Introduction
Esophagogastric junction adenocarcinoma (EJA) is a
prevalent malignant tumor of the digestive tract (1). The biological behavior of EJA differs
from that of its adjacent esophageal and gastric cancers, with a
worse prognosis compared with that of the other two (2,3). The
incidence of EJA has been alarmingly increasing worldwide in recent
years. However, due to its unique biological characteristics,
controversies surrounding the pathogenesis, pathology, clinical
treatment and prognosis of EJA remain. Most patients with EJA are
diagnosed at an advanced stage due to the lack of specific clinical
symptoms and effective early diagnostic methods. By this time, the
tumor may have already widely invaded and metastasized,
particularly in China where gastrointestinal malignancies are
highly prevalent. Consequently, most patients with EJA are
diagnosed at intermediate or late stages of tumor progression with
a 5-year survival rate <30% (4).
Therefore, identifying convenient and effective methods to improve
the rates of early diagnosis for EJA is crucial for enhancing
prognosis and survival rates.
Hydrogen sulfide (H2S) is the third
endogenous gaseous signaling molecule, following carbon monoxide
(5). Numerous studies have
demonstrated the diverse range of biological activities of
H2S in glucose metabolism, ischemia-reperfusion injury,
stress and endotoxemia (6–9). H2S actively participates in
various physiological and pathological processes within multiple
systems. It exists in vivo as H2S gas or sodium
hydrosulfide (NaHS). The dissociated H2S ions from NaHS
combine with hydrogen ions to generate H2S, maintaining
homeostasis within the body. Mammals possess more than one pathway
for producing H2S using L-cysteine (L-Cys substrate).
The primary pathways involve cystathionine-β-synthase (CBS) and
cystathionine-γ-lyase (CSE). A high concentration of NaHS can
reduce cellular oxidative stress levels, subsequently activating
the mitogen active protein kinase pathway while upregulating gene
expression. This induction prompts intestinal epithelial cells to
malignant transformation (10).
Interleukin-8 (IL-8) is a member of the chemokine
family that attracts neutrophil infiltration by acting on the C-X-C
chemokine receptor type 1 (11).
The immunosuppressive effect of the tumor microenvironment is
promoted by chemotaxis of neutrophils and myeloid suppressor cells
(12). Tumor cells secrete a
significant amount of IL-8 to facilitate the progression and
metastasis of tumor cells (13).
Known as Barrett's esophagus, metaplasia of the single columnar
epithelium is a precancerous lesion in the development of EJA
(14). The upregulation of IL-8
expression in Barrett's esophagus tissue inflammation is related to
intraepithelial neutrophil granulocytes, which may be affected by
the epithelial secretion of IL-8 (15). In addition, both IL-8 mRNA and
protein levels are upregulated in EJA (16). IL-8 can thus be used as an early
diagnostic marker for EJA (17).
Increased H2S concentrations have been
reported in colon cancer tissues compared with those in adjacent
normal tissues (18). Colorectal
cancer (CRC) cells are able to synthesize more H2S and
release it into the tumor microenvironment (19). Reportedly, H2S expression
increases in multidrug-resistant CRC cells (20). Moreover, H2S-producing
Clostridium and Bacillus fragilis are significantly
enriched in early-stage CRC, and H2S produced by
Clostridium nucleate and other microorganisms may promote
tumor development by destroying host cells and DNA (21). In recent years, different diagnostic
probes and combination therapy approaches, as well as tumor
treatment pathways mediated by H2S have been developed
(22). It has been observed that
H2S is expressed in the tumor tissues of colorectal
adenoma and bladder cancer making it a potential diagnostic marker
(23,24). However, the expression level of
H2S in EJA and its diagnostic value remain unexplored.
Gastrointestinal gas analysis holds promise as a diagnostic
technique. Therefore, the present study aimed to assess and compare
exhaled H2S in patients with EJA with that in healthy
controls. Additionally, correlations between exhaled H2S
and clinical diagnosis of EJA were analyzed to determine the
clinical significance of this biomarker.
