CD47 by the
CD47 antibody Inhibrix. Further studies are needed to determine
whether SLAMF7 is required to mediate CD47.
Clinical research has shown that CD47 is an
intrinsic immune checkpoint with high clinical development value
and promising application prospects. Numerous domestic and foreign
companies are actively developing drugs targeting CD47,
particularly mAbs, BsAbs, fusion proteins and small-molecule
antibodies, and many of them have already entered the clinical
research stage. A search of the PubMed database (https://pubmed.ncbi.nlm.nih.gov/) and online open
resources from the US National Clinical Trials Registry system
(www.clinical trials.gov) was
performed as part of the present review. Compared with previously
published reviews (48,94,95),
not only the names, structures and clinical trials of various
CD47-targeted representative drugs were summarized, but the current
state of clinical research and the results of clinical trials were
also outlined (Table I).
Immunotherapies targeting CD47 can be divided into
two categories: First, blocking or inhibiting the ‘do not eat me’
signal with SIRPα via antibodies to promote the phagocytosis of
tumor cells by macrophages (96);
second, the activation of innate and adaptive immune responses.
Tumor cells are recognized, taken up by antigen-presenting cells
(APCs) and delivered to the initial T cells, activating T cells. T
cells activate when APCs identify, pick up and transfer tumor cells
to initial T cells. Antibodies targeting CD47 can kill tumor cells
by inhibiting protein kinase A (97,98).
Closure of CD47 on tumor cells using mAbs targeting
CD47 or soluble SIRPα-Fc structures triggers macrophage
antibody-dependent cellular phagocytosis in vitro. It
significantly promotes the killing of tumor cells (99). In addition, the CD47-targeted fusion
protein SIRPαD1-Fc was found to inhibit the Akt/mTOR signaling
pathway, upregulate reactive oxygen species production and promote
autophagy in non-small cell lung cancer cells, thereby enhancing
the antitumor effect (100).
Furthermore, CD47-targeting antibodies can synergize
with various mAbs, and combining the two can provide a better
antitumor effect. Commonly used combinations include combination
therapy with other therapeutic antibodies, chemotherapy or
radiation therapy. A phase I clinical study revealed that the
combination of a CD47 mAb and Rituximab resulted in an objective
response rate (ORR) of 40% and a complete response rate (CRR) of
33% in patients with diffuse large B-cell lymphoma, with an ORR of
71% and a CRR of 43% in patients with follicular lymphoma (45).
In addition, several clinical trials have evaluated
the safety and efficacy of CD47-targeted drugs in different stages
and types of tumors. For instance, Lemzoparlimab (TJC4), which
targets CD47, was screened using a phage display system. A phase I
clinical trial is evaluating the efficacy effects of TJC4 alone or
in combination with Pembrolizumab or Rituximab in the treatment of
relapsed or refractory (R/R) advanced solid tumors and lymphomas
(103). The results of the
preclinical study demonstrated a favorable safety profile and
clinical efficacy in five patients with AML and high-risk MDS who
had received at least two treatments. Of particular note, one
patient with R/R AML achieved a morphologic leukemia-free status
after treatment with TJC4 (104).
Humanized CD47 antibody Ligufalimab (AK117) is an anti-CD47 mAb
with a unique structure, which not only has anti-tumor effects but
also eliminates erythrocyte agglutination and significantly reduces
phagocytosis of erythrocytes by macrophages. Phase I trials have
been completed in Australia and phase II trials are underway in
China and Australia. Results from a clinical trial enrolling 15
patients with advanced solid tumors showed that AK117 was safe and
well tolerated, with no infusion- or treatment-related adverse
effects observed (105). AO-176, a
mAb targeting CD47, is being evaluated in a phase I clinical trial
for treating R/R multiple myeloma (106,107). AO-176 binds preferentially to
tumor cells (rather than normal cells), can bind tumor cells more
efficiently in an acidic microenvironment and can kill tumor cells
directly in a cell-autonomous manner (108). Current clinical data show that of
the 27 patients treated with AO-176, one patient with endometrial
cancer did not respond to its treatment regimen and seven patients
had the best response of stable disease (SD) (109). CC-90002 is the first generation of
humanized CD47 antibody to enter clinical studies that block
CD47-SIRPα binding to achieve the killing of hematological tumor
cells (96). Clinical trials for
AML and MDS revealed that CC-90002 had poor efficacy and safety,
which led to its forced discontinuation. Researchers restarted
clinical trials after improving the CC-90002 treatment regimen and
safety (110,111). In a mouse transplantation tumor
model of multiple myeloma, CC-90002 showed significant
dose-dependent antitumor activity. In addition, in non-primate
animals, CC-90002 exhibited favorable pharmacokinetic properties
and toxicity (96). TTI-621 and
TTI-622 are SIRPs-Fc fusion proteins that have been used in the
treatment of hematologic malignancies, solid tumors and mycosis
fungoides (112), and such agents
are currently being evaluated in a phase I clinical trial for R/R
B-cell lymphomas (113). In 164
patients with B-cell non-Hodgkin's lymphoma (B-NHL), TT-621 plus
rituximab was used to treat the disease in a phase I trial. The
study showed that TT1-621 was well tolerated and that monotherapy
is a promising therapeutic option. The ORR for all patients treated
with TTI-621 monotherapy was 13%, while it was 29% for diffuse
large B-cell lymphoma and 25% for T-cell NHL (113). Clinical studies of TTI-622 in
patients with advanced R/R lymphomas showed that one patient with
non-growth center B cells who had received five prior therapies
achieved partial remission (PR) at week 8 and overall response at
week 36 (114). The SIRPs-Fc
fusion protein Evorpacept (ALX148) is presently undergoing
evaluation in several programs (95), such as a phase I/II trial for
patients with advanced solid tumors and a phase I trial for
patients with aggressive and indolent NHL (115). PR rates were 22% with trastuzumab
combination therapy in patients with Her2-positive gastric cancer
and 16% with pembrolizumab combination therapy in patients with
head and neck squamous cell carcinoma (116).
BsAbs are genetically engineered artificial
antibodies that contain two specific antigen-binding sites. The
BsAb backbone has two binding arms, one blocking the CD47-SIRPα
pathway and the other binding tumor-specific antigens, thus
ensuring the killing of tumor cells by BsAbs (99). Compared with combination therapy,
using BsAbs also reduces the cost of drug development and clinical
trials.
Several CD47-related BsAbs are in early clinical
trials. For instance, IMM0306, a BsAbs targeting CD20 and CD47,
avoids binding to CD47 in normal cells due to its high affinity for
CD20, thus reducing the toxicity associated with the CD47 target.
