Introduction
Hepatocellular carcinoma (HCC), the dominant type of
primary liver cancer, is the sixth most common type of cancer and
third leading cause of cancer-related deaths worldwide (1,2).
Several curative options are available for the treatment of
patients with early-stage HCC, including radiofrequency ablation,
surgical resection and liver transplantation (3). However, over two-thirds of patients
with HCC are diagnosed with unresectable HCC (uHCC) and curative
treatment may be less effective for these patients (4–6).
Various local and systemic therapies are available for uHCC based
on the Barcelona Clinic Liver Cancer Prognosis and Treatment
Strategy (3).
With significant progress in the treatment of HCC,
novel strategies for uHCC treatment continue to emerge.
Transarterial chemoembolization (TACE), tyrosine kinase inhibitors
(TKIs) and immune checkpoint inhibitors are widely recommended for
the treatment of intermediate to advanced HCC (6,7).
Sorafenib was the 1st TKI approved by the Food and Drug
Administration as a first-line treatment for uHCC and can target
multiple signaling pathways, such as the RAF/MEK/ERK pathway, to
achieve anti-angiogenesis (8,9). TACE
has been reported to induce to tumor cell necrosis by selectively
blocking the nutrient-supplying arteries of the tumor and by
delivering high concentrations of chemotherapeutic drugs into the
vessels feeding the tumor (10).
However, TACE has also been reported to induce tumor tissue hypoxia
and tumor angiogenesis, thus increasing the risk of tumor
progression (10,11). Therefore, TKIs that inhibit
angiogenesis may be beneficial when used in combination with TACE
to treat patients with uHCC.
In a phase III multicenter randomized trial,
lenvatinib, a TKI, was reported to have a significantly better
advantage in terms of progression-free survival (PFS), tumor
response and overall survival (OS) compared with sorafenib for the
treatment of patients with uHCC (12). Therefore, lenvatinib has also been
recommended as a first-line treatment for advanced HCC (12). Shimose et al (13) retrospectively studied 171 patients
with intermediate-stage HCC who were refractory to TACE and
received either lenvatinib or sorafenib. The aforementioned study
demonstrated that lenvatinib significantly prolonged the median PFS
time compared with sorafenib or TACE in patients with
TACE-refractory intermediate-stage HCC (lenvatinib, 5.8 months;
sorafenib, 3.8 months; TACE, 2.8 months) (13).
As the efficacy of lenvatinib and sorafenib has
received increased attention, several studies on the use of
combination therapy of lenvatinib or sorafenib with TACE for uHCC
have emerged in recent years. A prospective randomized trial
reported that lenvatinib combined with TACE did not significantly
improve survival outcomes in terms of OS but did improve the
time-to-progression (TTP) of HCC with portal vein tumor thrombus
(14). However, in our previous
meta-analysis, the use of lenvatinib with TACE was significantly
superior compared with sorafenib with TACE, in terms of OS, PFS and
tumor response in patients with uHCC (15). In contradiction to the
aforementioned results, a further network meta-analysis (NMA)
reported no statistically significant difference between the use of
lenvatinib with TACE compared with sorafenib with TACE in terms of
OS (16). A large proportion of
studies included in the aforementioned meta-analyses were
retrospective cohort studies, which could have introduced the
potential risk of selection bias and confounding bias. Therefore,
the present study aimed to perform an NMA based on randomized
controlled trials (RCTs). In particular, landmark phase III
clinical trials were used in the NMA of the present study for the
comparison of all therapies, including combination therapies using
either lenvatinib or sorafenib with TACE, and monotherapies using
TACE, lenvatinib or sorafenib in patients with uHCC.
Materials and methods
Study registration
The NMA performed in the present study complied with
guidelines specified by the Preferred Reporting Items for
Systematic reviews and Meta-Analyses statement (17). The present NMA was registered on the
PROSPERO database (www.crd.york.ac.uk/prospero/) of systematic reviews
with the identification no. CRD42023448995.
Search strategy and eligibility
criteria
The present study systematically searched for
available RCTs in the PubMed (https://pubmed.ncbi.nlm.nih.gov), Embase (www.embase.com), Web of Science (www.webofscience.com) and Cochrane Library (www.cochranelibrary.com) online databases
(Table SI). The paramount search
terms used were: ‘Hepatocellular carcinoma’, ‘liver neoplasms’,
‘chemoembolization’, ‘sorafenib’ and ‘lenvatinib’.