Patients and methods
Research participants
A total of 56 patients (36 males and 20 females;
mean age, 56.61±10.65 years) with EJA, who underwent surgical
treatment at Hebei Medical University Fourth Affiliated Hospital
(Shijiazhuang, China) from January 2019 to December 2021, were
included in the EJA group. All patients underwent gastroscopy and
received a pathological diagnosis confirming EJA without any
previous history of tumors. These patients were treated for the
first time without prior radiotherapy or chemotherapy. Early-stage
EJA was defined as AJCC Staging Manual 8th edition stages I and II
(1). Exclusion criteria consisted
of: i) Patients with other malignancies or severe cardiovascular,
pulmonary, or renal diseases where diagnosis was unclear; ii)
patients with a history of gastrointestinal surgery or trauma that
altered anatomy and function; iii) patients who had bowel surgery
or were preparing for bowel surgery, and those who were pregnant
and lactating; and iv) patients on antibiotics, lactulose, acid
suppressants, or drugs affecting gastrointestinal motility within
the past 4 weeks. Additionally, a healthy control group consisting
of 57 individuals (38 males and 19 females; mean age, 58.54±10.35
years), without any autoimmune diseases, tumors or organic lesions
(Table I) was also formed. The
present study adhered to the ethical standards set by the
responsible committee on human experimentation, namely, the Ethics
Committee of Hebei Medical University Fourth Affiliated Hospital,
Shijiazhuang, China; approval no. 2018MEC108), following the
principles outlined in The Declaration of Helsinki. All the
patients and their families were informed about the study details
and provided written informed consent.
 | Table I.Clinical characteristics of patients
with EJA and the healthy control group. |
Table I.
Clinical characteristics of patients
with EJA and the healthy control group.
|
Characteristics | EJA group | Healthy control
group |
|---|
| Number of
patients | 56 | 57 |
| Sex (male/female),
n | 36/20 | 38/19 |
| Age, years | 56.61±10.65 | 58.54±10.35 |
| IL-8, pg/ml |
2,200.80±641.69 | 243.64±70.36 |
| Stage I | 26 | 0 |
| Stage II | 30 | 0 |
Exhaled H2S
determination
Based on a previously published study (23), exhaled H2S tests were
performed using Nanocoulomb breath analyzer DA6000 (Sunvou Medical
Electronics Co., Ltd.). Briefly, all participants had to fast for
12 h before the test, and avoid exercise and smoking on the day of
the test. Participants wrapped their lips tightly with a disposable
filter. After inhaling through the filter, they held their breath
for 15 sec and then forcefully exhaled. Animation software
(Breathing Zone 13.0; Breathing Zone Limited) was used to
coordinate the exhalation rhythm. The analyzer automatically
collected the end-expiratory air. After the acquisition process was
complete, the analyzer automatically analyzed the exhaled gas and
displayed the results immediately. To eliminate the effects of
H2S in the environment, the participants first inhaled
through the H2S filter and then exhaled at a set flow
rate, expiratory pressure and exhalation time. Calibration with 50
parts per billion (PPB) and 200 PPB H2S/N2
standard gas, supplied by the manufacturer prior to daily testing,
was performed to ensure the accuracy of the test.
Enzyme-linked immunosorbent assay
(ELISA)
Patient serum was collected within 24 h of admission
before treatment and immediately stored at −80°C until use. Serum
IL-8 concentrations were measured using a commercial ELISA kit
(cat. no. H008-1-2; Nanjing Jiancheng Bioengineering Institute),
according to the manufacturer's instructions. All trained operators
were blinded to the characteristics of the patients with EJA and
healthy controls. Briefly, the preparation of blank wells, standard
holes and sample holes was performed. After washing, 100 µl of
enzymatic secondary antibody was added, incubated at 37°C for 60
min, washed repeatedly, and then color was added to the developing
solution, followed by incubation at room temperature for 20 min and
the addition of termination solution. The absorbance at 450 nm per
hole was measured using the developing method. The average OD
values were calculated after the difference in the duplicate wells
was <10%.
Statistical analysis
The experimental data are expressed as the mean ±
standard deviation. SPSS 21.0 (IBM Corp.) and GraphPad Prism 8.0
(GraphPad Software; Dotmatics) were used for data analysis.
Unpaired Student's t-test was used for comparison between the two
groups. Pearson correlation coefficient was used to analyze the
correlation between H2S and IL-8. The diagnostic value
of H2S or its combination with IL-8 was evaluated using
receiver operating characteristic (ROC) curves, according to the
area under the curve (AUC) with 95% confidence interval (CI).
P<0.05 was considered to indicate a statistically significant
difference. All experiments were performed three times.