IMM0306 has demonstrated vigorous antitumor activity in a mouse
model of human NHL transplantation tumor (117). It is currently being evaluated in
a phase I clinical trial in B-NHL (118). IBI322 is a drug that inhibits both
the programmed cell death 1 (PD-1)/PD-1 ligand 1 and CD47-SIRPα
signaling pathways for treating intermediate to advanced
malignancies. Repeated weekly injections of IBI322 showed good
tolerability in non-human primates (119). IBI322 is currently being evaluated
in a phase I clinical trial for advanced malignancies. HX009 is a
BsAb targeting PD-1 and CD47 for treating advanced tumors such as
gastric, colorectal and hepatocellular carcinomas and is currently
being evaluated in a phase I trial for advanced solid tumors
(120). Clinical studies
demonstrated that of the 18 patients with at least one
post-baseline tumor assessment, three patients achieved a PR and
six achieved SD (121). CC-96673,
a humanized BsAbs co-targeting CD47 and CD20, was able to
efficiently promote phagocytosis by macrophages by blocking
CD47-SIRPα interactions and mediated the selective removal of
CD20-expressing tumor cells by ADCC and CDC to selectively clear
CD20-expressing tumor cells. A phase I clinical trial is presently
assessing it for R/R NHL. NI-1701 is a novel BsAb constructed using
spinopore technology to target CD47 and CD19 (122). Previous studies have found that
NI-1701 selectively binds to CD47 and CD19 co-expressing cells and
has poor binding ability with normal cells by interacting poorly
with normal cells, avoiding binding to normal cells and thus
improving biosafety (121,123). SL-172154, a fusion protein
targeting SIRPs-Fc and CD40L, is being evaluated in a phase I
clinical trial for solid tumors (124).
Chimeric antigen receptor T cell (CAR T cell)
immunotherapy has made significant progress in oncology, and
combining CD47 blockade therapy with CAR T-cell therapy has become
a hot research topic. A previous review (125) described CAR T cells and their
future prospects and directions in detail; however, there is a lack
of description of the role of CD47-CAR T cells in various types of
tumor. CAR T-cell therapy is a cell-over-cell immunotherapy that
does not depend on major histocompatibility complex (126). Beckett et al (127) examined the role of CD47 in CAR
T-cell function by knocking down CD47 in T cells for downstream
functional analysis. They showed that CD47 expression is critical
for CAR T-cell survival in vivo and is required for
successful overt T-cell therapy. Golubovskaya et al
(128) reported that CD47 CAR T
cells had antitumor activity and significantly inhibited the growth
of transplanted pancreatic cancer tumors. Shu et al
(129) constructed a CAR T cell
targeting both CD47 and tumor-associated glycoprotein 72 (TAG-72),
which showed vigorous antitumor activity in both in vitro
and in vivo models of ovarian cancer. The specific targeting
of TAG-72 could reduce its killing of normal cells. Chen et
al (130) developed a SIRPα-Fc
fusion protein CAR T cell, which promoted the phagocytosis of
macrophages, recruited more DCs into tumor tissues, inhibited the
apoptosis of CAR T cells themselves and reduced the expression of
PD-1 on the surface of CAR T cells, thus enhancing the antitumor
effect.
The understanding of chimeric antigen receptor
macrophages (CAR-Ms) is minimal. CAR-Ms is the engineering of
macrophages to modify CARs in order to enhance macrophage
antigen-specific phagocytosis and tumor clearance (131,132). Klichinsky et al (133) first proposed the CAR-M concept,
constructed CAR-Ms and reported that CAR-Ms have strong antitumor
effects and can promote the secretion of proinflammatory factors,
promote M2-type to M1-type polarization and increase T-lymphocyte
antigen presentation (134).
In addition, a new therapeutic strategy for
targeting CD47 has emerged in recent years, namely reprogramming
the immunogenicity of cancer cells, whereby specific
chemotherapeutic agents or radiation therapy stimulate tumor cells
to undergo tumor immunogenic cell death (ICD), which is a form of
apoptosis that activates the immune system (9). Abdel-Bar et al (135) developed nucleic acid lipid
particles for the delivery of ICD-inducing Adriamycin and CD47
proteins, which could enhance phagocytosis by macrophages by
increasing the amount of cell surface calreticulin.
Due to its high expression on the surface of tumor
cells, CD47 has become an ideal target for tumor immunotherapy, and
antitumor drugs targeting CD47 were shown to have promising
applications. However, chemotherapeutic drugs targeting CD47 have
numerous adverse effects, a limitation that makes targeted CD47
therapy a significant challenge. First, CD47 is widely expressed on
the surface of tumor cells and normal cells, leading to inevitable
injury to normal red blood cells in the process of killing tumor
cells. Many red blood cells will become the best ‘cover’ for tumor
cells, and red blood cells will be exhausted by targeted drugs
before tumor cells, resulting in adverse effects such as red
blood-cell aggregation, anemia and thrombocytopenia (100,136). The degree of toxicity is dose-,
time- and patient-specific and can be reduced by optimizing the
dosage and combining drugs with erythropoietin. Second, there may
be differences in the level of CD47 expression on the surface of
different tumor cells, resulting in different sensitivities to
targeted CD47 therapy (137,138). Finally, due to the presence of
multiple immunosuppressive cells in the human body, such as
myeloid-derived suppressor cells, tumor-associated macrophages and
tumor-associated DCs, tumor cells may evade the surveillance of
immune cells by upregulating the expression other immune checkpoint
molecules (139–141), thus altering the therapeutic
efficacy of targeting CD47 (6).
Challenges in immunotherapy targeting CD47 have led
to the proposal of new therapeutic regimens to improve the
effectiveness of treatment. One such approach is to combine
CD47-targeting drugs with other immune checkpoint inhibitors to
reduce immune escape by tumor cells (142,143). Furthermore, the development of
BsAbs has provided new ideas for achieving improved specificity of
targeted therapy (144,145). In addition, solutions to modulate
the TME to enhance the efficacy of CD47-targeted therapies are also
being explored (141,146,147). These solutions are expected to
improve the efficacy of CD47-targeted therapies and reduce
resistance.
In recent years, an increasing number of studies on
CD47 have been conducted, and this topic has become a significant
hotspot in various research fields. CD47 binds to SIRPα to activate
a signaling pathway that regulates DC activation and antigen
presentation in both directions and regulates macrophage
phagocytosis during erythrocyte and HSC transplantation.
Upregulating or downregulating the expression of CD47 has an
essential regulatory role in tumor-cell growth or death, and
blocking CD47 expression also initiates an adaptive immune response
that kills tumor cells (148).