Studies were included in the present NMA if they
fulfilled the following criteria: i) The study population comprised
of patients diagnosed with uHCC; ii) the interventions of interest
included lenvatinib with TACE, sorafenib with TACE, or TACE,
lenvatinib and sorafenib monotherapies; iii) the outcomes assessed
were OS, PFS, objective response rate (ORR) and disease control
rate (DCR); and iv) the publication type was classed as an RCT.
Studies were excluded from the present NMA if they fulfilled the
following criteria: i) Patients with uHCC had received any previous
systemic therapy; and ii) studies lacked adequate outcome data or
did not report outcomes of interest as specified in the
aforementioned inclusion criteria.
Data extraction and quality
assessment
The following data were independently extracted from
each study by two reviewers in a standardized manner: i) General
characteristics (number of patients and nationality of study
population); ii) patient characteristics (age, sex, Child-Pugh
class, viral hepatitis, portal vein tumor thrombosis and
extrahepatic metastasis); iii) clinical outcomes (OS, PFS, ORR and
DCR); and iv) adverse events (AEs). The Cochrane risk-of-bias tool
was used to independently investigate potential biases for each
study (18).
Statistical analysis
The present study used a Bayesian approach to
analyze the relevant data using R (version 4.2.1; RStudio, Inc.)
and Stata/MP (version 17.0; StataCorp LP). Quality assessment was
performed using RevMan (version 5.3; The Cochrane Collaboration).