Results
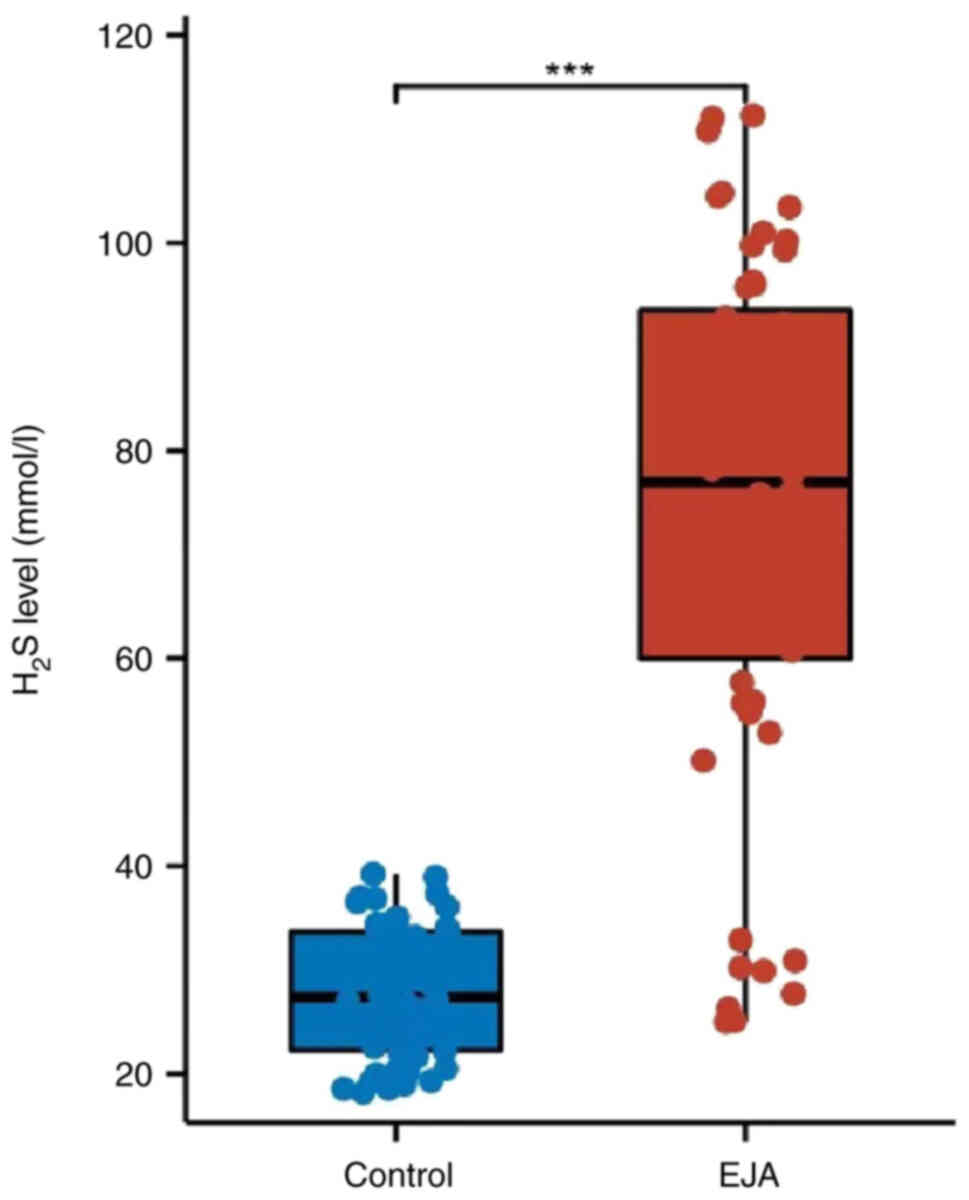
Exhaled H2S is increased in
patients with EJA
The exhaled indicator results showed significantly
higher levels of H2S exhaled in patients with EJA
compared with those in healthy controls (Fig. 1; P<0.05).
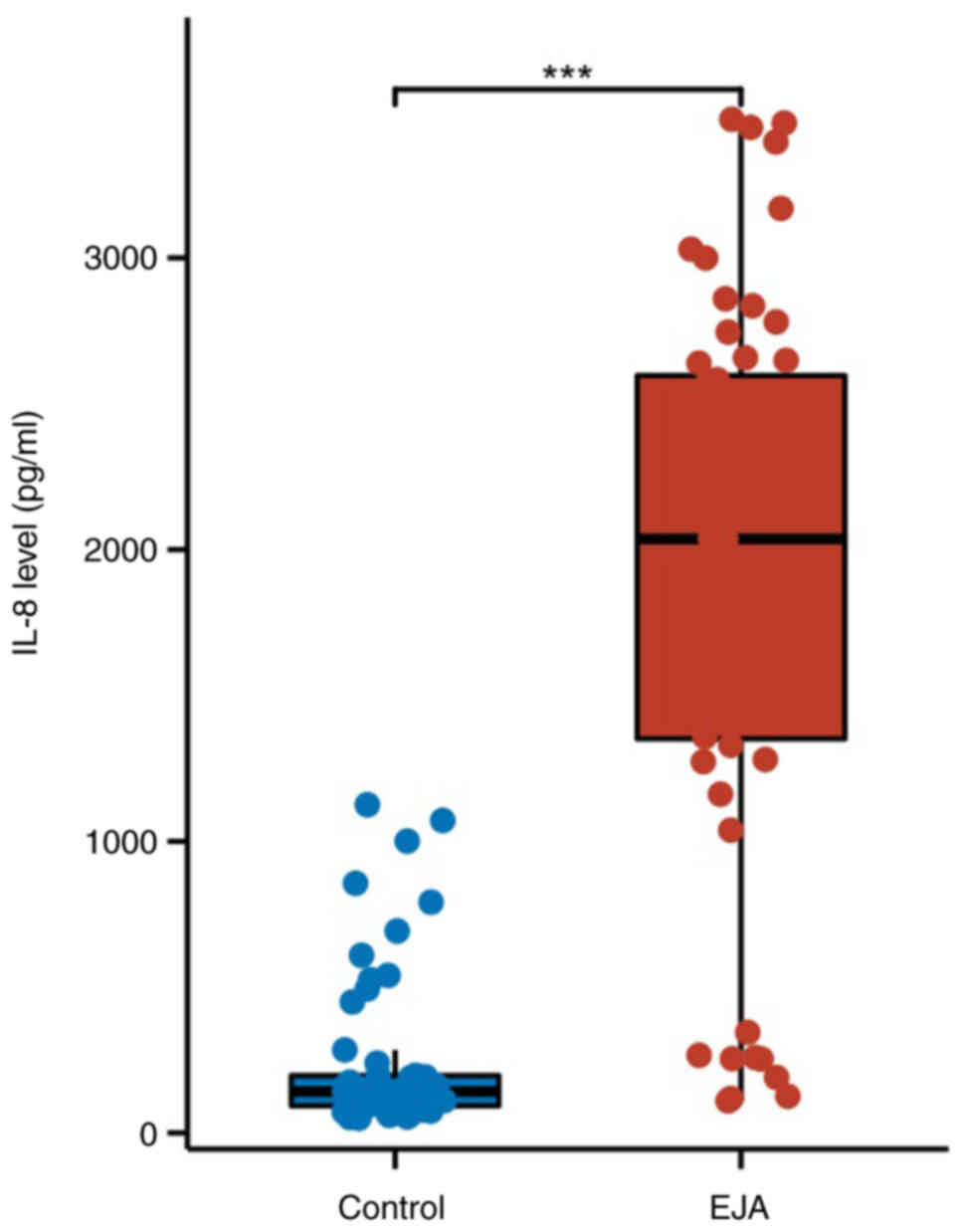
Expression levels of IL-8 in patients
with EJA
The expression levels of IL-8 were determined using
ELISA. The results revealed that the levels of IL-8 in patients
