Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common malignancy and fourth leading cause of cancer-related death
worldwide. According to the National Cancer Institute SEER
database, the average 5-year survival rate of patients with HCC in
the USA is 19.6%, but can be as low as 2.5% for advanced,
metastatic disease (1,2). A total of >90% of HCC cases develop
in individuals with preexisting chronic liver conditions, making
this cancer a significant contributor to morbidity and mortality
among patients with cirrhosis (3).
The poor prognosis for patients with HCC has traditionally stemmed
from a combination of late-stage diagnosis and limited treatment
effectiveness at advanced stages.
Systemic therapy continues to be the preferred
treatment modality for patients diagnosed with unresectable HCC
(uHCC). Among the available options, tyrosine kinase inhibitors
(TKIs), such as sorafenib and lenvatinib, demonstrate similar
overall survival (OS) outcomes for patients with uHCC (range,
10–13.5 months) (4–6). Lenvatinib, a multi-TKI, acts on
receptors that include platelet-derived growth factor receptor-α,
rearranged during transfection and tyrosine-kinase receptor
(7). This inhibition effectively
curtails angiogenesis and hinders tumour growth. Its robust
suppression of the fibroblast growth factor receptor (FGFR) pathway
is the key approach to managing liver cancer. In the REFLECT trial,
lenvatinib exhibited superior response rates, extended
progression-free survival and demonstrated non-inferiority in OS
compared with sorafenib (4).
Although lenvatinib has shown superiority to sorafenib in improving
OS in patients with HCC in clinical trials (clinical trials nos.
NCT01761266 and NCT04127396), its effectiveness has been limited by
resistance and side effects (4).
Further improvements in efficacy are required.
In recent years, immune checkpoint inhibitors have
been widely studied in various cancers. Programmed death-1 (PD-1)
and PD-ligand 1 (PD-L1) checkpoint inhibitors have emerged as
promising treatment strategies for HCC (8). Currently, an increasing body of
evidence indicates that most TKIs exert anti-angiogenic activity in
cancer treatment, and when used in combination with
immunosuppressants, including PD-1 blockade, sirolimus and
everolimus, they exhibit a synergistic effect (9). TKIs can promote the expression of
major histocompatibility complex antigens on tumour cells, thereby
enhancing sensitivity to T-cell-mediated tumour cell elimination.
This effect is particularly pronounced when used in combination
with PD-1 inhibitors as it facilitates T-cell-mediated tumour cell
destruction (10). In vitro
studies have shown that lenvatinib and PD-1 inhibitors can exert
synergistic antitumour effects (11,12).
In addition, an early clinical trial regarding the combination of
lenvatinib and PD-1 inhibitors for advanced HCC has reported
favourable safety and effectiveness (13). However, to date, evidence-based
information on the efficacy and safety of lenvatinib combined with
PD-1/PD-L1 inhibitors in treating HCC is lacking. A meta-analysis
of the clinical survival indicators, adverse reactions and safety
of lenvatinib combined with PD-1 inhibitors in liver cancer
treatment was conducted, providing objective and effective
evidence-based medical information for clinical use. The present
study is anticipated to guide clinical application.
Materials and methods
Literature inclusion and exclusion
criteria
The inclusion criteria were the following: i)
Regarding the study design, randomized controlled trials (RCTs),
non-RCTs and single-arm trial studies related to the combined
treatment of lenvatinib and PD-1/PD-L1 inhibitors for HCC were
included and the language was limited to English; ii) regarding the
study subjects, patients with a confirmed diagnosis of primary
liver cancer were included without restrictions on sex, race, or
age; iii) regarding interventions, in the control group, patients
received lenvatinib monotherapy, while in the experimental group,
patients received combination therapy using lenvatinib and
PD-1/PD-L1 inhibitors for liver cancer; and iv) regarding outcome
measures, complete response (CR), partial response (PR), stable
disease (SD), progression disease (PD), objective response rate
(ORR), disease control rate (DCR), safety measures, including
adverse reactions, were assessed. The exclusion criteria were the
following: i) Case reports, review articles and previously
published duplicate studies; ii) animal experiments and basic
research; iii) literature that did not align with the inclusion
criteria; iv) studies with flawed research designs or treatment
measures unrelated to the experiment; and v) literature that could
not provide valid information and data.
Literature search
The Embase, PubMed and Cochrane Library databases
were used for literature search from inception to September 2023.
The search terms were a combination of Medical Subject Heading
terms and entry terms. The search terms included ‘lenvatinib’ AND
‘nivolumab’, ‘pembrolizumab’, ‘tremelimumab, ‘atezolizumab,
‘durvalumab’, ‘camrelizumab’, ‘PD1’, ‘PDL1’ AND ‘hepatocellular
carcinoma’.
Data extraction
Two researchers independently conducted the
screening and data extraction based on the aforementioned inclusion
and exclusion criteria. In case of discrepancies between the two
researchers, disagreements were resolved through discussion or, if
needed, by consulting a third researcher.
Literature quality assessment
Two researchers independently conducted literature
quality evaluations using the Newcastle-Ottawa Scale (NOS) for
cohort studies (14). NOS includes
four items (4 points) for ‘Research Subject Selection’, one item (2
points) for ‘Comparability between Groups’ and three items (3
points) for ‘Result Measurement’. It has a full score of 9 points,
with ≥7 regarded as high-quality literature and <7, low-quality
literature. The meta-analysis was performed based on the related
items of the Preferred Reporting Items for Systematic Reviews and
Meta-analysis guidelines (15).
Data synthesis and statistical
analysis
Data were analysed using Review Manager (version
5.3; http://revman.cochrane.org) and
processed using STATA 15.1 (StataCorp LP). The differences in
treatment outcomes in RCTs were assessed using the relative risk
(RR) and its 95% confidence interval (CI). I2 was used
to evaluate cell heterogeneity. If the test for heterogeneity was
P≥0.1 and I2≤50%, homogeneity between studies was
indicated, and the studies were analysed together using a fixed
effects model. If the test was P<0.1 and I2>50%,
significant heterogeneity within this group was indicated; if there
was a difference, the source of the difference was identified using
sensitivity analysis. A random-effects model was used for the
pooling effect in the present meta-analysis. Publication bias was
analysed using funnel plots and Egger's test with P>0.05
indicating no publication bias.
Results
Literature search results
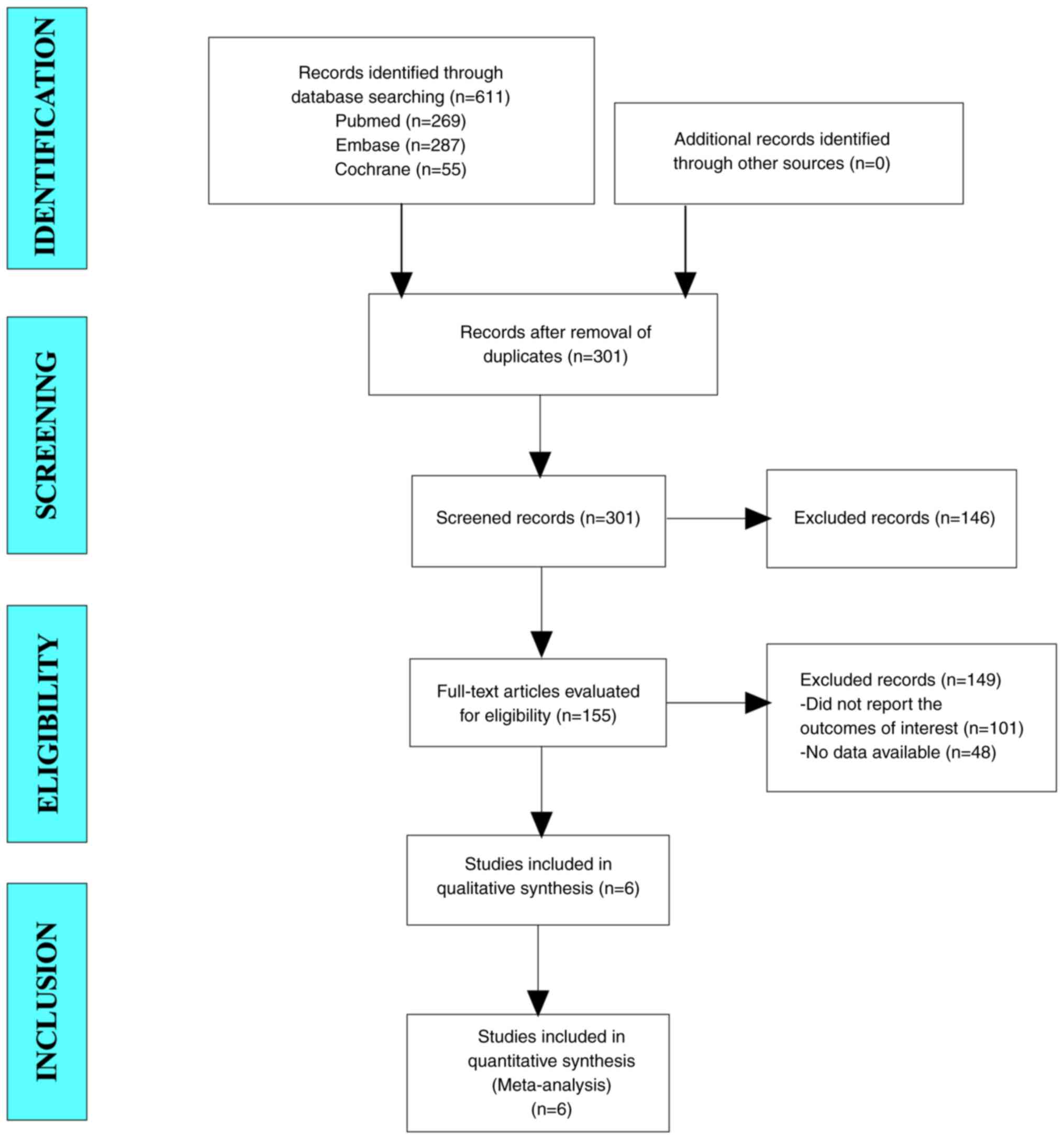
A total of 611 articles were collected for the
present study. After excluding duplicate studies, 301 articles
remained of which, 155 articles were identified following
eligibility screening of titles and abstracts. After reading the
full text, 101 studies that did not report the outcomes of interest
and 48 studies with no data available were excluded. Finally, six
studies were included in the present meta-analysis (Fig. 1).