The primary outcomes (OS and PFS) were presented as hazard ratios
(HR) with corresponding 95% CI. The ORR and DCR were evaluated
according to the modified Response Evaluation Criteria in Solid
Tumors assessment (19) and were
expressed as risk ratios (RR) with the corresponding 95% CI.
Brooks-Gelman-Rubin diagnostics, traces and density plots were used
to assess the model convergence. A total of 50,000 iterations per
chain were performed, of which the first 10,000 iterations were
used for annealing the algorithm to remove the effects of the
initial value. The ranking probabilities of the various treatments
were used to estimate the hierarchy of treatments. A node-splitting
approach was used to assess the local consistency of the models.
The comparison results of the different treatments were expressed
as forest plots. According to Q statistics and the I2 statistic
index, studies with P>0.05 or I2<50% were considered to have
low heterogeneity (20). The
random-effects model was used regardless of the I2 value. P<0.05
was considered to indicate a statistically significant
difference.
Results
Study selection and study
characteristics
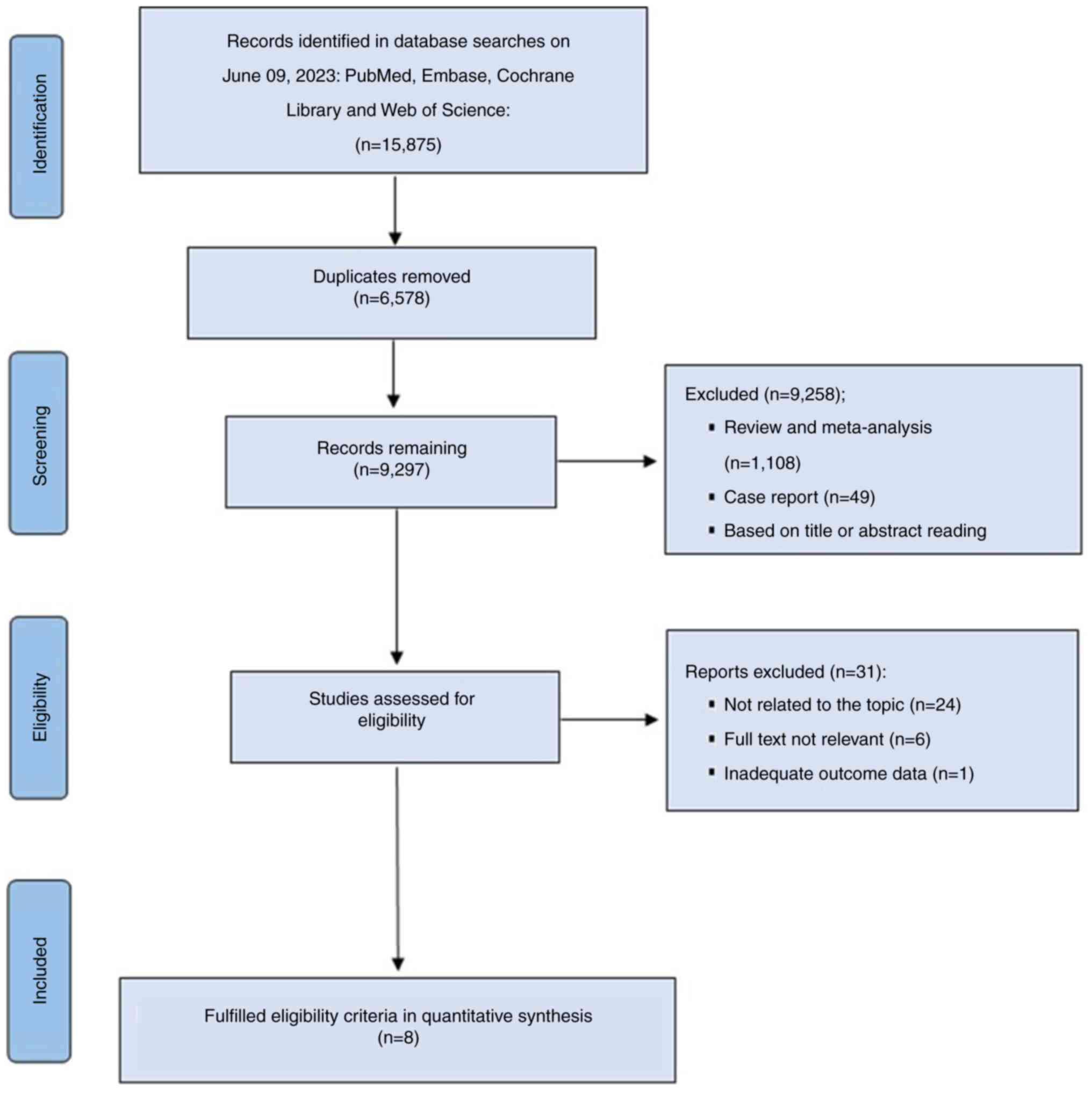
A total of 15,875 relevant records were initially
identified, of which 39 were assessed for eligibility after
removing duplicate articles and screening the titles and abstracts
to ensure they met the inclusion and exclusion criteria. The
present NMA included eight RCTs that fulfilled the eligibility
criteria and screening process for quantitative synthesis: Kudo