with EJA were significantly higher than those in the control group
(Fig. 2; P<0.05).
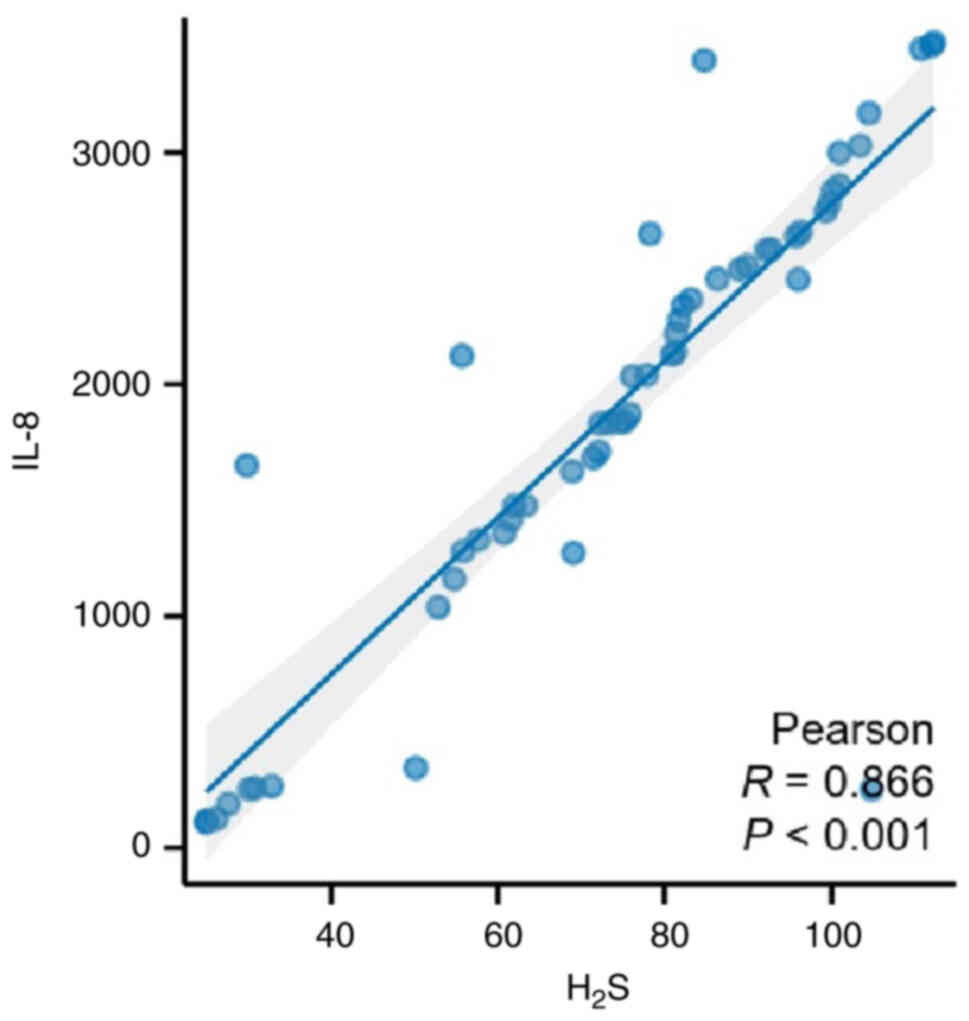
Exhaled H2S is positively
correlated with IL-8 expression in patients with EJA
Pearson's correlation coefficient was used to
analyze the correlation between H2S exhaled and serum
IL-8 in patients with EJA. The results showed that exhaled
H2S was positively correlated with IL-8 expression
(Fig. 3; P<0.001).