Although the combination of targeted CD47-SIRPα axis blockade
therapy with other antibody drugs or therapies has shown good
antitumor efficacy, CD47 is widely expressed in erythrocytes,
myeloid cells and other hematopoietic cells, and anemia remains the
most significant challenge associated with CD47-targeted drug
therapy (149); furthermore,
relevant antibody drugs have shown good efficacy. These drugs
effectively attenuate the adverse effects of CD47-SIRPα blockade
and significantly improve safety (117,143). However, much progress is needed
before immunotherapy targeting the CD47-SIRPα axis can be applied
in the clinic. To date, numerous clinical studies have shown that
metabolic reprogramming has an essential role in the regulation of
macrophage activation and study of the regulation of phagocytosis
by the CD47-SIRPα axis from the point of view of metabolic
reprogramming will be a promising direction; however, the
underlying mechanisms of metabolism during phagocytosis, which are
associated with the CD47-SIRPα axis, remain elusive (9). Further scientific research will
clarify the mechanisms of action.
Not applicable.
This work was supported by the Natural Science Research Project
of the Anhui Educational Committee (grant no. KJ2020ZD49) and the
512 Talent Cultivation Program of Bengbu Medical College (grant no.
by51201103).
Not applicable.
FW was involved in writing of the original draft
and searching the literature. HP, FL and MH performed the
literature search and reviewed the draft. JT and CS were involved
in supervision, writing and editing. All of the authors discussed
the article, and have read and approved the final version of the
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Chao MP, Weissman IL and Majeti R: The
CD47-SIRPα pathway in cancer immune evasion and potential
therapeutic implications. Curr Opin Immunol. 24:225–232. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khandelwal S, van Rooijen N and Saxena RK:
Reduced expression of CD47 during murine red blood cell (RBC)
senescence and its role in RBC clearance from the circulation.
Transfusion. 47:1725–1732. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matlung HL, Szilagyi K, Barclay NA and van
den Berg TK: The CD47-SIRPα signaling axis as an innate immune
checkpoint in cancer. Immunol Rev. 276:145–164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jia X, Yan B, Tian X, Liu Q, Jin J, Shi J
and Hou Y: CD47/SIRPα pathway mediates cancer immune escape and
immunotherapy. Int J Biol Sci. 17:3281–3287. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin F, Xiong M, Hao W, Song Y, Liu R, Yang
Y, Yuan X, Fan D, Zhang Y, Hao M, et al: A novel blockade CD47
antibody with therapeutic potential for cancer. Front Oncol.
10:6155342020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Q, Guo X and Ma W: Opportunities and
challenges of CD47-targeted therapy in cancer immunotherapy. Oncol
Res. 32:49–60. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J, Meng Z, Xu T, Kuerban K, Wang S,
Zhang X, Fan J, Ju D, Tian W, Huang X, et al: A SIRPαFc fusion
protein conjugated with the Collagen-Binding domain for targeted
immunotherapy of non-small cell lung cancer. Front Immunol.
13:8452172022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ozaniak A, Smetanova J, Bartolini R, Rataj
M, Capkova L, Hacek J, Fialova M, Krupickova L, Striz I, Lischke R,
et al: A novel anti-CD47-targeted blockade promotes immune
activation in human soft tissue sarcoma but does not potentiate
anti-PD-1 blockade. J Cancer Res Clin Oncol. 149:3789–3801. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hao Y, Zhou X, Li Y, Li B and Cheng L: The
CD47-SIRPα axis is a promising target for cancer immunotherapies.
Int Immunopharmacol. 120:1102552023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brown E, Hooper L, Ho T and Gresham H:
Integrin-associated protein: A 50-kD plasma membrane antigen
physically and functionally associated with integrins. J Cell Biol.
111:2785–2794. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lindberg FP, Bullard DC, Caver TE, Gresham
HD, Beaudet AL and Brown EJ: Decreased resistance to bacterial
infection and granulocyte defects in IAP-deficient mice. Science.
274:795–798. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Helden MJ, Zwarthoff SA, Arends RJ,
Reinieren-Beeren IMJ, Paradé MCBC, Driessen-Engels L, de Laat-Arts
K, Damming D, Santegoeds-Lenssen EWH, van Kuppeveld DWJ, et al:
BYON4228 is a pan-allelic antagonistic SIRPα antibody that
potentiates destruction of antibody-opsonized tumor cells and lacks
binding to SIRPγ on T cells. J Immunother Cancer. 11:e0065672023.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Navarro-Alvarez N and Yang YG: CD47: A new
player in phagocytosis and xenograft rejection. Cell Mol Immunol.
8:285–288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deng H, Wang G, Zhao S, Tao Y, Zhang Z,
Yang J and Lei Y: New hope for tumor immunotherapy: The
macrophage-related ‘do not eat me’ signaling pathway. Front
Pharmacol. 14:12289622023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hatherley D, Graham SC, Turner J, Harlos
K, Stuart DI and Barclay AN: Paired receptor specificity explained
by structures of signal regulatory proteins alone and complexed
with CD47. Mol Cell. 31:266–277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hatherley D, Harlos K, Dunlop DC, Stuart
DI and Barclay AN: The structure of the macrophage signal
regulatory protein alpha (SIRPalpha) inhibitory receptor reveals a
binding face reminiscent of that used by T cell receptors. J Biol
Chem. 282:14567–14575. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lymn JS, Patel MK, Clunn GF, Rao SJ,
Gallagher KL and Hughes AD: Thrombospondin-1 differentially induces
chemotaxis and DNA synthesis of human venous smooth muscle cells at
the receptor-binding level. J Cell Sci. 115:4353–4360. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chung J, Gao AG and Frazier WA:
Thrombspondin acts via integrin-associated protein to activate the
platelet integrin alphaIIbbeta3. J Biol Chem. 272:14740–14746.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hayat SMG, Bianconi V, Pirro M, Jaafari
MR, Hatamipour M and Sahebkar A: CD47: Role in the immune system
and application to cancer therapy. Cell Oncol (Dordr). 43:19–30.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang T, Wang F, Xu L and Yang YG:
Structural-functional diversity of CD47 proteoforms. Front Immunol.
15:13295622024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sadallah S, Eken C, Martin PJ and
Schifferli JA: Microparticles (ectosomes) shed by stored human
platelets downregulate macrophages and modify the development of
dendritic cells. J Immunol. 186:6543–6552. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aversa R, Sorrentino A, Esposito R,
Ambrosio MR, Amato A, Zambelli A, Ciccodicola A, D'Apice L and
Costa V: Alternative splicing in adhesion- and motility-related
genes in breast cancer. Int J Mol Sci. 17:1212016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reinhold MI, Lindberg FP, Plas D, Reynolds
S, Peters MG and Brown EJ: In vivo expression of alternatively
spliced forms of integrin-associated protein (CD47). J Cell Sci.
108:3419–3425. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barclay AN and Van den Berg TK: The
interaction between signal regulatory protein alpha (SIRPα) and
CD47: Structure, function, and therapeutic target. Annu Rev
Immunol. 32:25–50. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee EH, Hsieh YP, Yang CL, Tsai KJ and Liu
CH: Induction of integrin-associated protein (IAP) mRNA expression
during memory consolidation in rat hippocampus. Eur J Neurosci.