et al (21), SPACE (22), TACE-2 (23), REFLECT (12), STAH (24), Ding et al (14), TACTICS (25) and LAUNCH (26) (Fig.
1).
The eight trials included in the present NMA
enrolled 2,929 patients in total (Table
I). Direct comparisons included sorafenib with TACE vs. TACE
monotherapy or in combination with a placebo, lenvatinib vs.
sorafenib, sorafenib with TACE vs. sorafenib monotherapy,
lenvatinib with TACE vs. sorafenib with TACE and lenvatinib with
TACE vs. lenvatinib monotherapy. The majority of patients with uHCC
enrolled in the eight included trials were male and had Child-Pugh
class A liver function and an Eastern Cooperative Oncology Group
Performance Status score of 0.
 | Table I.Demographic characteristics of the
trials included in the network meta-analysis. |
Table I.
Demographic characteristics of the
trials included in the network meta-analysis.
| First author,
year | Trial name | Intervention | Number of
patients | Age, years | Male sex, % | Eastern cooperative
oncology group performance status (0/1), % | Child-pugh class
(A/B), % | Hepatitis B virus,
% | Hepatitis C virus,
% | Barcelona clinic
liver cancer stage (A/B), % | Portal vein tumor
thrombosis, % | Extrahepatic
metastasis, % | (Refs.) |
|---|
| Kudo et al,
2011 | NA | Sorafenib +
TACE | 229 | 69a,b | 76 | 87.8/12.2 | 100/0 | 20.5 | 60.7 | NA | 0 | 0 | (21) |
|
|
| TACE + placebo | 229 | 70a,b | 73.4 | 88.2/11.8 | 100/0 | 22.7 | 64.6 | NA | 0 | 0 |
|
| Lencioni et
al, 2016 | SPACE | Sorafenib +
TACE | 154 | 64.5a | 87.7 | 100/0 | 99.4/0.6 | 35.7 | 25.3 | 0/100 | 0 | 0 | (22) |