Baseline characteristics and quality
assessment of the included studies
Six cohort studies were included in the present
meta-analysis. The sample size range was 39–92 and the total number
of patients was 427; 203 in the combination treatment group and 224
in the lenvatinib group. The age range of patients in the
combination treatment group was 50.5–58.5 years, and that in the
lenvatinib monotherapy group was 50.0–63.7 years suggesting that
ages were comparable. The PD-1/PD-L1 inhibitors used included
camrelizumab, nivolumab, toripalimab, sintilimab, tislelizumab and
pembrolizumab. The NOS scores used for quality assessment were all
>7, indicating that the quality of the included studies was high
(Table I) (16–21).
 | Table I.Baseline characteristics and quality
assessment of the included studies. |
Table I.
Baseline characteristics and quality
assessment of the included studies.
|
|
| Sample size, n | Age, years | Sex, n
(male/female) | Barcelona clinic
liver cancer stage, n (A/B/C) | Child-Pugh, n
(A/B) | HBV infection, n
(Yes/No) |
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| First author,
year | Study design | Combination treatment
group | Lenva-tinib
group | Combination treatment
group | Lenvatinib group | Combination treatment
group | Lenva-tinib
group | Combination treatment
group | Lenva-tinib
group | Combination treatment
group | Lenva-tinib
group | Combination treatment
group | Lenva-tinib
group | PD-1/PD-L1
inhibitors | NOS score | (Refs.) |
|---|
| Zhu et al,
2021 | Retrospective | 30 | 39 | 50.5±10.6 | 50.0±13.3 | 28/2 | 37/2 | 5/5/20 | 6/11/22 | 4/26 | 7/32 | 65/7 | 33/6 |
Toripalimab/camrelizumab/sintilimab/tislelizumab/pembrolizumab | 7 | (16) |
| Wei et al,
2021 | Retrospective | 21 | 27 | / | / | 19/2 | 24/3 | / | / | 19/2 | 25/2 | 17/4 | 25/2 | Camrelizumab | 7 | (17) |
| Zhu et al,
2021 | Retrospective | 18 | 21 | 57.4 (32–79) | 63.7 (40–84) | 15/3 | 18/3 | 0/2/16 | 0/4/17 | 16/2 | 20/1 | / | / | / | 8 | (18) |
| Li et al,
2022 | Retrospective | 48 | 44 | 53.8±15.8 | 54.9±18.3 | 43/5 | 40/4 | 0/6/42 | 0/7/37 | 41/7 | 40/4 | 41/7 | 38/6 | Camrelizumab | 7 | (19) |
| Wen et al,
2022 | Prospective | 46 | 46 | / | / | / | / | / | / | / | / | / | / | Nivolumab | 8 | (20) |
| Wu et al,
2022 | Retrospective | 40 | 47 | 58.5±13.8 | 70.6±13.3 | 29/11 | 32/15 | 0/17/23 | 0/22/25 | 25/15 | 35/12 | 31/9 | 26/21 | Nivolumab | 8 | (21) |
Results of the meta-analysis
CR
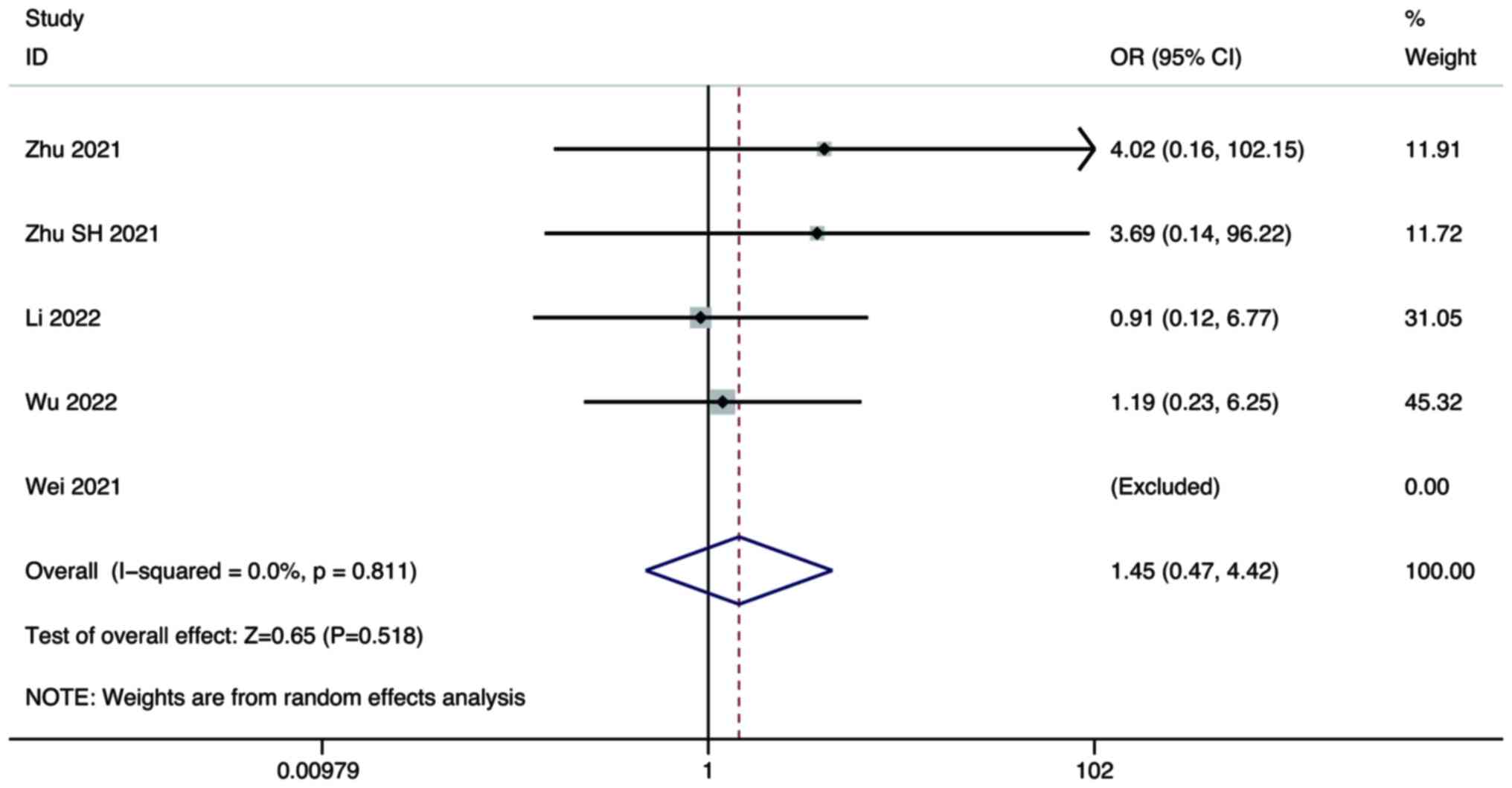
Five studies compared the CR of combination therapy
using lenvatinib and PD-1/PD-L1 inhibitors, with lenvatinib
monotherapy in the treatment of HCC. A random-effects model was
used for the meta-analysis. The pooled results showed that there
was no significant difference in the CR between combination therapy
and lenvatinib monotherapy (OR, 1.45; 95% CI, 0.47–4.42; P=0.518;
Fig. 2).