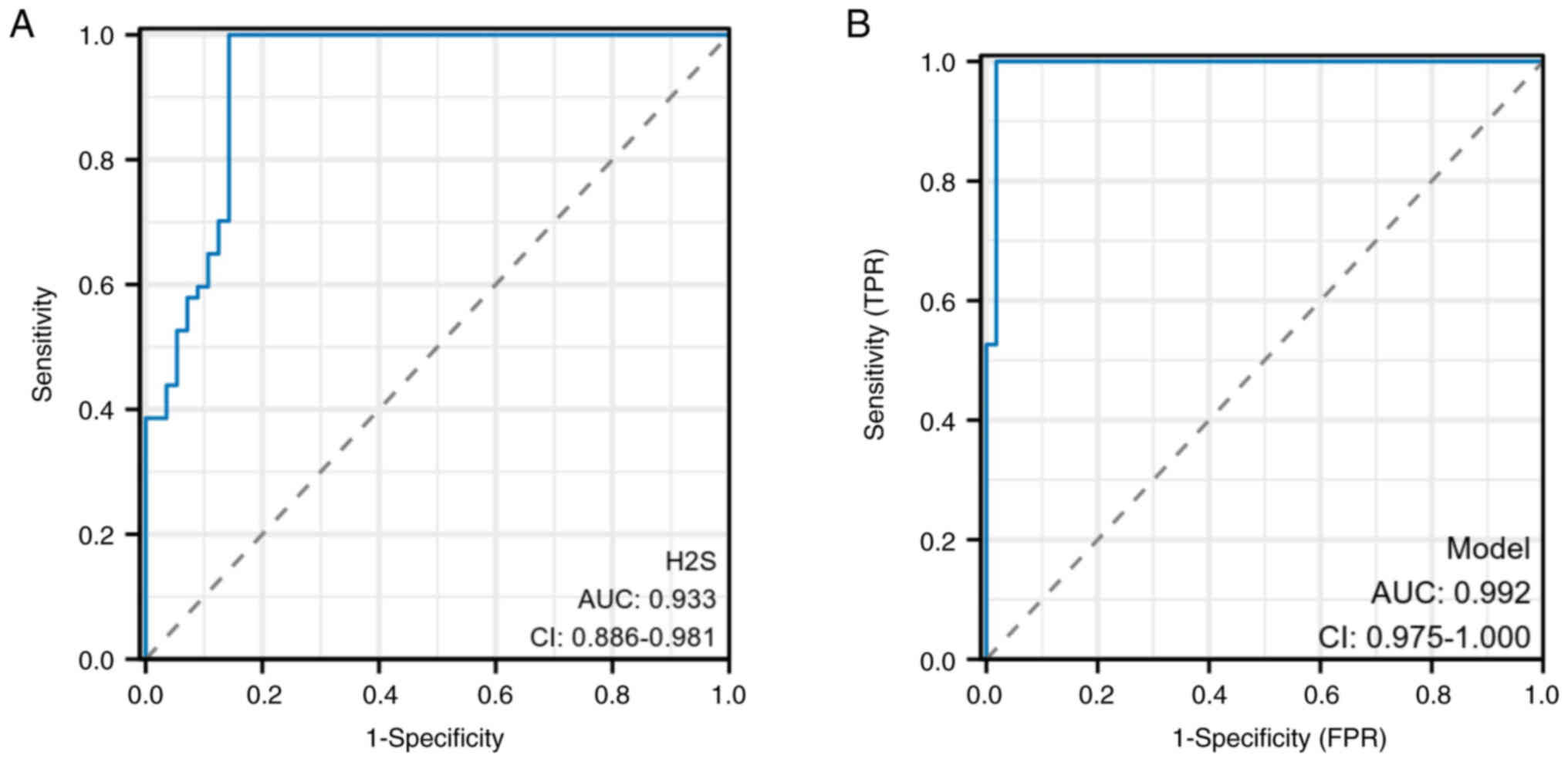
Evaluation of the clinical value of
miR-29c and miR-146a in diagnosing EJA
To evaluate the diagnostic value of exhaled
H2S, ROC curves were generated based on the two groups.