|
|
| TACE + placebo | 153 | 63a | 82.4 | 100/0 | 100/0 | 32.7 | 26.8 | 0/100 | 0 | 0 |
|
| Meyer et al,
2017 | TACE-2 | Sorafenib +
TACE | 157 | 65 (57–71)c | 89 | 62/37 | 93/4 | 5 | 12 | NA | NA | 0 | (23) |
|
|
| TACE + placebo | 156 | 68 (63–74)c | 88 | 62/37 | 95/2 | 6 | 9 | NA | NA | 0 |
|
| Kudo et al,
2018 | REFLECT | Lenvatinib | 478 | 63 (20–88)c | 85 | 64/36 | 99/1 | 53 | 19 | NA | 23 | 61 | (12) |
|
|
| Sorafenib | 476 | 62 (22–88)c | 84 | 63/37 | 99/1 | 48 | 26 | NA | 19 | 62 |
|
| Park et al,
2019 | STAH | Sorafenib +
TACE | 170 | 60.2 (9.6)d | 80 | 80/19.4 | 87.1/12.9 | 78.8 | 4.7 | 1.8/22.9 | 40 | 36.5 | (24) |
|
|
| Sorafenib | 169 | 61.3 (9.6)d | 87 | 82.8/16.6 | 86.9/13 | 71 | 9.5 | 0/26 | 37.3 | 34.9 |
|
| Ding et al,
2021 | NA | Lenvatinib +
TACE | 32 | 57 (11)d | 78.1 | 75/25 | 68.8/31.2 | 93.8 | 3.1 | NA | 100 | 40.6 | (14) |
|
|
| Sorafenib +
TACE | 32 | 56 (11)d | 84.4 | 68.8/31.2 | 87.5/12.5 | 90.6 | 9.4 | NA | 100 | 28.1 |
|
| Kudo et al,
2022 | TACTICS | Sorafenib +
TACE | 80 | 72 (36–85)c | 78.8 | 88.8/11.2 | 98.8/1.2 | 12.5 | 47.5 | 33.8/55 | 0 | 0 | (25) |
|
|
| TACE | 76 | 73 (53–86)c | 72.4 | 88.2/11.8 | 93.5/5.6 | 2.6 | 69.7 | 43.4/44.7 | 0 | 0 |
|
| Peng et al,
2023 | LAUNCH | Lenvatinib +
TACE | 170 | 54 (46–64)c | 18.8 | 52.4/47.6 | 100/0 | 87.1 | 2.4 | NA | 71.8 | 55.3 | (26) |
|
|
| Lenvatinib | 168 | 56 (48–63)c | 78.6 | 58.9/41.1 | 100/0 | 85.7 | 3.6 | NA | 69.6 | 56.5 |
|
OS and PFS
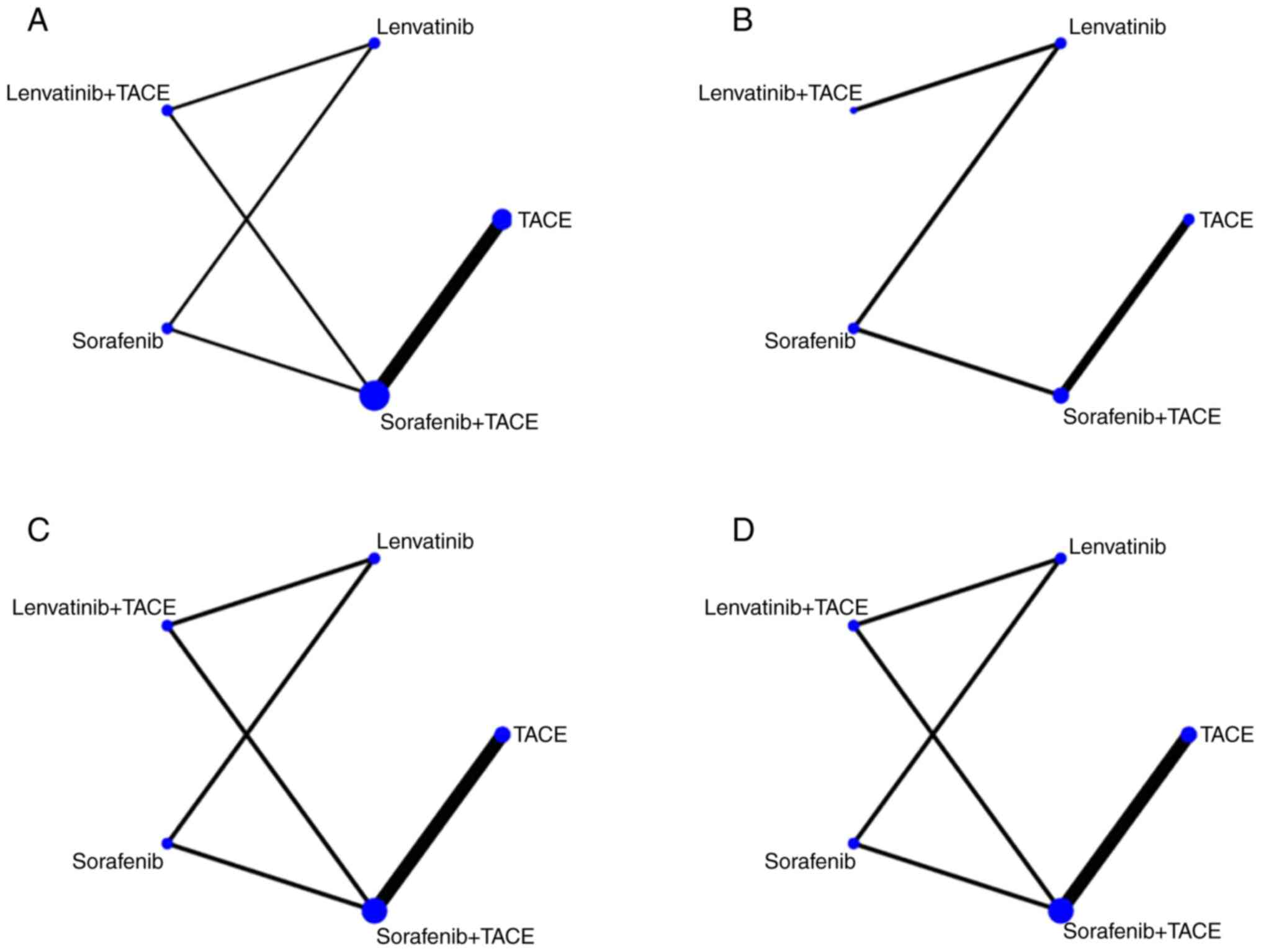