PR
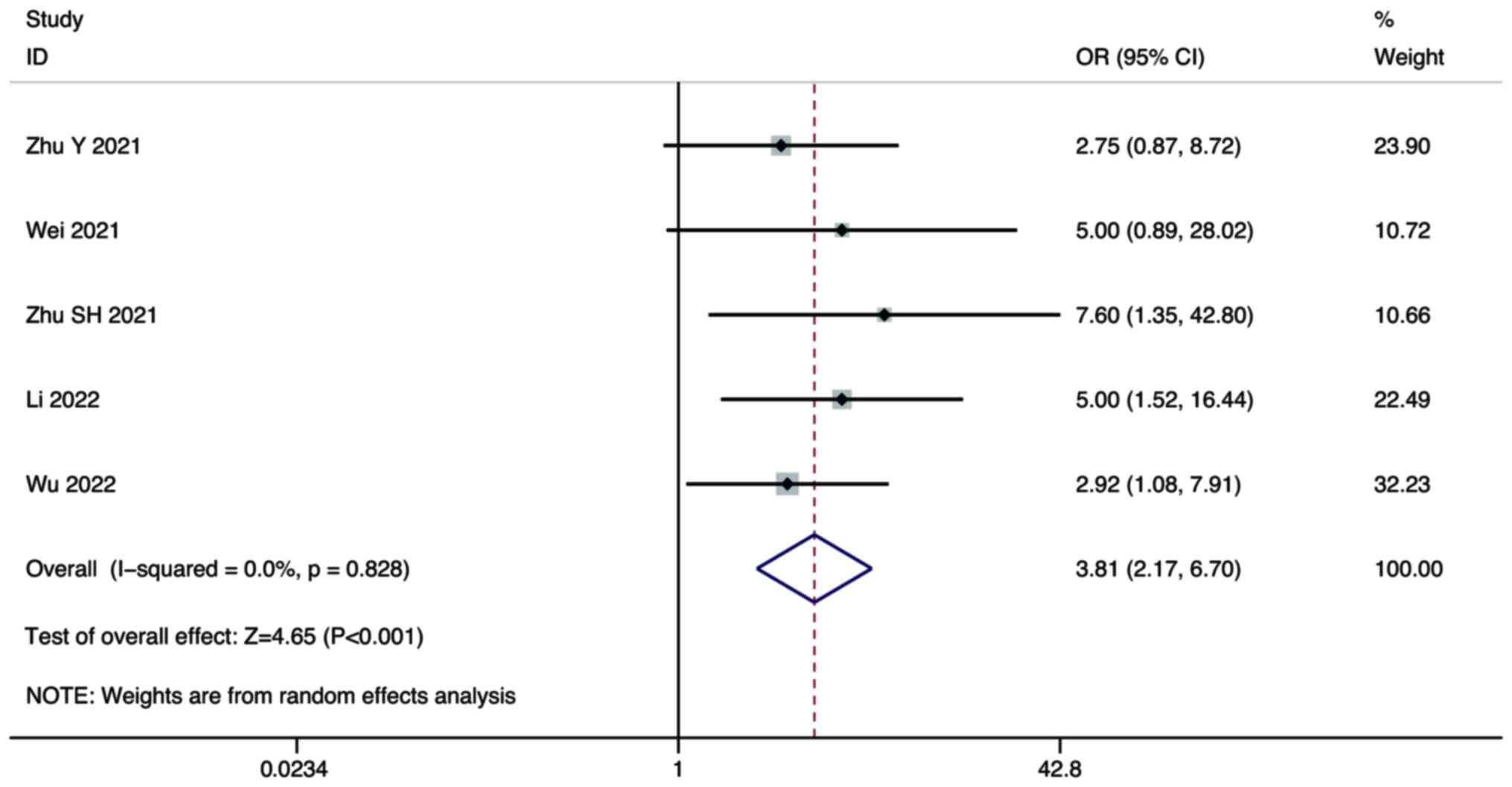
Five studies compared the PR of combination therapy
and monotherapy in the treatment of HCC. A random-effect model was
used for the meta-analysis. The pooled results showed that the PR
of combination therapy was significantly higher than that of
lenvatinib monotherapy (OR, 3.81; 95% CI, 2.17–6.70; P<0.001;
Fig. 3).
SD
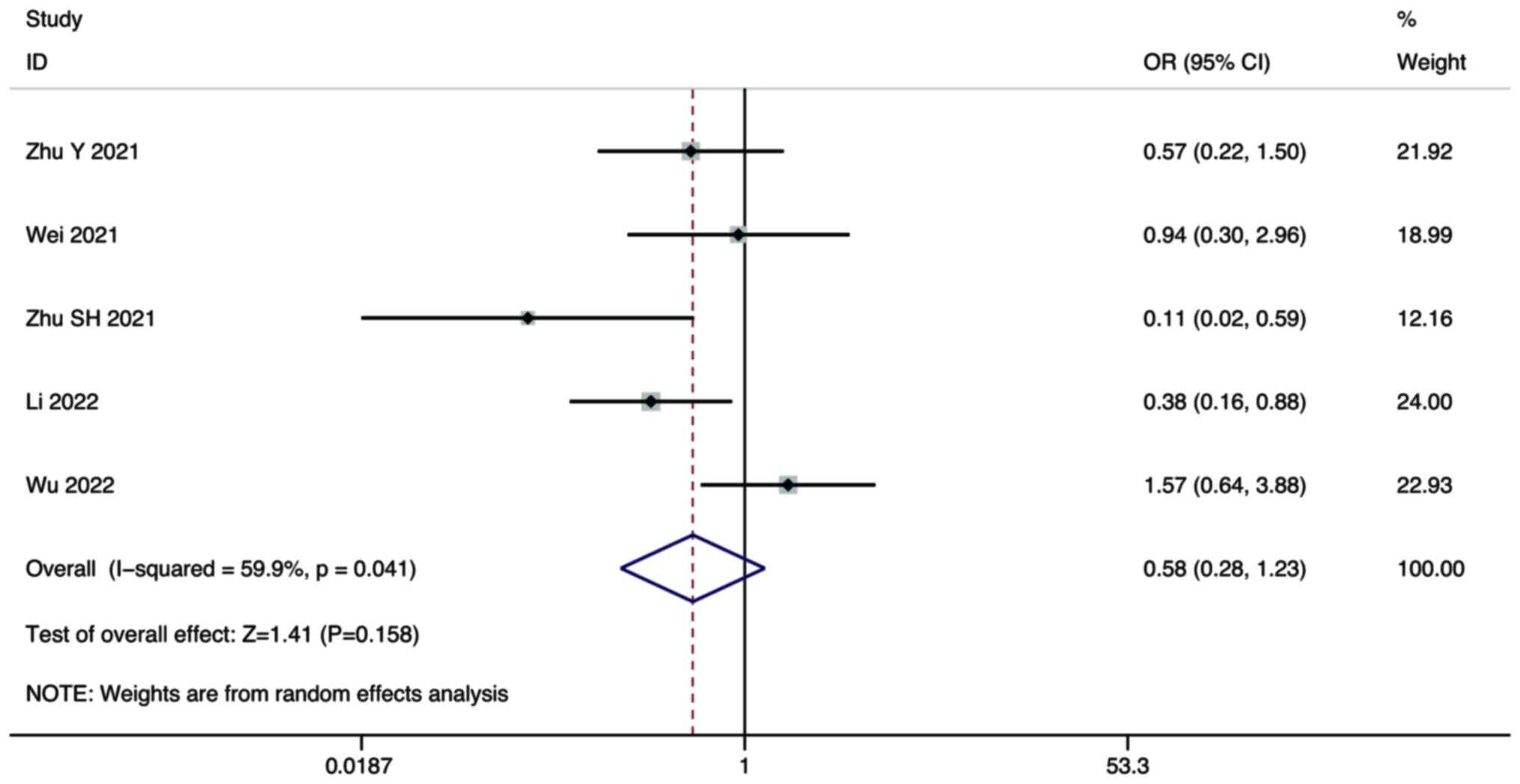
Five studies compared the SD of combination therapy
and lenvatinib monotherapy in the treatment of HCC. A random-effect
model was used for the meta-analysis. The pooled results showed
that there was no significant difference in the SD between
combination therapy and lenvatinib monotherapy (OR, 0.58; 95% CI,
0.28–1.23; P=0.158; Fig. 4).