12:1105–1112. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ratnikova NM, Lezhnin YN, Frolova EI,
Kravchenko JE and Chumakov SP: CD47 receptor as a primary target
for cancer therapy. Mol Biol (Mosk). 51:251–261. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Frazier WA, Gao AG, Dimitry J, Chung J,
Brown EJ, Lindberg FP and Linder ME: The thrombospondin receptor
integrin-associated protein (CD47) functionally couples to
heterotrimeric Gi. J Biol Chem. 274:8554–8560. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
N'Diaye EN and Brown EJ: The
ubiquitin-related protein PLIC-1 regulates heterotrimeric G protein
function through association with Gbetagamma. J Cell Biol.
163:1157–1165. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sick E, Boukhari A, Deramaudt T, Rondé P,
Bucher B, André P, Gies JP and Takeda K: Activation of CD47
receptors causes proliferation of human astrocytoma but not normal
astrocytes via an Akt-dependent pathway. Glia. 59:308–319. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mateo V, Brown EJ, Biron G, Rubio M,
Fischer A, Deist FL and Sarfati M: Mechanisms of CD47-induced
caspase-independent cell death in normal and leukemic cells: Link
between phosphatidylserine exposure and cytoskeleton organization.
Blood. 100:2882–2890. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Soto-Pantoja DR, Kaur S and Roberts DD:
CD47 signaling pathways controlling cellular differentiation and
responses to stress. Crit Rev Biochem Mol Biol. 50:212–230. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brown EJ and Frazier WA:
Integrin-associated protein (CD47) and its ligands. Trends Cell
Biol. 11:130–135. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Murata Y, Saito Y, Kotani T and Matozaki
T: Blockade of CD47 or SIRPα: A new cancer immunotherapy. Expert
Opin Ther Targets. 24:945–951. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Manna PP and Frazier WA: The mechanism of
CD47-dependent killing of T cells: Heterotrimeric Gi-dependent
inhibition of protein kinase A. J Immunol. 170:3544–3553. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lindberg FP, Gresham HD, Reinhold MI and
Brown EJ: Integrin-associated protein immunoglobulin domain is
necessary for efficient vitronectin bead binding. J Cell Biol.
134:1313–1322. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brittain JE, Han J, Ataga KI, Orringer EP
and Parise LV: Mechanism of CD47-induced alpha4beta1 integrin
activation and adhesion in sickle reticulocytes. J Biol Chem.
279:42393–42402. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Orazizadeh M, Lee HS, Groenendijk B,
Sadler SJ, Wright MO, Lindberg FP and Salter DM: CD47 associates
with alpha 5 integrin and regulates responses of human articular
chondrocytes to mechanical stimulation in an in vitro model.
Arthritis Res Ther. 10:R42008. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Koenigsknecht J and Landreth G: Microglial
phagocytosis of fibrillar beta-amyloid through a beta1
integrin-dependent mechanism. J Neurosci. 24:9838–9846. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang K, Li M, Yin L, Fu G and Liu Z: Role
of thrombospondin-1 and thrombospondin-2 in cardiovascular diseases
(Review). Int J Mol Med. 45:1275–1293. 2020.PubMed/NCBI
|
|
40
|
Adams JC and Lawler J: The
thrombospondins. Cold Spring Harb Perspect Biol. 3:a0097122011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Leclair P and Lim CJ: CD47-independent
effects mediated by the TSP-derived 4N1K peptide. PLoS One.
9:e983582014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Isenberg JS, Romeo MJ, Yu C, Yu CK, Nghiem
K, Monsale J, Rick ME, Wink DA, Frazier WA and Roberts DD:
Thrombospondin-1 stimulates platelet aggregation by blocking the
antithrombotic activity of nitric oxide/cGMP signaling. Blood.
111:613–623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jeanne A, Sarazin T, Charlé M, Moali C,
Fichel C, Boulagnon-Rombi C, Callewaert M, Andry MC, Diesis E,
Delolme F, et al: Targeting ovarian carcinoma with TSP-1: CD47
antagonist TAX2 activates Anti-Tumor immunity. Cancers (Basel).
13:50192021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kharitonenkov A, Chen Z, Sures I, Wang H,
Schilling J and Ullrich A: A family of proteins that inhibit
signalling through tyrosine kinase receptors. Nature. 386:181–186.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Advani R, Flinn I, Popplewell L, Forero A,
Bartlett NL, Ghosh N, Kline J, Roschewski M, LaCasce A, Collins GP,
et al: CD47 Blockade by Hu5F9-G4 and rituximab in Non-Hodgkin's
lymphoma. N Engl J Med. 379:1711–1721. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Barclay AN and Brown MH: The SIRP family
of receptors and immune regulation. Nat Rev Immunol. 6:457–464.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Feng M, Jiang W, Kim BYS, Zhang CC, Fu YX
and Weissman IL: Phagocytosis checkpoints as new targets for cancer
immunotherapy. Nat Rev Cancer. 19:568–586. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhao H, Song S, Ma J, Yan Z, Xie H, Feng Y
and Che S: CD47 as a promising therapeutic target in oncology.
Front Immunol. 13:7574802022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nakaishi A, Hirose M, Yoshimura M, Oneyama
C, Saito K, Kuki N, Matsuda M, Honma N, Ohnishi H, Matozaki T, et
al: Structural insight into the specific interaction between murine
SHPS-1/SIRP alpha and its ligand CD47. J Mol Biol. 375:650–660.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Vernon-Wilson EF, Kee WJ, Willis AC,
Barclay AN, Simmons DL and Brown MH: CD47 is a ligand for rat
macrophage membrane signal regulatory protein SIRP (OX41) and human
SIRPalpha 1. Eur J Immunol. 30:2130–2137. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Han X, Sterling H, Chen Y, Saginario C,
Brown EJ, Frazier WA, Lindberg FP and Vignery A: CD47, a ligand for
the macrophage fusion receptor, participates in macrophage
multinucleation. J Biol Chem. 275:37984–37992. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rebres RA, Vaz LE, Green JM and Brown EJ:
Normal ligand binding and signaling by CD47 (integrin-associated
protein) requires a long range disulfide bond between the
extracellular and membrane-spanning domains. J Biol Chem.