All eight RCTs included in the present NMA reported
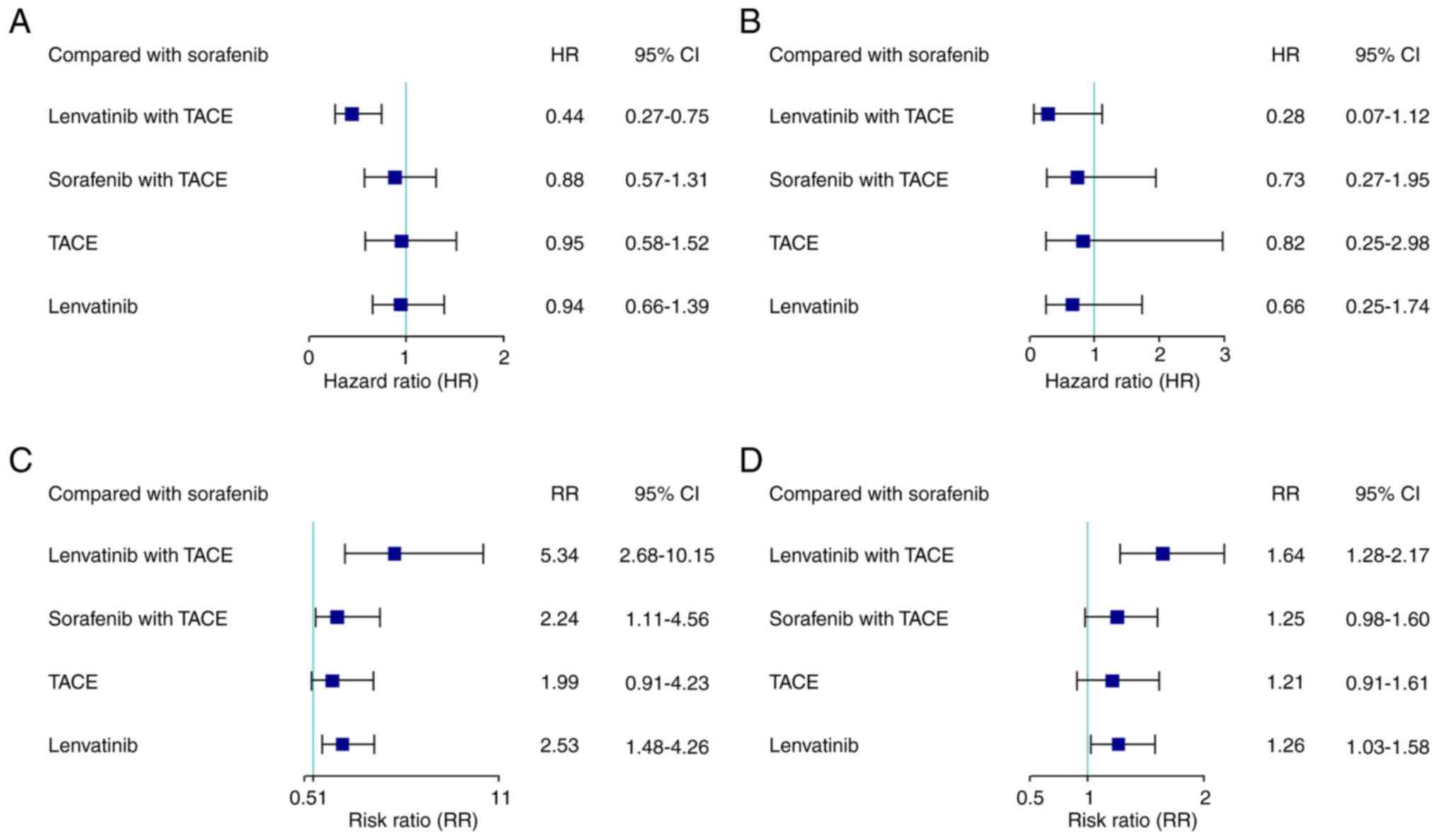
the OS of patients with uHCC as a survival outcome (Fig. 2A). Compared with sorafenib
monotherapy, lenvatinib with TACE demonstrated significantly
increased OS (HR, 0.44; 95% CI, 0.27–0.75), while all other
treatment strategies showed no significant differences in OS
compared with sorafenib monotherapy (Fig. 3A). Moreover, the present study found
that lenvatinib with TACE was associated with a significantly
increased OS compared with sorafenib with TACE, TACE alone and
lenvatinib monotherapy (HR, 0.50; 95% CI, 0.30–0.89; HR, 0.46; 95%
CI, 0.27–0.86; HR, 0.47; 95% CI, 0.31–0.72, respectively; Table II). In addition, a significant
survival advantage was observed between sorafenib with TACE and all
the other monotherapies, including TACE, sorafenib and lenvatinib.
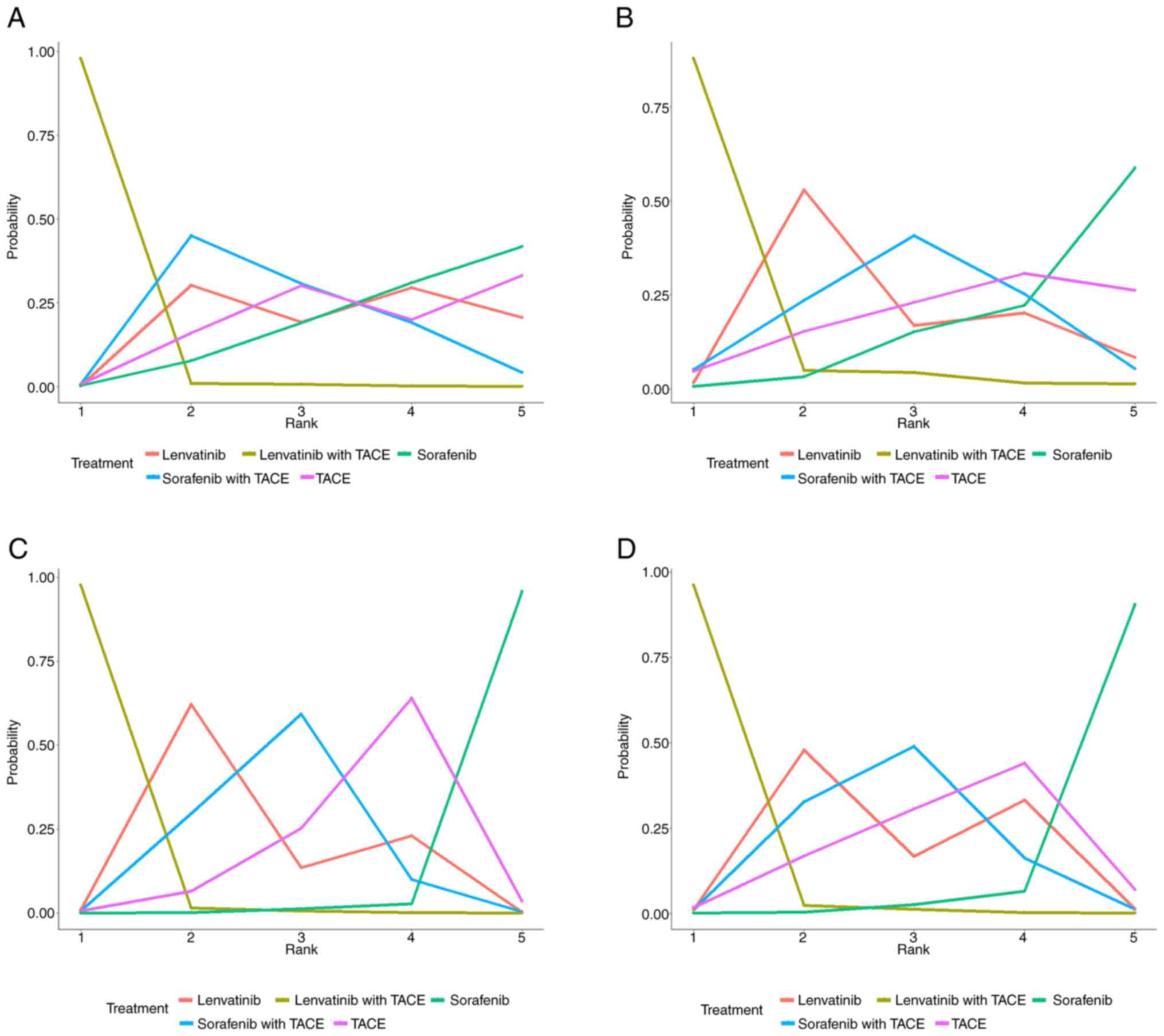
In the treatment ranking analysis, the combination of lenvatinib
with TACE had a 97.92% probability of delivering a increased OS and
was most effective compared with all other treatments examined
(Fig. 4A; Table III). In the present NMA, five RCTs
reported PFS as a survival outcome of patients with uHCC; however,
there were no closed loops in the network (Fig. 2B). No significant differences in PFS
were observed among all treatments examined (Fig. 3B; Table
II). Similarly, lenvatinib in combination with TACE had the
highest likelihood of providing maximal PFS compared with all other
therapies (rank probability, 87.8%; Fig. 4B; Table III).