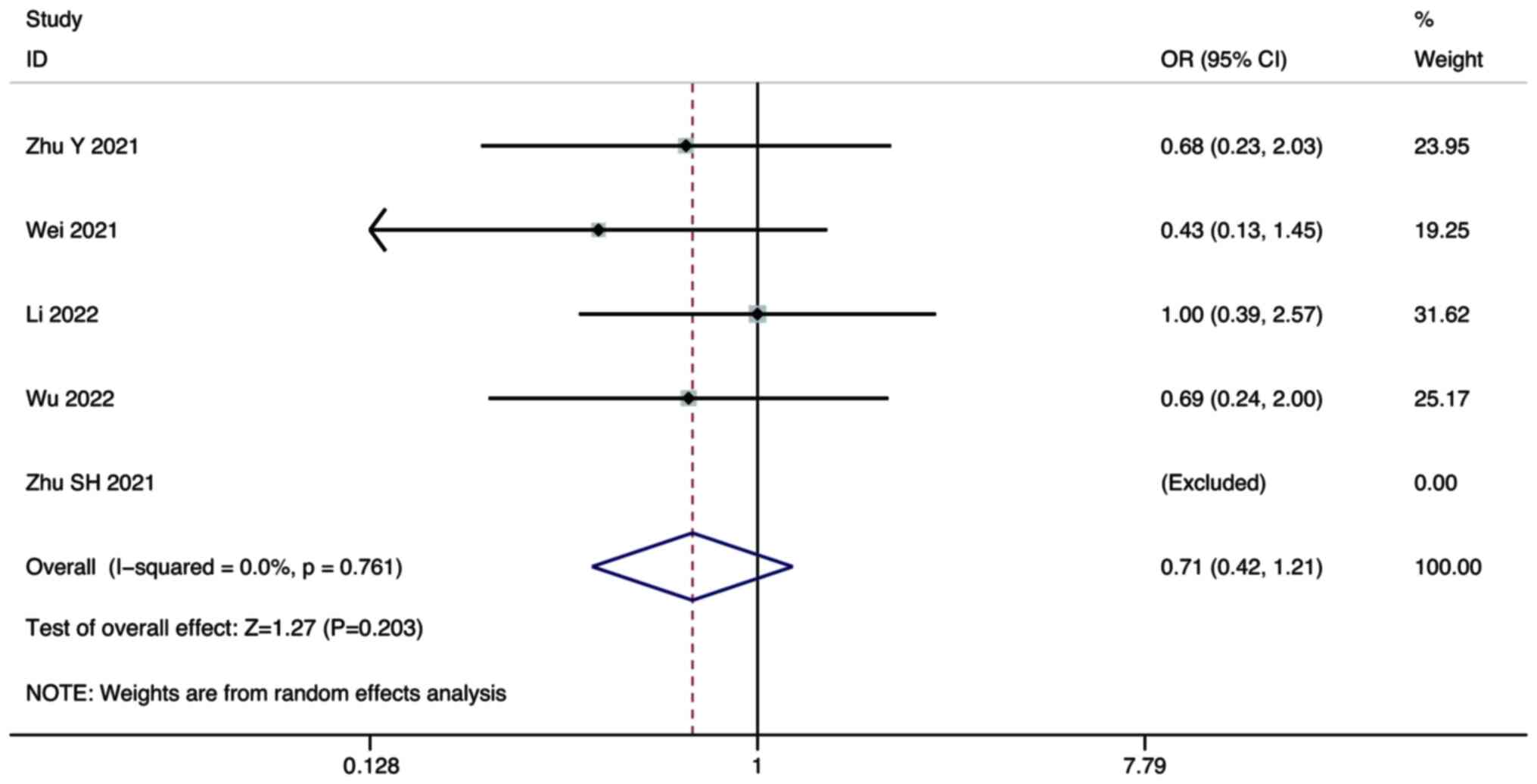
PD
Five studies compared the PD of combination therapy
and monotherapy in the treatment of HCC. A random-effect model was
used for the meta-analysis. The pooled results showed that there
was no significant difference in the PD between combination therapy
and lenvatinib monotherapy (OR, 0.71; 95% CI, 0.42–1.21; P=0.203;
Fig. 5).
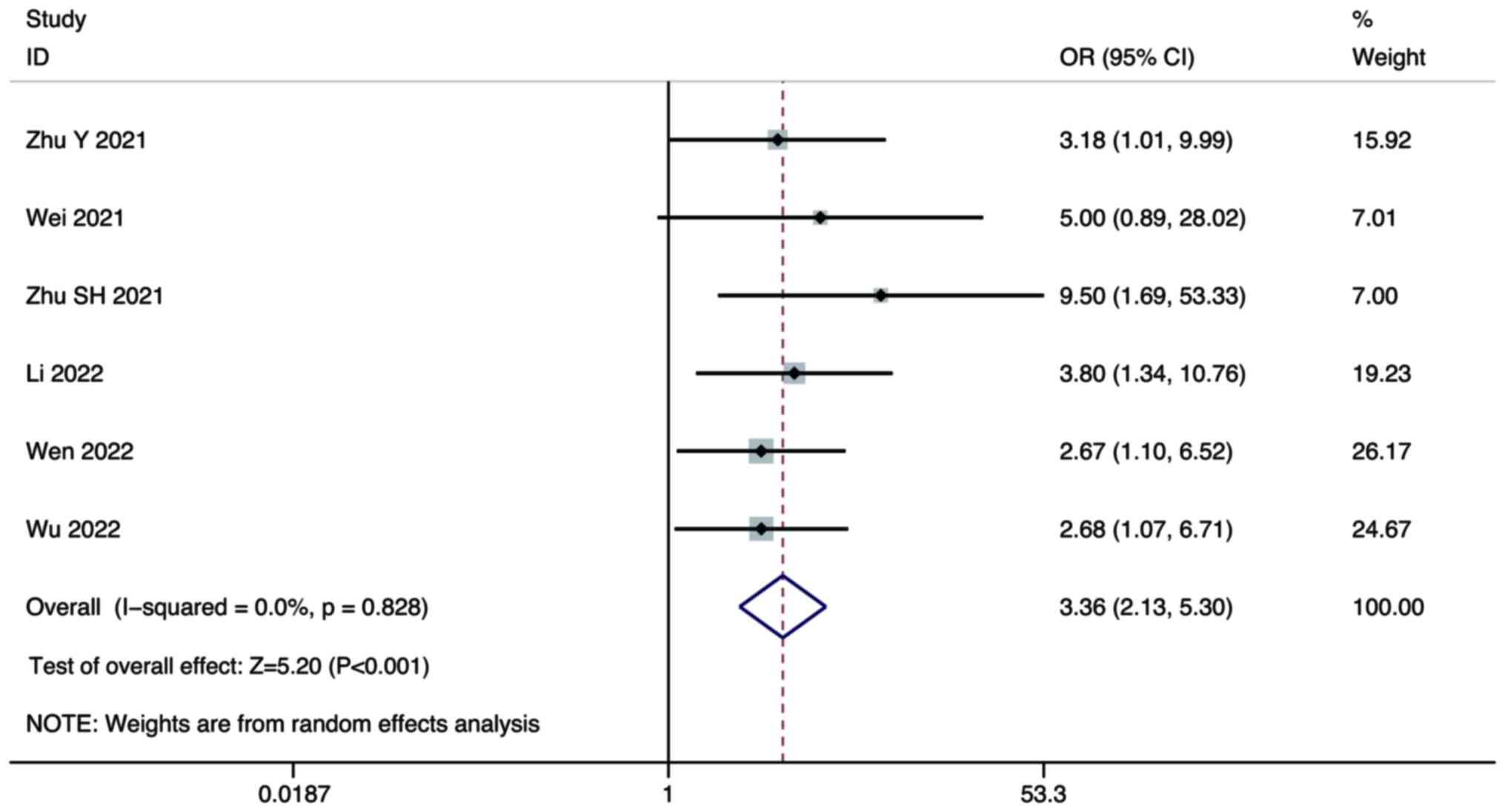
ORR
Six studies compared the ORR of combination therapy
and monotherapy in the treatment of HCC. A random-effect model was
used for the meta-analysis. The pooled results showed that the ORR
of combination therapy was significantly higher than that of
lenvatinib monotherapy (OR, 3.36; 95% CI, 2.13–5.30; P<0.001;
Fig. 6).