276:34607–34616. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hatherley D, Graham SC, Harlos K, Stuart
DI and Barclay AN: Structure of signal-regulatory protein alpha: A
link to antigen receptor evolution. J Biol Chem. 284:26613–26619.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Takada T, Matozaki T, Takeda H, Fukunaga
K, Noguchi T, Fujioka Y, Okazaki I, Tsuda M, Yamao T, Ochi F and
Kasuga M: Roles of the complex formation of SHPS-1 with SHP-2 in
insulin-stimulated mitogen-activated protein kinase activation. J
Biol Chem. 273:9234–9242. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tsai RK and Discher DE: Inhibition of
‘self’ engulfment through deactivation of myosin-II at the
phagocytic synapse between human cells. J Cell Biol. 180:989–1003.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sato-Hashimoto M, Saito Y, Ohnishi H,
Iwamura H, Kanazawa Y, Kaneko T, Kusakari S, Kotani T, Mori M,
Murata Y, et al: Signal regulatory protein α regulates the
homeostasis of T lymphocytes in the spleen. J Immunol. 187:291–297.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Latour S, Tanaka H, Demeure C, Mateo V,
Rubio M, Brown EJ, Maliszewski C, Lindberg FP, Oldenborg A, Ullrich
A, et al: Bidirectional negative regulation of human T and
dendritic cells by CD47 and its cognate receptor signal-regulator
protein-alpha: Down-regulation of IL-12 responsiveness and
inhibition of dendritic cell activation. J Immunol. 67:2547–2554.
2001. View Article : Google Scholar
|
|
58
|
Saito Y, Iwamura H, Kaneko T, Ohnishi H,
Murata Y, Okazawa H, Kanazawa Y, Sato-Hashimoto M, Kobayashi H,
Oldenborg PA, et al: Regulation by SIRPα of dendritic cell
homeostasis in lymphoid tissues. Blood. 116:3517–3525. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Maile LA, DeMambro VE, Wai C, Lotinun S,
Aday AW, Capps BE, Beamer WG, Rosen CJ and Clemmons DR: An
essential role for the association of CD47 to SHPS-1 in skeletal
remodeling. J Bone Miner Res. 26:2068–2081. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Oldenborg PA, Zheleznyak A, Fang YF,
Lagenaur CF, Gresham HD and Lindberg FP: Role of CD47 as a marker
of self on red blood cells. Science. 288:2051–2054. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Clevers H, Loh KM and Nusse R: Stem cell
signaling. An integral program for tissue renewal and regeneration:
Wnt signaling and stem cell control. Science. 346:12480122014.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Murata Y, Kotani T, Ohnishi H and Matozaki
T: The CD47-SIRPα signalling system: Its physiological roles and
therapeutic application. J Biochem. 155:335–344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ferrari D, Gorini S, Callegari G and la
Sala A: Shaping immune responses through the activation of
dendritic cells' P2 receptors. Purinergic Signal. 3:99–107. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Logtenberg MEW, Scheeren FA and Schumacher
TN: The CD47-SIRPα Immune Checkpoint. Immunity. 52:742–752. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Okazawa H, Motegi S, Ohyama N, Ohnishi H,
Tomizawa T, Kaneko Y, Oldenborg PA, Ishikawa O and Matozaki T:
Negative regulation of phagocytosis in macrophages by the
CD47-SHPS-1 system. J Immunol. 174:2004–2011. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ishikawa-Sekigami T, Kaneko Y, Okazawa H,
Tomizawa T, Okajo J, Saito Y, Okuzawa C, Sugawara-Yokoo M,
Nishiyama U, Ohnishi H, et al: SHPS-1 promotes the survival of
circulating erythrocytes through inhibition of phagocytosis by
splenic macrophages. Blood. 107:341–348. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yamao T, Noguchi T, Takeuchi O, Nishiyama
U, Morita H, Hagiwara T, Akahori H, Kato T, Inagaki K, Okazawa H,
et al: Negative regulation of platelet clearance and of the
macrophage phagocytic response by the transmembrane glycoprotein
SHPS-1. J Biol Chem. 277:39833–39839. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang C, Wang H, Ide K, Wang Y, Van Rooijen
N, Ohdan H and Yang YG: Human CD47 expression permits survival of
porcine cells in immunodeficient mice that express SIRPα capable of
binding to human CD47. Cell Transplant. 20:1915–1920. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Griesemer A, Yamada K and Sykes M:
Xenotransplantation: Immunological hurdles and progress toward
tolerance. Immunol Rev. 258:241–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Takenaka K, Prasolava TK, Wang JC,
Mortin-Toth SM, Khalouei S, Gan OI, Dick JE and Danska JS:
Polymorphism in Sirpa modulates engraftment of human hematopoietic
stem cells. Nat Immunol. 8:1313–1323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kwong LS, Brown MH, Barclay AN and
Hatherley D: Signal-regulatory protein α from the NOD mouse binds
human CD47 with an exceptionally high affinity-implications for
engraftment of human cells. Immunology. 143:61–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Theocharides AP, Jin L, Cheng PY,
Prasolava TK, Malko AV, Ho JM, Poeppl AG, van Rooijen N, Minden MD,
Danska JS, et al: Disruption of SIRPα signaling in macrophages
eliminates human acute myeloid leukemia stem cells in xenografts. J
Exp Med. 209:1883–1899. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Rodriguez PL, Harada T, Christian DA,
Pantano DA, Tsai RK and Discher DE: Minimal ‘Self’ peptides that
inhibit phagocytic clearance and enhance delivery of nanoparticles.
Science. 339:971–975. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Iwamoto C, Takenaka K, Urata S, Yamauchi
T, Shima T, Kuriyama T, Daitoku S, Saito Y, Miyamoto T, Iwasaki H,
et al: The BALB/c-specific polymorphic SIRPA enhances its affinity
for human CD47, inhibiting phagocytosis against human cells to
promote xenogeneic engraftment. Exp Hematol. 42:163–171.e1. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ishikawa-Sekigami T, Kaneko Y, Saito Y,
Murata Y, Okazawa H, Ohnishi H, Oldenborg PA, Nojima Y and Matozaki
T: Enhanced phagocytosis of CD47-deficient red blood cells by
splenic macrophages requires SHPS-1. Biochem Biophys Res Commun.