The results revealed that exhaled H2S had high
diagnostic accuracy, with an AUC of 0.933 (sensitivity, 92.9%; and
specificity, 85.7%) (Fig. 4A).
Moreover, exhaled H2S combined with serum IL-8 exhibited
greater diagnostic value with an AUC of 0.992 (sensitivity, 99.1%;
and specificity, 98.2%) (Fig. 4B),
indicating that this combination led to a significant improvement
in diagnostic ability.
Discussion
In the present study, a small quantity of
H2S was detected in patients with EJA, indicating
endogenous origin as there was no H2S detected in the
inhaled and swallowed air of the healthy individuals. There are two
pathways for H2S synthesis in the intestinal tissue: i)
Enzymatic and ii) non-enzymatic. The primary enzymatic pathway
involves endogenous H2S production by CBS and CSE
enzymes and involves fermentation of sulfur-containing amino acids
and sulfates by H2S. Increased production of
H2S is a characteristic pathophysiological feature of
colorectal adenoma (25). Overall
there is an upward trend in the production of H2S among
patients with EJA. Additionally, it has been reported that human
colon cancer cell lines (HCT-116 and SW-480) produce H2S
(26). In the present study,
exhaled H2S levels were examined in patients with EJA
for the first time, revealing higher levels compared with those in
healthy individuals. This may be attributed to an increase in
endogenous H2S synthesis within tumor cells, accompanied
by alterations in gut microbiota metabolism, thus favoring enhanced
production of H2S.
The endogenous H2S molecule is small and
has a solubility in fat-soluble solvents that is five times higher
than that in water, enabling it to freely traverse cell membranes
(5). H2S exists in the
body in two forms, 1/3 is in the form of gas H2S and 2/3
are in the form of NaHS. NaHS is not only the donor of
H2S, but also its precursor and a dynamic balance is
formed between these two forms (27). Within mitochondria, glutathione
catalyzes the oxidation of most H2S into sulfate and
thiosulfate. In the cytoplasm, small amounts of H2S are
converted into less toxic methylmercaptan and dimethylsulfate
through methylation, with these metabolites being excreted by the
kidneys, intestines and lungs. Consequently, under normal
physiological conditions, there is minimal accumulation of
H2S (28). Reportedly,
H2S promotes tumor proliferation, metastasis,
differentiation and neovascularization while providing nutrition
for tumor cell growth and facilitating tumor progression (29,30).
Furthermore, in certain gastrointestinal cancers such as CRC, where
increased levels of H2S occurs due to enzymatic
synthesis within colon cells or release from intestinal
microorganisms followed by oxidation within colon cell mitochondria
(31), upregulation of endogenous
synthase may be responsible for elevated levels of H2S
in cancer cells. The high concentration of colonic cavity-derived
H2S has been suggested to contribute to CRC pathogenesis
(32). However, limited research
exists on the association between H2S and EJA. The
present study discovered that exhaled H2S was
significantly elevated in patients with EJA, suggesting potential
involvement in EJA development. This needs to be further analyzed
and confirmed in future experiments. In addition, the ROC curve in
the present study showed that the AUC, sensitivity and specificity
of H2S diagnosis of EJA were 0.933, 92.9 and 85.7%,
respectively, indicating that H2S has diagnostic
potential for EJA. Serum levels of IL-8 revealed significant
results in the diagnosis of early-stage EJA (17). In the present study, exhaled
H2S combined with patient serum IL-8 was also used to
diagnose early-stage EJA. The results demonstrated that combined
detection was superior to single detection, and it significantly
improved sensitivity, specificity and AUC.
The present study has some limitations. First, the
patients enrolled in this study were all diagnosed with EJA and
whether exhaling H2S could be used for diagnosis in
asymptomatic individuals needs to be analyzed in future studies.
Second, the present study did not analyze whether there were
differences in H2S between patients with stage I and II
EJA. Therefore, whether H2S levels change with different
stages of the disease will be the topic of future research. In
addition, the sample size of this study was limited and may not be
representative of all patients with EJA. The high AUC values in
this study may have potential overfitting. The sample size needs to
be expanded to further clarify the diagnostic value of exhaled
H2S in an EJA population. Therefore, the authors of the
present study anticipate to further expand the sample size in
future experiments. Moreover, further in vitro experiments
are required to confirm the underlying mechanism between
H2S and EJA. Therefore, expanding the sample size in
future studies may further comprehensively identify the role played
by H2S in EJA through additional experiments.