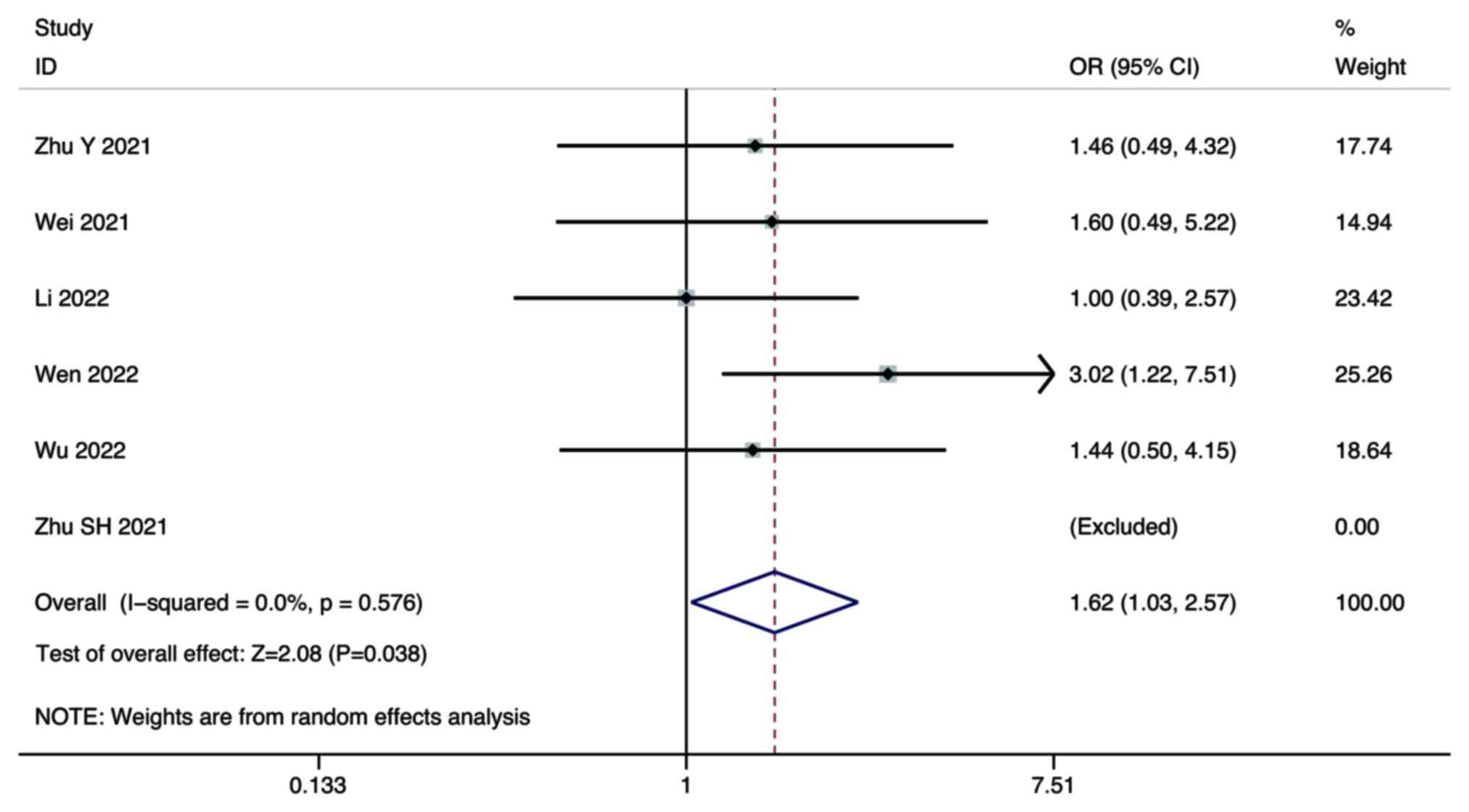
DCR
Six studies compared the DCR of combination therapy
and monotherapy in the treatment of HCC. A random-effect model was
used for the meta-analysis. The pooled results showed that the DCR
of combination therapy was significantly higher than that of
lenvatinib monotherapy (OR, 1.62; 95% CI, 1.03–2.57; P=0.038;
Fig. 7).
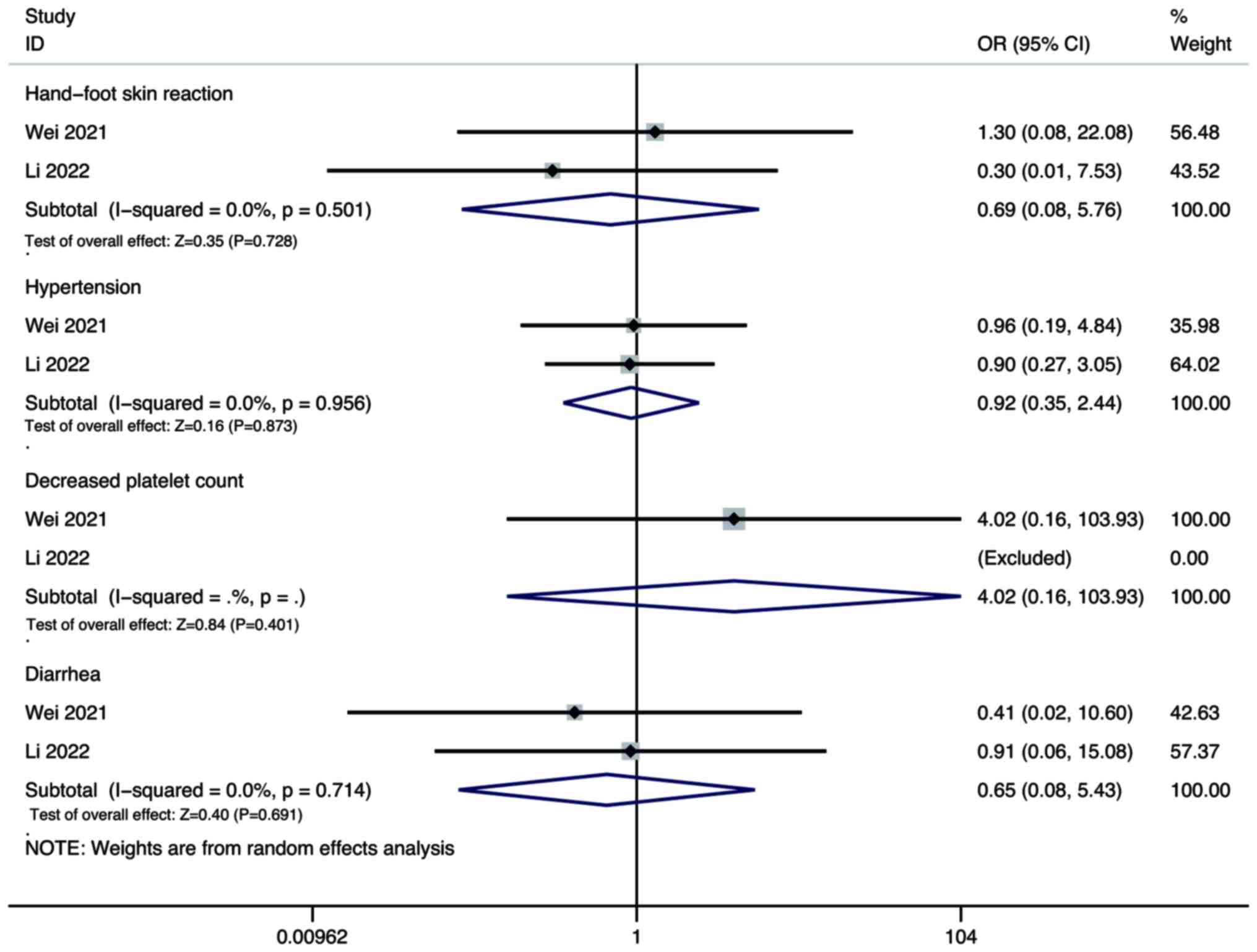
Adverse events
Pooled results showed that there was no significant
difference in the incidence rate of hand-foot skin reaction (OR,
0.69; 95% CI, 0.08–5.76; P=0.728), hypertension (OR, 0.92; 95% CI,
0.35–2.44; P=0.873), decreased platelet count (OR, 4.02; 95% CI,
0.16–103.93; P=0.401), and diarrhoea (OR, 0.65; 95% CI, 0.08–5.43;
P=0.691) between the combination therapy group and the lenvatinib
monotherapy group (Fig. 8).
Subgroup analysis
In addition, subgroup analyses of the ORR and DCR
based on different PD-1/PD-L1 inhibitors were performed.