343:1197–1200. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Chao MP, Alizadeh AA, Tang C, Myklebust
JH, Varghese B, Gill S, Jan M, Cha AC, Chan CK, Tan BT, et al:
Anti-CD47 antibody synergizes with rituximab to promote
phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 142:699–713.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Xiao Z, Chung H, Banan B, Manning PT, Ott
KC, Lin S, Capoccia BJ, Subramanian V, Hiebsch RR, Upadhya GA, et
al: Antibody mediated therapy targeting CD47 inhibits tumor
progression of hepatocellular carcinoma. Cancer Lett. 360:302–309.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhang H, Lu H, Xiang L, Bullen JW, Zhang
C, Samanta D, Gilkes DM, He J and Semenza GL: HIF-1 regulates CD47
expression in breast cancer cells to promote evasion of
phagocytosis and maintenance of cancer stem cells. Proc Natl Acad
Sci USA. 112:E6215–6223. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Liu L, Zhang L, Yang L, Li H, Li R, Yu J,
Yang L, Wei F, Yan C, Sun Q, et al: Anti-CD47 antibody as a
targeted therapeutic agent for human lung cancer and cancer stem
cells. Front Immunol. 8:4042017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Russ A, Hua AB, Montfort WR, Rahman B,
Riaz IB, Khalid MU, Carew JS, Nawrocki ST, Persky D and Anwer F:
Blocking ‘don't eat me’ signal of CD47-SIRPα in hematological
malignancies, an in-depth review. Blood Rev. 32:480–489. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yang K, Xu J, Liu Q, Li J and Xi Y:
Expression and significance of CD47, PD1 and PDL1 in T-cell acute
lymphoblastic lymphoma/leukemia. Pathol Res Pract. 215:265–271.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Abe H, Saito R, Ichimura T, Iwasaki A,
Yamazawa S, Shinozaki-Ushiku A, Morikawa T, Ushiku T, Yamashita H,
Seto Y and Fukayama M: CD47 expression in Epstein-Barr
virus-associated gastric carcinoma: Coexistence with tumor immunity
lowering the ratio of CD8+/Foxp3+ T cells.
Virchows Arch. 472:643–651. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yu L, Ding Y, Wan T, Deng T, Huang H and
Liu J: Significance of CD47 and its association with tumor immune
microenvironment heterogeneity in ovarian cancer. Front Immunol.
12:7681152021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Casey SC, Tong L, Li Y, Do R, Walz S,
Fitzgerald KN, Gouw AM, Baylot V, Gütgemann I, Eilers M and Felsher
DW: MYC regulates the antitumor immune response through CD47 and
PD-L1. Science. 352:227–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Betancur PA, Abraham BJ, Yiu YY,
Willingham SB, Khameneh F, Zarnegar M, Kuo AH, McKenna K, Kojima Y,
Leeper NJ, et al: A CD47-associated super-enhancer links
pro-inflammatory signalling to CD47 upregulation in breast cancer.
Nat Commun. 8:148022017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Suzuki S, Yokobori T, Tanaka N, Sakai M,
Sano A, Inose T, Sohda M, Nakajima M, Miyazaki T, Kato H and Kuwano
H: CD47 expression regulated by the miR-133a tumor suppressor is a
novel prognostic marker in esophageal squamous cell carcinoma.
Oncol Rep. 28:465–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Rastgoo N, Wu J, Liu A, Pourabdollah M,
Atenafu EG, Reece D, Chen W and Chang H: Targeting CD47/TNFAIP8 by
miR-155 overcomes drug resistance and inhibits tumor growth through
induction of phagocytosis and apoptosis in multiple myeloma.
Haematologica. 105:2813–2823. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Huang CY, Ye ZH, Huang MY and Lu JJ:
Regulation of CD47 expression in cancer cells. Transl Oncol.
13:1008622020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ma R, Ortiz Serrano TP, Davis J, Prigge AD
and Ridge KM: The cGAS-STING pathway: The role of self-DNA sensing
in inflammatory lung disease. FASEB J. 34:13156–13170. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
von Roemeling CA, Wang Y, Qie Y, Yuan H,
Zhao H, Liu X, Yang Z, Yang M, Deng W, Bruno KA, et al: Therapeutic
modulation of phagocytosis in glioblastoma can activate both innate
and adaptive antitumour immunity. Nat Commun. 11:15082020.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Xu MM, Pu Y, Han D, Shi Y, Cao X, Liang H,
Chen X, Li XD, Deng L, Chen ZJ, et al: Dendritic cells but not
macrophages sense tumor mitochondrial DNA for cross-priming through
signal regulatory protein α signaling. Immunity. 47:363–373.e5.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chen J, Zhong MC, Guo H, Davidson D,
Mishel S, Lu Y, Rhee I, Pérez-Quintero LA, Zhang S, Cruz-Munoz ME,
et al: SLAMF7 is critical for phagocytosis of haematopoietic tumour
cells via Mac-1 integrin. Nature. 544:493–497. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
He Y, Bouwstra R, Wiersma VR, de Jong M,
Jan Lourens H, Fehrmann R, de Bruyn M, Ammatuna E, Huls G, van
Meerten T and Bremer E: Cancer cell-expressed SLAMF7 is not
required for CD47-mediated phagocytosis. Nat Commun. 10:5332019.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Yang Y, Yang Z and Yang Y: Potential role
of CD47-directed bispecific antibodies in cancer immunotherapy.
Front Immunol. 12:6860312021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhang W, Huang Q, Xiao W, Zhao Y, Pi J, Xu
H, Zhao H, Xu J, Evans CE and Jin H: Advances in anti-tumor
treatments targeting the CD47/SIRPα axis. Front Immunol. 11:182020.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Narla RK, Modi H, Bauer D, Abbasian M,
Leisten J, Piccotti JR, Kopytek S, Eckelman BP, Deveraux Q, Timmer
J, et al: Modulation of CD47-SIRPα innate immune checkpoint axis
with Fc-function detuned anti-CD47 therapeutic antibody. Cancer
Immunol Immunother. 71:473–489. 202 View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Kuo TC, Chen A, Harrabi O, Sockolosky JT,
Zhang A, Sangalang E, Doyle LV, Kauder SE, Fontaine D, Bollini S,
et al: Targeting the myeloid checkpoint receptor SIRPα potentiates
innate and adaptive immune responses to promote anti-tumor
activity. J Hematol Oncol. 13:1602020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Bian HT, Shen YW, Zhou YD, Nagle DG, Guan
YY, Zhang WD and Luan X: CD47: Beyond an immune checkpoint in
cancer treatment. Biochim Biophys Acta Rev Cancer. 1877:1887712022.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Luo X, Shen Y, Huang W, Bao Y, Mo J, Yao L
and Yuan L: Blocking CD47-SIRPα signal axis as promising
immunotherapy in ovarian cancer. Cancer Control.
30:107327482311597062023. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liu Y, Wang Y, Yang Y, Weng L, Wu Q, Zhang
J, Zhao P, Fang L, Shi Y and Wang P: Emerging phagocytosis
checkpoints in cancer immunotherapy. Signal Transduct Target Ther.
8:1042023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Maute R, Xu J and Weissman IL:
CD47-SIRPα-targeted therapeutics: Status and prospects. Immunooncol
Technol. 13:1000702022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kayser S and Levis MJ: The clinical impact
of the molecular landscape of acute myeloid leukemia.
Haematologica. 108:308–320. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Berlin J, Harb W, Adjei A, Xing Y,
Swiecicki P, Seetharam M, Nandagopal L, Gopal A, Xu C, Meng Y, et
al: 385 A first-in-human study of lemzoparlimab, a differentiated
anti-CD47 antibody, in subjects with relapsed/refractory
malignancy: Initial monotherapy results. J Immuno Ther Res Cancer.