 | Table II.Indirect comparisons of outcomes
among different treatments. |
Table II.
Indirect comparisons of outcomes
among different treatments.
| A, Overall
survival |
|---|
|
|---|
| Drug treatment | Lenvatinib +
TACE | Sorafenib +
TACE | TACE | Lenvatinib |
|---|
| Sorafenib +
TACE | 0.50
(0.30–0.89) |
|
|
|
| TACE | 0.46
(0.27–0.86) | 0.92
(0.72–1.18) |
|
|
| Lenvatinib | 0.47
(0.31–0.72) | 0.93
(0.55–1.48) | 1.02
(0.57–1.71) |
|
| Sorafenib | 0.44
(0.27–0.75) | 0.88
(0.57–1.31) | 0.95
(0.58–1.52) | 0.94
(0.66–1.39) |
|
| B,
Progression-free survival |
|
| Drug
treatment | Lenvatinib +
TACE | Sorafenib +
TACE | TACE |
Lenvatinib |
|
| Sorafenib +
TACE | 0.39
(0.07–2.14) |
|
|
|
| TACE | 0.35
(0.05–2.11) | 0.89
(0.4–1.76) |
|
|
| Lenvatinib | 0.43
(0.16–1.14) | 1.10
(0.28–4.37) | 1.23
(0.27–6.48) |
|
| Sorafenib | 0.28
(0.07–1.12) | 0.73
(0.27–1.95) | 0.82
(0.25–2.98) | 0.66
(0.25–1.74) |
|
| C, Objective
response rate |
|
| Drug
treatment | Lenvatinib +
TACE | Sorafenib +
TACE | TACE |
Lenvatinib |
|
| Sorafenib +
TACE | 2.36
(1.19–4.75) |
|
|
|
| TACE | 2.66
(1.26–5.77) | 1.13
(0.84–1.57) |
|
|
| Lenvatinib | 2.10
(1.23–3.47) | 0.89
(0.43–1.86) | 0.79
(0.35–1.74) |
|
| Sorafenib | 5.34
(2.68–10.15) | 2.24
(1.11–4.56) | 1.99
(0.91–4.23) | 2.53
(1.48–4.26) |
|
| D, Disease
control rate |
|
| Drug
treatment | Lenvatinib +
TACE | Sorafenib +
TACE | TACE |
Lenvatinib |
|
| Sorafenib +
TACE | 1.31
(1.02–1.73) |
|
|
|
| TACE | 1.35
(1.02–1.86) | 1.03
(0.89–1.21) |
|
|
| Lenvatinib | 1.30
(1.06–1.63) | 0.99
(0.75–1.29) | 0.96 (0.7–1.3) |
|
| Sorafenib | 1.64
(1.28–2.17) | 1.25
(0.98–1.60) | 1.21
(0.91–1.61) | 1.26
(1.03–1.58) |
 | Table III.Analysis of treatment ranking
probability in patients with unresectable hepatocellular
carcinoma. |
Table III.