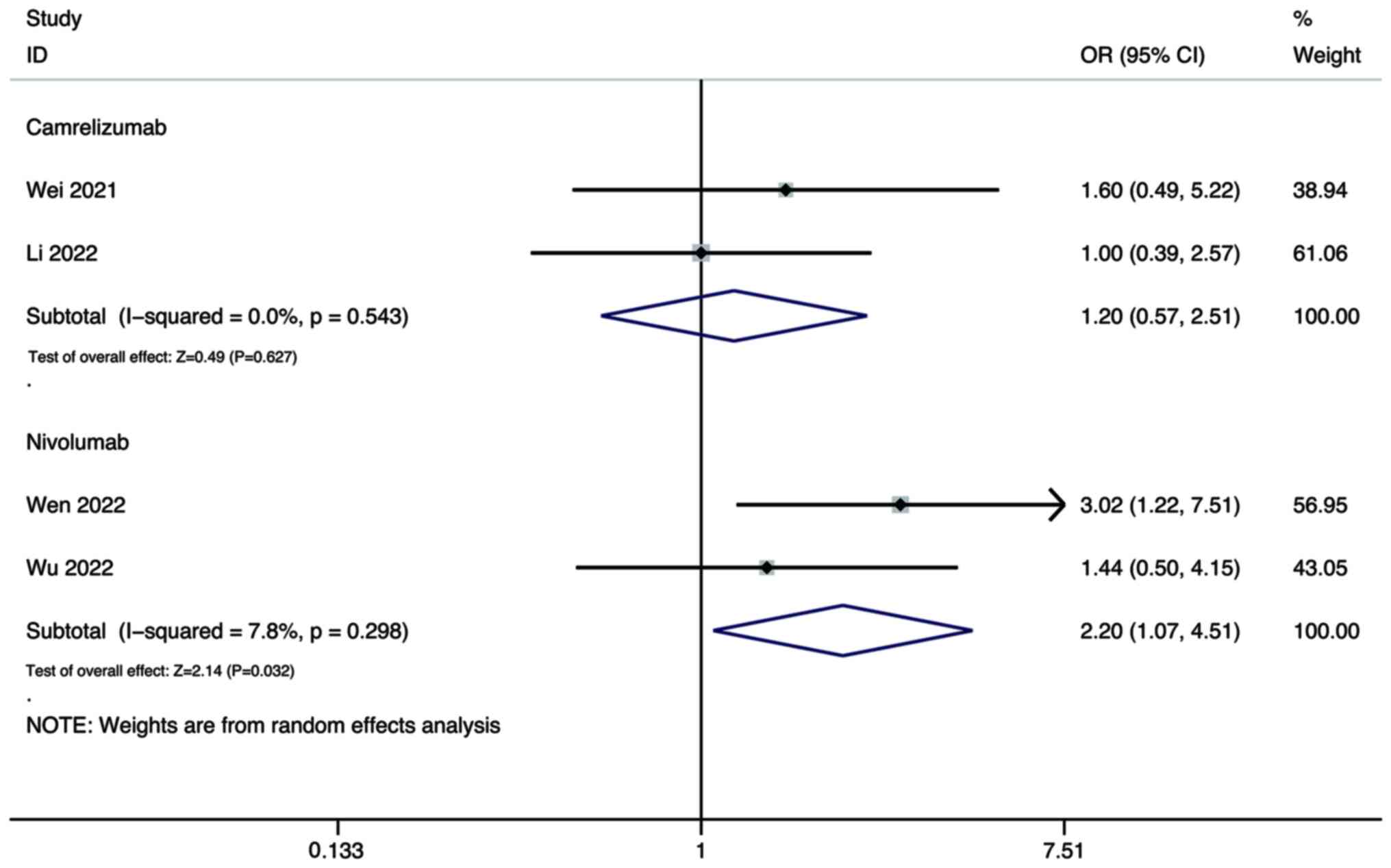
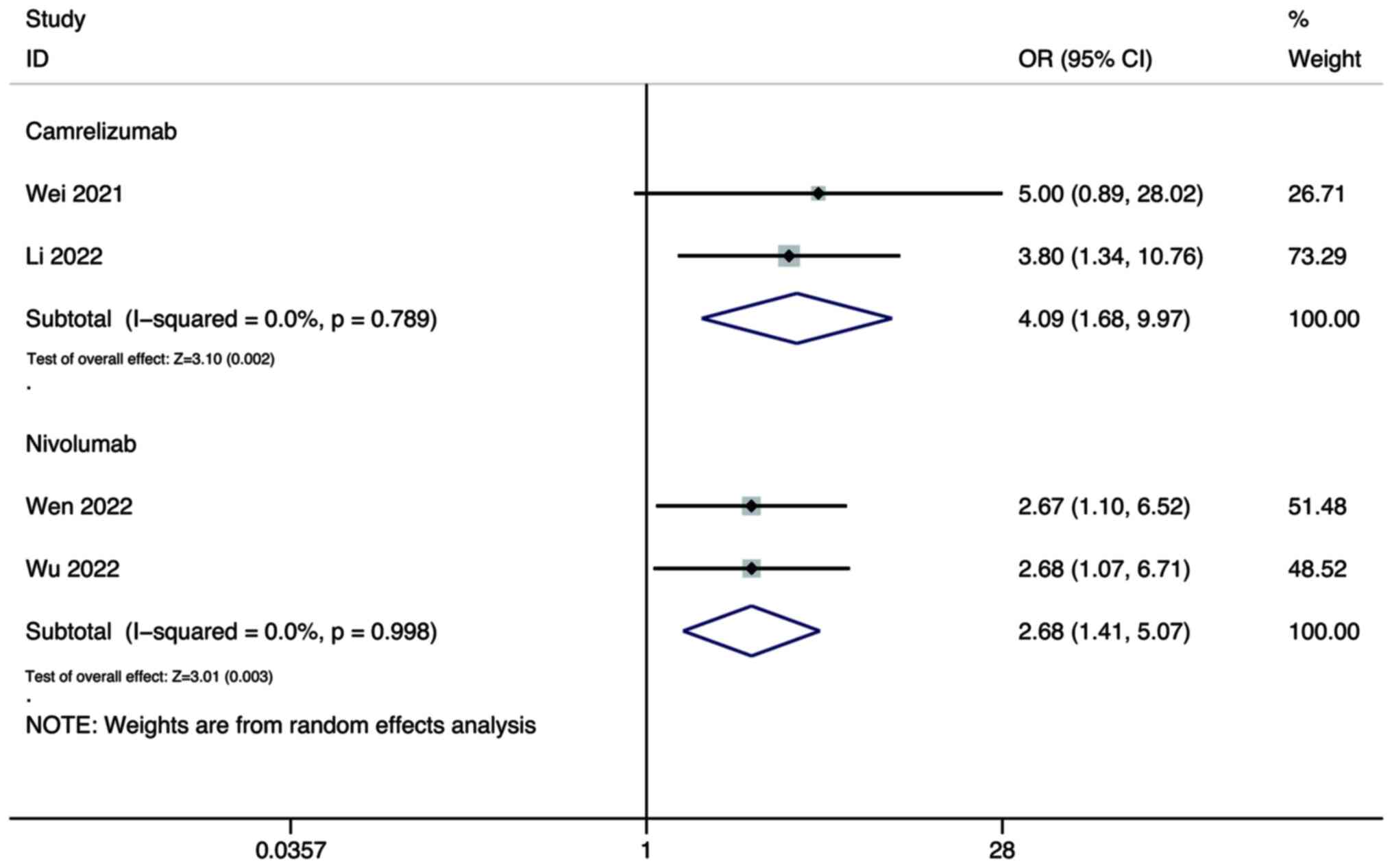
For the ORR, the pooled results showed that the DCR
of combination therapy using lenvatinib and either camrelizumab
(OR, 4.09; 95% CI, 1.68–9.97; P=0.002) or nivolumab (OR, 2.68; 95%
CI, 1.41–5.07; P=0.003) were all significantly higher than that of
lenvatinib monotherapy (Fig.
9).
As for the DCR, the pooled results showed that the
DCR of combination therapy using lenvatinib and nivolumab was
significantly higher than that of lenvatinib monotherapy (OR, 2.20;
95% CI, 1.07–4.51; P=0.032; Fig.
9), while there was no significant difference between
combination therapy using lenvatinib and camrelizumab, and
monotherapy (OR, 1.20; 95% CI, 0.57–2.51; P=0.627; Fig. 10).
Sensitivity analysis
A sensitivity analysis was performed to exclude
every trial individually before performing a combined analysis of
the remaining trials. It was found that no study had a great
influence on the results, suggesting that the results of the
present study are reliable and stable.