8 (Suppl 3):A233–A234. 2020.
|
|
104
|
Qi J, Li J, Jiang B, Jiang B, Liu H, Cao
X, Zhang M, Meng Y, MA X, Jia Y, et al: A Phase I/IIa study of
lemzoparlimab, a monoclonal antibody targeting CD47, in patients
with relapsed and/or refractory acute myeloid leukemia (AML) and
myelodysplastic syndrome (MDS): Initial phase I results. Blood.
136:30–31. 2020. View Article : Google Scholar
|
|
105
|
Gan HK, Coward J, Mislang A, Cosman R,
Nagrial A, Jin X, Li B, Wang ZM, Kwek KY, Xia D and Xia Y: Safety
of AK117, an anti-CD47 monoclonal antibody, in patients with
advanced or metastatic solid tumors in a phase I study. J Clini
Oncol. 39 (Suppl 15):S26302021. View Article : Google Scholar
|
|
106
|
Jiang Z, Sun H, Yu J, Tian W and Song Y:
Targeting CD47 for cancer immunotherapy. J Hematol Oncol.
14:1802021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Qu T, Li B and Wang Y: Targeting
CD47/SIRPα as a therapeutic strategy, where we are and where we are
headed. Biomark Res. 10:202022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Puro RJ, Bouchlaka MN, Hiebsch RR,
Capoccia BJ, Donio MJ, Manning PT, Frazier WA, Karr RW and Pereira
DS: Development of AO-176, a Next-Generation Humanized Anti-CD47
antibody with novel anticancer properties and negligible red blood
cell binding. Mol Cancer Ther. 19:835–846. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
III HAB, Spira AI, Taylor MH, Yeku OO, Liu
JF, Munster P, Hamilton EP, Thomas JS, Gatlin F, Penson RT, et al:
A first-in-human study of AO-176, a highly differentiated anti-CD47
antibody, in patients with advanced solid tumors. J Clin Oncol. 39
(15_Suppl):S25162021. View Article : Google Scholar
|
|
110
|
Zeidan AM, DeAngelo DJ, Palmer J, Seet CS,
Tallman MS, Wei X, Raymon H, Sriraman P, Kopytek S, Bewersdorf JP,
et al: Phase 1 study of anti-CD47 monoclonal antibody CC-90002 in
patients with relapsed/refractory acute myeloid leukemia and
high-risk myelodysplastic syndromes. Ann Hematol. 101:557–569.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Zeidan AM, DeAngelo DJ, Palmer JM,
DeAngelo DJ, Palmer JM, Seet CS, Tallman MS, Wei X, Li YF, Hock R,
et al: A Phase I study of CC-90002, a monoclonal antibody targeting
CD47, in patients with relapsed and/or refractory (R/R) acute
myeloid leukemia (AML) and High-risk myelodysplastic syndromes
(MDS): Final results. Blood. 134:13202019. View Article : Google Scholar
|
|
112
|
Velliquette RW, Aeschlimann J, Kirkegaard
J, Shakarian G, Lomas-Francis C and Westhoff CM: Monoclonal
anti-CD47 interference in red cell and platelet testing.
Transfusion. 59:730–737. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ansell SM, Maris MB, Lesokhin AM, Chen RW,
Flinn IW, Sawas A, Minden MD, Villa D, Percival MM, Advani AS, et
al: Phase I study of the CD47 Blocker TTI-621 in patients with
relapsed or refractory hematologic malignancies. Clin Cancer Res.
27:2190–2199. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Patel K, Maris MB, Cheson BD, Zonder JA,
Lesokhin AM, Keudell GV, Seymour EK, Lin GHY, Catalano T, Shou Y,
et al: Ongoing, first-in-human, phase I dose escalation study of
the investigational CD47-blocker TTI-622 in patients with advanced
relapsed or refractory lymphoma. J Clin Oncol. 38
(15_Suppl):S30302020. View Article : Google Scholar
|
|
115
|
Yang H, Xun Y and You H: The landscape
overview of CD47-based immunotherapy for hematological
malignancies. Biomark Res. 11:152023. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Chow LQ, Gainor J, Lakhani N, Chunget HC,
Lee KW, Lee J, Lorusso P, Bang YJ, Hodi FS, Fanning P, et al: A
phase 1 study of ALX148, a CD47 blocker, in combination with
established anticancer antibodies in patients with advanced
malignancy. Safety. 1:362019.
|
|
117
|
Piccione EC, Juarez S, Liu J, Tseng S,
Ryan CE, Narayanan C, Wang L, Weiskopf K and Majeti R: A bispecific
antibody targeting CD47 and CD20 selectively binds and eliminates
dual antigen expressing lymphoma cells. MAbs. 7:946–956. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Yu J, Li S, Chen D, Guo H, Yang C, Zhang
W, Zhang L, Zhao G, Tu X, Peng L, et al: IMM0306, a fusion protein
of CD20 mAb with the CD47 binding domain of SIRPα, exerts excellent
cancer killing efficacy by activating both macrophages and NK cells
via blockade of CD47-SIRPα interaction and FcɣR engagement by
simultaneously binding to CD47 and CD20 of B cells. Leukemia.
37:695–698. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Wang Y, Ni H, Zhou S, He K, Gao Y, Wu W,
Wu M, Wu Z, Qiu X, Zhou Y, et al: Tumor-selective blockade of CD47
signaling with a CD47/PD-L1 bispecific antibody for enhanced
anti-tumor activity and limited toxicity. Cancer Immunol
Immunother. 70:365–376. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Ke H, Zhang F, Wang J, Xiong L, An X, Tu
X, Chen C, Wang Y, Mao M, Guo S, et al: HX009, a novel BsAb dual
targeting PD1 × CD47, demonstrates potent anti-lymphoma activity in
preclinical models. Sci Rep. 13:54192023. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Roohullah A, Ganju V, Zhang F, Zhang L, Yu
T, Wilkinson K, Cooper A and de Souza P: First-in-human phase 1
dose escalation study of HX009, a novel recombinant humanized
anti-PD-1 and CD47 bispecific antibody, in patients with advanced
malignancies. J Clin Oncol. 39:2517. 2021. View Article : Google Scholar
|
|
122
|
Dheilly E, Moine V, Broyer L,
Salgado-Pires S, Johnson Z, Papaioannou A, Cons L, Calloud S,
Majocchi S, Rousseau F, et al: Selective blockade of the ubiquitous
checkpoint receptor CD47 is enabled by dual-targeting bispecific
antibodies. Mol Ther. 25:523–533. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Buatois V, Johnson Z, Salgado-Pires S,
Papaioannou A, Hatterer E, Chauchet X, Richard F, Barba L, Daubeuf
B, Cons L, et al: Preclinical development of a bispecific antibody
that safely and effectively targets CD19 and CD47 for the treatment
of B-Cell lymphoma and leukemia. Mol Cancer Ther. 17:1739–1751.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
de Silva S, Fromm G, Shuptrine CW,
Johannes K, Patel A, Yoo KJ, Huang K and Schreiber TH: CD40
enhances type I interferon responses downstream of CD47 blockade,
bridging innate and adaptive immunity. Cancer Immunol Res.