In conclusion, the results indicated that increased
levels of exhaled H2S in patients with EJA may indicate
its involvement in the occurrence and development of EJA. Exhaled
H2S holds promise as an early diagnostic indicator for
EJA. Furthermore, combining H2S and IL-8 detection serum
can enhance diagnostic efficacy. These results provided broader
prospect for the early diagnosis of EJA.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
FL and GL made substantial contributions to the
conception, design and draft of the manuscript. QL contributed in
the acquisition, analysis and interpretation of the data. LW and BZ
made substantial contributions to the conception of the study and
its critical revision for important intellectual content. XS and JJ
made substantial contributions to the conception, design and
supervision of the study, and reviewed and edited the manuscript.
FL and GL confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript. All authors
have participated sufficiently in the work to take public
responsibility for appropriate portions of the content and agreed
to be accountable for all aspects of the work in ensuring that
questions related to its accuracy.
Ethics approval and consent to
participate
The study adhered to ethical standards set by the
responsible committee on human experimentation, namely the Ethics
Committee of Hebei Medical University Fourth Affiliated Hospital,
Shijiazhuang, China; approval no. 2018MEC108), following the
principles outlined in The Declaration of Helsinki. All patients
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hasegawa S and Yoshikawa T: Adenocarcinoma
of the esophagogastric junction: Incidence, characteristics, and
treatment strategies. Gastric Cancer. 13:63–73. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McManus DT, Olaru A and Meltzer SJ:
Biomarkers of esophageal adenocarcinoma and Barrett's esophagus.
Cancer Res. 64:1561–1569. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou Y, Zhang Z, Zhang Z, Wu J, Ren D, Yan
X, Wang Q, Wang Y, Wang H, Zhang J, et al: A rising trend of
gastric cardia cancer in Gansu Province of China. Cancer Lett.
269:18–25. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao JJ and Liu FL: Laparoscopic proximal
gastrectomy and lymph node resection in adenocarcinoma of the
esophagogastric junction. Zhonghua Wei Chang Wai Ke Za Zhi.
25:114–119. 2022.(In Chinese). PubMed/NCBI
|
|
5
|
Guo FF, Yu TC, Hong J and Fang JY:
Emerging roles of hydrogen sulfide in inflammatory and neoplastic
colonic diseases. Front Physiol. 7:1562016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Landry AP, Ballou DP and Banerjee R:
Hydrogen sulfide oxidation by sulfide quinone oxidoreductase.
Chembiochem. 22:949–960. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilkie SE, Borland G, Carter RN, Morton NM
and Selman C: Hydrogen sulfide in ageing, longevity and disease.
Biochem J. 478:3485–3504. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dilek N, Papapetropoulos A, Toliver-Kinsky
T and Szabo C: Hydrogen sulfide: An endogenous regulator of the
immune system. Pharmacol Res. 161:1051192020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang YX, Jing MR, Cai CB, Zhu SG, Zhang
CJ, Wang QM, Zhai YK, Ji XY and Wu DD: Role of hydrogen sulphide in
physiological and pathological angiogenesis. Cell Prolif.
56:e133742023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ye M, Yu M, Yang D, Li J, Wang H, Chen F,
Yu H, Shen T, Zhu Q and Zhou C: Exogenous hydrogen sulfide donor
NaHS alleviates nickel-induced epithelial-mesenchymal transition
and the migration of A549 cells by regulating TGF-β1/Smad2/Smad3
signaling. Ecotoxicol Environ Saf. 195:1104642020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alfaro C, Sanmamed MF, Rodríguez-Ruiz ME,
Teijeira Á, Oñate C, González Á, Ponz M, Schalper KA, Pérez-Gracia
JL and Melero I: Interleukin-8 in cancer pathogenesis, treatment
and follow-up. Cancer Treat Rev. 60:24–31. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsushima K, Yang D and Oppenheim JJ:
Interleukin-8: An evolving chemokine. Cytokine. 153:1558282022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fousek K, Horn LA and Palena C:
Interleukin-8: A chemokine at the intersection of cancer
plasticity, angiogenesis, and immune suppression. Pharmacol Ther.