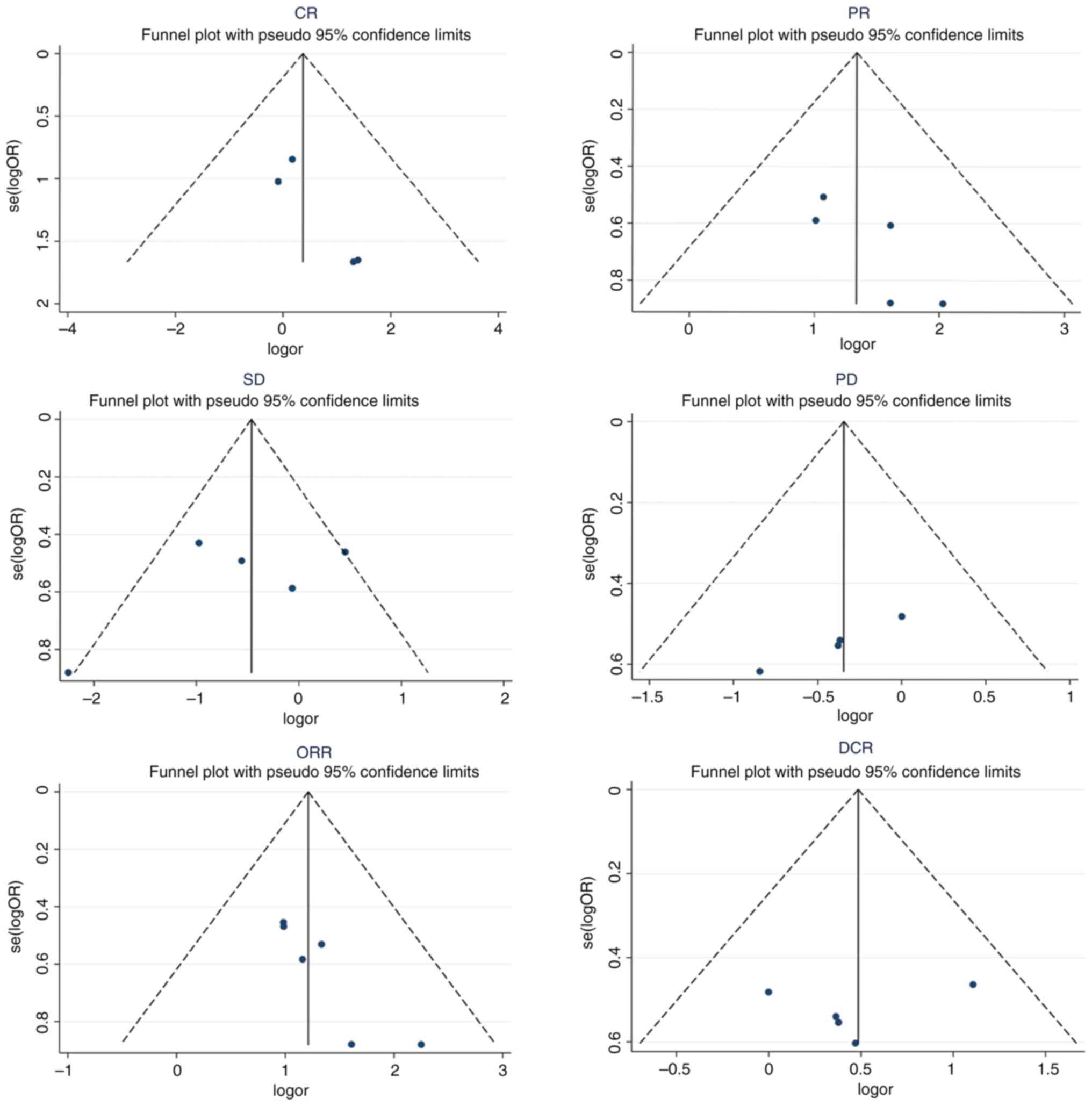
Publication bias
The funnel plots of the CR, PR, SD, PD and DCR were
symmetrically distributed, and the P-values of Egger's test were
0.097, 0.111, 0.412, 0.203 and 0.633, respectively, indicating
there was no obvious publication bias in these studies. Meanwhile,
the funnel plot of the ORR was not completely symmetric (P=0.015),
suggesting that there may be publication bias in the study
(Fig. 11).
Discussion
The growing body of evidence underscores the
significance of FGF pathway signalling activation in HCC
development and advancement (22).
Matsuki et al (23)
conducted a thorough investigation, using in vitro
experiments with human HCC cell lines and in vivo studies
with mouse xenograft models. That study confirmed that the
FGF19-FGFR4 axis plays a pivotal role in markedly augmenting the
proliferation and growth of HCC. These discoveries provide a
compelling explanation for the treatment responses witnessed with
lenvatinib, a potent inhibitor designed to target FGFR 1–4,
particularly in advanced HCC cases (4).
Lenvatinib, similar to other multi-kinase
inhibitors, has demonstrated its capacity for immunomodulation
(24). Notably, VEGF-A and β-FGF
have been identified as potent inducers of immune checkpoint
markers while concurrently inhibiting the secretion of IFN-γ and
granzyme B, consequently dampening T-cell cytotoxicity (25). Notably, lenvatinib can reverse this
immunosuppressive effect (11).
Additionally, Adachi et al (25) elucidated that the activation of FGFR
signalling leads to the suppression of the JAK/STAT pathway,
resulting in IFN-γ secretion reduction. Nevertheless, the use of
lenvatinib, which inhibits FGFR signalling, efficiently reinstates
the stimulation of IFN-γ (25).
Furthermore, multiple studies have shown that lenvatinib elevates
the proportion of activated CD8+ T cells that secrete
IFN-γ and granzyme B (25,26). The antitumour efficacy of lenvatinib
was diminished in immunodeficient mice when CD8+ T cells
were depleted (26). Conversely,
lenvatinib reduced the percentage of monocytes and the population
of macrophages, including tumour-associated macrophages (TAMs)
(25,26). In summary, lenvatinib has been
observed to enhance antitumour immunity by increasing the
population of IFN-γ-producing CD8+ T-cells and reducing
the presence of TAMs. These findings suggest that lenvatinib may
hold the potential for combination therapy with immunotherapy
approaches.
The present meta-analysis included six studies to
analyse the efficacy and safety of lenvatinib combined with
PD-1/PD-L1 inhibitors and lenvatinib monotherapy in HCC treatment.
In summary, the current study demonstrates the potential
synergistic effects of combining lenvatinib with PD-1/PD-L1
inhibitors in HCC treatment. The improved ORR and DCR observed in
the combination therapy group compared with those in the lenvatinib
monotherapy group suggest that this approach is promising for
enhancing treatment outcomes. However, the increased efficacy
primarily manifested as PR, and the difference in CRs between the
combination therapy and lenvatinib monotherapy groups was not
statistically significant. The failure of combination therapy to
significantly enhance CR rates suggests that specific biological or
microenvironmental factors, such as immunosuppressive cells
(27) and proinflammatory cytokines
(28), in HCC may contribute to
resistance against complete eradication. Understanding the
mechanisms behind this limitation could direct future therapeutic
strategies. Further research and clinical trials are warranted to
explore the mechanistic basis of these observations, optimize
patient selection criteria, and assess the long-term benefits and
potential toxicities associated with this therapeutic approach.
The present subgroup analyses, which focused on the
PD-1/PD-L1 inhibitors nivolumab and camrelizumab provided valuable
insights into the variations in DCR associated with these specific
combinations. The pooled results clearly demonstrated that
combination therapy using lenvatinib and nivolumab led to a
significantly higher DCR than that of lenvatinib monotherapy. This
finding underscores the potential synergy between lenvatinib and
nivolumab in effectively controlling the progression of HCC. The
improved DCR suggests that this combination may be particularly
beneficial for patients with HCC who seek enhanced disease control
and tumour burden reduction. By contrast, the current analysis did
not reveal a significant difference in DCR between combination
therapy using lenvatinib and camrelizumab, and lenvatinib
monotherapy. While this result might suggest a limited additive
effect when using lenvatinib and camrelizumab in terms of DCR, it
is noteworthy that clinical outcomes may be influenced by various
factors, including patient heterogeneity and tumour
characteristics. Overall, these subgroup analyses emphasize the
need for the careful selection of PD-1/PD-L1 inhibitors when
combining them with lenvatinib in the treatment of HCC. The choice
of PD-1/PD-L1 inhibitor could significantly impact therapeutic
response, and further research is warranted to explore the
underlying mechanisms behind these observed differences.
Personalized treatment approaches, tailored to individual patient
profiles, may ultimately provide the most effective outcomes in the
context of combination therapies for HCC.