8:230–245. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Golubovskaya V: CAR-T cells targeting
immune checkpoint pathway players. Front Biosci (Landmark Ed).
27:1212022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Feins S, Kong W, Williams EF, Milone MC
and Fraietta JA: An introduction to chimeric antigen receptor (CAR)
T-cell immunotherapy for human cancer. Am J Hematol. 94
(Suppl):S3–S9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Beckett AN, Chockley P, Pruett-Miller SM,
Nguyen P, Vogel P, Sheppard H, Krenciute G, Gottschalk S and
DeRenzo C: CD47 expression is critical for CAR T-cell survival in
vivo. J Immunother Cancer. 11:e0058572023. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Golubovskaya V, Berahovich R, Zhou H, Xu
S, Harto H, Li L, Chao CC, Mao MM and Wu L: CD47-CAR-T cells
effectively kill target cancer cells and block pancreatic tumor
growth. Cancers (Basel). 9:1392017. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Shu R, Evtimov VJ, Hammett MV, Nguyen NN,
Zhuang J, Hudson PJ, Howard MC, Pupovac A, Trounson AO and Boyd RL:
Engineered CAR-T cells targeting TAG-72 and CD47 in ovarian cancer.
Mol Ther Oncolytics. 20:325–341. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Chen H, Yang Y, Deng Y, Wei F, Zhao Q, Liu
Y, Liu Z, Yu B and Huang Z: Delivery of CD47 blocker SIRPα-Fc by
CAR-T cells enhances antitumor efficacy. J Immunother Cancer.
10:e0037372022. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Sloas C, Gill S and Klichinsky M:
Engineered CAR-macrophages as adoptive immunotherapies for solid
tumors. Front Immunol. 12:7833052021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Chen Y, Yu Z, Tan X, Jiang H, Xu Z, Fang
Y, Han D, Hong W, Wei W and Tu J: CAR-macrophage: A new
immunotherapy candidate against solid tumors. Biomed Pharmacother.
139:1116052021. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Klichinsky M, Ruella M, Shestova O, Lu XM,
Best A, Zeeman M, Schmierer M, Gabrusiewicz K, Anderson NR, Petty
NE, et al: Human chimeric antigen receptor macrophages for cancer
immunotherapy. Nat Biotechnol. 38:947–953. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Zhang L, Tian L, Dai X, Yu H, Wang J, Lei
A, Zhu M, Xu J, Zhao W, Zhu Y, et al: Pluripotent stem cell-derived
CAR-macrophage cells with antigen-dependent anti-cancer cell
functions. J Hematol Oncol. 13:1532020. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Abdel-Bar HM, Walters AA, Lim Y, Rouatbi
N, Qin Y, Gheidari F, Han S, Osman R, Wang JT and Al-Jamal KT: An
‘eat me’ combinatory nano-formulation for systemic immunotherapy of
solid tumors. Theranostics. 11:8738–8754. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Chen YC, Shi W, Shi JJ and Lu JJ: Progress
of CD47 immune checkpoint blockade agents in anticancer therapy: A
hematotoxic perspective. J Cancer Res Clin Oncol. 148:1–14. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Yan X, Lai B, Zhou X, Yang S, Ge Q, Zhou
M, Shi C, Xu Z and Ouyang G: The differential expression of CD47
may be related to the pathogenesis from myelodysplastic syndromes
to acute myeloid leukemia. Front Oncol. 12:8729992022. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Shi M, Gu Y, Jin K, Fang H, Chen Y, Cao Y,
Liu X, Lv K, He X, Lin C, et al: CD47 expression in gastric cancer
clinical correlates and association with macrophage infiltration.
Cancer Immunol Immunother. 70:1831–1840. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Li K, Shi H, Zhang B, Ou X, Ma Q, Chen Y,
Shu P, Li D and Wang Y: Myeloid-derived suppressor cells as
immunosuppressive regulators and therapeutic targets in cancer.
Signal Transduct Target Ther. 6:3622021. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Xu S, Wang C, Yang L, Wu J, Li M, Xiao P,
Xu Z, Xu Y and Wang K: Targeting immune checkpoints on
tumor-associated macrophages in tumor immunotherapy. Front Immunol.
14:11996312023. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Zhang H, Liu L, Liu J, Dang P, Hu S, Yuan
W, Sun Z, Liu Y and Wang C: Roles of tumor-associated macrophages
in anti-PD-1/PD-L1 immunotherapy for solid cancers. Mol Cancer.
22:582023. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Torres ETR and Emens LA: Emerging
combination immunotherapy strategies for breast cancer: Dual immune
checkpoint modulation, antibody-drug conjugates and bispecific
antibodies. Breast Cancer Res Treat. 191:291–302. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Chen SH, Dominik PK, Stanfield J, Ding S,
Yang W, Kurd N, Llewellyn R, Heyen J, Wang C, Melton Z, et al: Dual
checkpoint blockade of CD47 and PD-L1 using an affinity-tuned
bispecific antibody maximizes antitumor immunity. J Immunother
Cancer. 9:e0034642021. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
van de Donk N and Zweegman S:
T-cell-engaging bispecific antibodies in cancer. Lancet.
402:142–158. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Zhang T, Lin Y and Gao Q: Bispecific
antibodies targeting immunomodulatory checkpoints for cancer
therapy. Cancer Biol Med. 20:181–195. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Olaoba OT, Ayinde KS, Lateef OM,
Akintubosun MO, Lawal KA and Adelusi TI: Is the new angel better
than the old devil? Challenges and opportunities in
CD47-SIRPα-based cancer therapy. Crit Rev Oncol Hematol.
184:1039392023. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Cao A, Yi J, Tang X, Szeto CW, Wu R, Wan
B, Fang X, Li S, Wang L, Wang L, et al: CD47-blocking antibody
ZL-1201 promotes Tumor-associated macrophage phagocytic activity
and enhances the efficacy of the therapeutic antibodies and
chemotherapy. Cancer Res Commun. 2:1404–1417. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Chen Q, Sun L and Chen ZJ: Regulation and
function of the cGAS-STING pathway of cytosolic DNA sensing. Nat
Immunol. 17:1142–1149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Brierley CK, Staves J, Roberts C, Johnson
H, Vyas P, Goodnough LT and Murphy MF: The effects of monoclonal
anti-CD47 on RBCs, compatibility testing, and transfusion
requirements in refractory acute myeloid leukemia. Transfusion.
59:2248–2254. 2019. View Article : Google Scholar : PubMed/NCBI
|