219:1076922021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McQuaid KR, Laine L, Fennerty MB, Souza R
and Spechler SJ: Systematic review: The role of bile acids in the
pathogenesis of gastro-oesophageal reflux disease and related
neoplasia. Aliment Pharmacol Ther. 34:146–165. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Isomoto H, Saenko VA, Kanazawa Y, Nishi Y,
Ohtsuru A, Inoue K, Akazawa Y, Takeshima F, Omagari K, Miyazaki M,
et al: Enhanced expression of interleukin-8 and activation of
nuclear factor kappa-B in endoscopy-negative gastroesophageal
reflux disease. Am J Gastroenterol. 99:589–597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jenkins GJS, Mikhail J, Alhamdani A, Brown
TH, Caplin S, Manson JM, Bowden R, Toffazal N, Griffiths AP, Parry
JM and Baxter JN: Immunohistochemical study of nuclear
factor-kappaB activity and interleukin-8 abundance in oesophageal
adenocarcinoma; a useful strategy for monitoring these biomarkers.
J Clin Pathol. 60:1232–1237. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Z, Xu H, Yu J, Liu C, Zheng C, Zeng R,
Xu L, Li E, Peng Y and Xu Y: The early diagnostic value of serum
interleukin-8 in esophagogastric junction adenocarcinoma. Cancer
Control. 28:107327482110048832021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang Y, Yan C, Zhao Q, Xu J, Liu Z, Gao J,
Zhu H, Dai Z, Wang D and Tang D: The roles of microbial products in
the development of colorectal cancer: A review. Bioengineered.
12:720–735. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yue T, Li J, Zhu J, Zuo S, Wang X, Liu Y,
Liu J, Liu X, Wang P and Chen S: Hydrogen sulfide creates a
favorable immune microenvironment for colon cancer. Cancer Res.
83:595–612. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ascenção K, Lheimeur B and Szabo C:
Regulation of CyR61 expression and release by 3-mercaptopyruvate
sulfurtransferase in colon cancer cells. Redox Biol. 56:1024662022.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hale VL, Jeraldo P, Mundy M, Yao J, Keeney
G, Scott N, Cheek EH, Davidson J, Greene M, Martinez C, et al:
Synthesis of multi-omic data and community metabolic models reveals
insights into the role of hydrogen sulfide in colon cancer.
Methods. 149:59–68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Zhou J, Wang L and Xie Z: Endogenous
hydrogen sulfide-triggered MOF-based nanoenzyme for synergic cancer
therapy. ACS Appl Mater Interfaces. 12:30213–30220. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu N, Tseng Y, Zhang H and Chen J: The
role of exhaled hydrogen sulfide in the diagnosis of colorectal
adenoma. Can J Infect Dis Med Microbiol. 2021:80463682021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gai JW, Qin W, Liu M, Wang HF, Zhang M, Li
M, Zhou WH, Ma QT, Liu GM, Song W, et al: Expression profile of
hydrogen sulfide and its synthases correlates with tumor stage and
grade in urothelial cell carcinoma of bladder. Urol Oncol.
34:166.e15–e120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao X, Ding L, Xie ZZ, Yang Y, Whiteman M,
Moore PK and Bian JS: A review of hydrogen sulfide synthesis,
metabolism, and measurement: Is modulation of hydrogen sulfide a
novel therapeutic for cancer? Antioxid Redox Signal. 31:1–38. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cai WJ, Wang MJ, Ju LH, Wang C and Zhu YC:
Hydrogen sulfide induces human colon cancer cell proliferation:
Role of Akt, ERK and p21. Cell Biol Int. 34:565–572. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Furne J, Springfield J, Koenig T, DeMaster
E and Levitt MD: Oxidation of hydrogen sulfide and methanethiol to
thiosulfate by rat tissues: A specialized function of the colonic
mucosa. Biochem Pharmacol. 62:255–259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Munteanu C, Turnea MA and Rotariu M:
Hydrogen Sulfide: An emerging regulator of oxidative stress and
cellular homeostasis-a comprehensive one-year review. Antioxidants
(Basel). 12:17372023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khattak S, Rauf MA, Khan NH, Zhang QQ,
Chen HJ, Muhammad P, Ansari MA, Alomary MN, Jahangir M, Zhang CY,
et al: Hydrogen sulfide biology and its role in cancer. Molecules.
27:33892022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shackelford RE, Mohammad IZ, Meram AT, Kim
D, Alotaibi F, Patel S, Ghali GE and Kevil CG: Molecular functions
of hydrogen sulfide in cancer. Pathophysiology. 28:437–456. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin H, Yu Y, Zhu L, Lai N, Zhang L, Guo Y,
Lin X, Yang D, Ren N, Zhu Z and Dong Q: Implications of hydrogen
sulfide in colorectal cancer: Mechanistic insights and diagnostic
and therapeutic strategies. Redox Biol. 59:1026012023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sakuma S, Minamino S, Takase M, Ishiyama
Y, Hosokura H, Kohda T, Ikeda Y and Fujimoto Y: Hydrogen sulfide
donor GYY4137 suppresses proliferation of human colorectal cancer
Caco-2 cells by inducing both cell cycle arrest and cell death.
Heliyon. 5:e022442019. View Article : Google Scholar : PubMed/NCBI
|