In addition, the pooled results showed that there
was no significant difference in the incidence rate of adverse
events from combination therapy using lenvatinib and PD-1/PD-L1
inhibitors, and lenvatinib monotherapy in the treatment of HCC,
suggesting that patients should be fully informed of possible
adverse reactions before treatment and that further treatment
should be carried out after patient consent. Timely treatment is
needed to prevent possible adverse reactions.
The current study still had some limitations: i) The
sample size of the included literature was small, which may lead to
the risk of bias in the results; a large sample and multicentre RCT
studies are needed; ii) the intervention was lenvatinib combined
with PD-1 inhibitors, however, due to the difference in dosage
between studies and the different response effects of patients with
liver cancer to related drugs, the results of the present
meta-analysis were affected to some extent; and iii) there is a
possible publication bias in the analysis of the ORR, hence, it is
necessary to continue to include new studies in future studies to
exclude the risk of bias.
Compared with lenvatinib monotherapy, lenvatinib
combined with PD-1/PD-L1 inhibitors significantly improved the ORR,
mainly PR, and DCR in patients with HCC. It is noteworthy that
lenvatinib was mainly combined with nivolumab to increase the DCR
of lenvatinib monotherapy for HCC. In addition, there was no
significant difference in the incidence rate of adverse reactions
between combination therapy and lenvatinib monotherapy for HCC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets are available from the corresponding
author on reasonable request.
Authors' contributions
YL and BZ wrote the manuscript and created the
figures. BZ and LS participated in the acquisition and analysis of
data. YL conceived the final version of manuscript. YL and BZ
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brown ZJ, Tsilimigras DI, Ruff SM, Mohseni
A, Kamel IR, Cloyd JM and Pawlik TM: Management of hepatocellular
carcinoma: A review. JAMA Surg. 158:410–420. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chidambaranathan-Reghupaty S, Fisher PB
and Sarkar D: Hepatocellular carcinoma (HCC): Epidemiology,
etiology and molecular classification. Adv Cancer Res. 149:1–61.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kumar A, Acharya SK, Singh SP, Arora A,
Dhiman RK, Aggarwal R, Anand AC, Bhangui P, Chawla YK, Gupta SD, et
al: 2019 update of Indian national association for study of the
liver consensus on prevention, diagnosis, and management of
hepatocellular carcinoma in India: The puri II recommendations. J
Clin Exp Hepatol. 10:43–80. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao Y, Zhang YN, Wang KT and Chen L:
Lenvatinib for hepatocellular carcinoma: From preclinical
mechanisms to anti-cancer therapy. Biochim Biophys Acta Rev Cancer.
1874:1883912020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ikeda M, Okusaka T, Mitsunaga S, Ueno H,
Tamai T, Suzuki T, Hayato S, Kadowaki T, Okita K and Kumada H:
Safety and pharmacokinetics of lenvatinib in patients with advanced
hepatocellular carcinoma. Clin Cancer Res. 22:1385–1394. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
El-Khoueiry AB, Sangro B, Yau T, Crocenzi
TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling THR, et al:
Nivolumab in patients with advanced hepatocellular carcinoma
(CheckMate 040): An open-label, non-comparative, phase 1/2 dose
escalation and expansion trial. Lancet. 389:2492–2502. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fukumura D, Kloepper J, Amoozgar Z, Duda
DG and Jain RK: Enhancing cancer immunotherapy using
antiangiogenics: Opportunities and challenges. Nat Rev Clin Oncol.
15:325–340. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng AL, Hsu C, Chan SL, Choo SP and Kudo
M: Challenges of combination therapy with immune checkpoint
inhibitors for hepatocellular carcinoma. J Hepatol. 72:307–319.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deng H, Kan A, Lyu N, Mu L, Han Y, Liu L,
Zhang Y, Duan Y, Liao S, Li S, et al: Dual vascular endothelial
growth factor receptor and fibroblast growth factor receptor
inhibition elicits antitumor immunity and enhances programmed cell
death-1 checkpoint blockade in hepatocellular carcinoma. Liver
Cancer. 9:338–357. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kudo M: Scientific rationale for combined
immunotherapy with PD-1/PD-L1 antibodies and VEGF inhibitors in
advanced hepatocellular carcinoma. Cancers (Basel). 12:10892020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Finn RS, Ikeda M, Zhu AX, Sung MW, Baron
AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, et al:
Phase Ib study of lenvatinib plus pembrolizumab in patients with
unresectable hepatocellular carcinoma. J Clin Oncol. 38:2960–2970.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cook DA and Reed DA: Appraising the
quality of medical education research methods: The medical
education research study quality instrument and the
newcastle-ottawa scale-education. Acad Med. 90:1067–1076. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bernardo WM: PRISMA statement and
PROSPERO. Int Braz J Urol. 43:383–384. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu Y, Sun P, Wang K, Xiao S and Cheng Y,
Li X, Wang B, Li J, Yu W and Cheng Y: Efficacy and safety of
lenvatinib monotreatment and lenvatinib-based combination therapy
for patients with unresectable hepatocellular carcinoma: A
retrospective, real-world study in China. Cancer Cell Int.
21:5032021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei F, Huang Q, He J, Luo L and Zeng Y:
Lenvatinib plus Camrelizumab versus Lenvatinib monotherapy as
post-progression treatment for advanced hepatocellular carcinoma: A
short-term prognostic study. Cancer Manag Res. 13:4233–4240. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu S, Liu C, Dong Y, Shao J, Liu B and
Shen J: A retrospective study of lenvatinib monotherapy or combined
with programmed cell death protein 1 antibody in the treatment of
patients with hepatocellular carcinoma or intrahepatic
cholangiocarcinoma in China. Front Oncol. 11:7886352021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Q, Cao M, Yuan G, Cheng X, Zang M, Chen
M, Hu X, Huang J, Li R, Guo Y, et al: Lenvatinib Plus Camrelizumab
vs. Lenvatinib Monotherapy as first-line treatment for unresectable
hepatocellular carcinoma: A multicenter retrospective cohort study.
Front Oncol. 12:8097092022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wen S, Zeng J, Zhong L, Ye J and Lai X:
The efficacy and adverse effects of nivolumab and lenvatinib in the
treatment of advanced hepatocellular carcinoma. Cell Mol Biol
(Noisy-le-grand). 68:53–57. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu WC, Lin TY, Chen MH, Hung YP, Liu CA,
Lee RC, Huang YH, Chao Y and Chen SC: Lenvatinib combined with
nivolumab in advanced hepatocellular carcinoma-real-world
experience. Invest New Drugs. 40:789–797. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miura S, Mitsuhashi N, Shimizu H, Kimura
F, Yoshidome H, Otsuka M, Kato A, Shida T, Okamura D and Miyazaki
M: Fibroblast growth factor 19 expression correlates with tumor
progression and poorer prognosis of hepatocellular carcinoma. BMC
Cancer. 12:562012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsuki M, Hoshi T, Yamamoto Y,
Ikemori-Kawada M, Minoshima Y, Funahashi Y and Matsui J: Lenvatinib
inhibits angiogenesis and tumor fibroblast growth factor signaling
pathways in human hepatocellular carcinoma models. Cancer Med.
7:2641–2653. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin YY, Tan CT, Chen CW, Ou DL, Cheng AL
and Hsu C: Immunomodulatory effects of current targeted therapies
on hepatocellular carcinoma: Implication for the future of
immunotherapy. Semin Liver Dis. 38:379–388. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adachi Y, Kamiyama H, Ichikawa K,
Fukushima S, Ozawa Y, Yamaguchi S, Goda S, Kimura T, Kodama K,
Matsuki M, et al: Inhibition of FGFR reactivates IFNgamma signaling
in tumor cells to enhance the combined antitumor activity of
lenvatinib with Anti-PD-1 antibodies. Cancer Res. 82:292–306. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kimura T, Kato Y, Ozawa Y, Kodama K, Ito
J, Ichikawa K, Yamada K, Hori Y, Tabata K, Takase K, et al:
Immunomodulatory activity of lenvatinib contributes to antitumor
activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci.
109:3993–4002. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meng Y, Ye F, Nie P, Zhao Q, An L, Wang W,
Qu S, Shen Z, Cao Z, Zhang X, et al: Immunosuppressive CD10+ALPL+
neutrophils promote resistance to anti-PD-1 therapy in HCC by
mediating irreversible exhaustion of T cells. J Hepatol.
79:1435–1449. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma H, Kang Z, Foo TK, Shen Z and Xia B:
Disrupted BRCA1-PALB2 interaction induces tumor immunosuppression
and T-lymphocyte infiltration in HCC through cGAS-STING pathway.
Hepatology. 77:33–47. 2023. View Article : Google Scholar : PubMed/NCBI
|