Analysis of treatment ranking
probability in patients with unresectable hepatocellular
carcinoma.
| A, Overall
survival |
|---|
|
|---|
| Treatment | Rank 1 | Rank 2 | Rank 3 | Rank 4 | Rank 5 |
|---|
| Lenvatinib +
TACE | 0.97915 | 0.00980 | 0.00750 | 0.00265 | 0.00090 |
| Sorafenib +
TACE | 0.00735 | 0.45000 | 0.30815 | 0.19185 | 0.04265 |
| TACE | 0.00760 | 0.15960 | 0.30160 | 0.19970 | 0.33150 |
| Lenvatinib | 0.00200 | 0.30335 | 0.19255 | 0.29515 | 0.20695 |
| Sorafenib | 0.00390 | 0.07725 | 0.19020 | 0.31065 | 0.41800 |
|
| B,
Progression-free survival |
|
|
Treatment | Rank 1 | Rank 2 | Rank 3 | Rank 4 | Rank 5 |
|
| Lenvatinib +
TACE | 0.87800 | 0.04880 | 0.04325 | 0.01610 | 0.01385 |
| Sorafenib +
TACE | 0.05125 | 0.23590 | 0.40665 | 0.25240 | 0.05380 |
| TACE | 0.04740 | 0.15330 | 0.22965 | 0.30735 | 0.26230 |
| Lenvatinib | 0.01660 | 0.52900 | 0.16900 | 0.20185 | 0.08355 |
| Sorafenib | 0.00675 | 0.03300 | 0.15145 | 0.22230 | 0.58650 |
|
| C, Objective
response rate |
|
|
Treatment | Rank 1 | Rank 2 | Rank 3 | Rank 4 | Rank 5 |
|
| Lenvatinib +
TACE | 0.97680 | 0.01565 | 0.00615 | 0.00115 | 0.00025 |
| Sorafenib +
TACE | 0.00725 | 0.29610 | 0.59255 | 0.10045 | 0.00365 |
| TACE | 0.00670 | 0.06525 | 0.25320 | 0.64000 | 0.03485 |
| Lenvatinib | 0.00900 | 0.62180 | 0.13505 | 0.23060 | 0.00355 |
| Sorafenib | 0.00025 | 0.00120 | 0.01305 | 0.02780 | 0.95770 |
|
| D, Disease
control rate |
|
|
Treatment | Rank 1 | Rank 2 | Rank 3 | Rank 4 | Rank 5 |
|
| Lenvatinib +
TACE | 0.96265 | 0.02255 | 0.01230 | 0.00205 | 0.00045 |
| Sorafenib +
TACE | 0.00995 | 0.32730 | 0.48945 | 0.16155 | 0.01175 |
| TACE | 0.01620 | 0.16805 | 0.30575 | 0.44025 | 0.06975 |
| Lenvatinib | 0.01050 | 0.47860 | 0.16665 | 0.33180 | 0.01245 |
| Sorafenib | 0.00070 | 0.00350 | 0.02585 | 0.06435 | 0.90560 |
Tumor response
The present NMA demonstrated that seven of the eight
RCTs reported five different treatment strategies for both ORR and
DCR (Fig. 2C and D). Compared with
sorafenib monotherapy, the combination therapies and lenvatinib and
TACE monotherapies had a significantly higher ORR and DCR, while
lenvatinib with TACE combination therapy demonstrated a
significantly improved tumor response compared with the other
treatments examined (Fig. 3). Based
on the ranking plot, lenvatinib with TACE had the highest
probability of delivering the maximum ORR (rank probability,
97.68%) and DCR (rank probability, 96.27%), followed by lenvatinib
monotherapy, sorafenib with TACE, TACE and sorafenib monotherapy
for both ORR and DCR, respectively (Fig. 4; Table
III).
Adverse events
In the analysis of AEs, reported grade 3/4 AEs in
all included studies were limited and different, so the present NMA
analyzed several common AEs amongst the studies (Table SII). The three most common AEs in
lenvatinib with TACE group were elevated aspartate transaminase
(AST) levels, elevated alanine aminotransferase (ALT) levels and
hypertension. In the sorafenib with TACE group the three most
common AEs were elevated AST, ALT and hypertension. In the
sorafenib monotherapy group, hypertension, hand-foot skin reaction
(HFSR) and elevated AST were the most common AEs. Furthermore,
comparisons of the commonly reported AEs between different
treatments in included trials were conducted (Table SIII). There were no significant
differences in reported AEs between lenvatinib with TACE and
sorafenib with TACE [(HFSR: RR, 0.28; 95% CI, 0.07–1.03);
(diarrhea: RR, 1.03; 95% CI, 0.22–4.80); (hypertension: RR, 3.23;
95% CI, 0.63–26.29); (elevated AST: RR, 0.69; 95% CI, 0.32–1.48);
(abdominal pain: RR, 0.69; 95% CI, 0.31–1.50); (fatigue: HR, 0.68;
95% CI, 0.31–1.46); and (nausea: RR, 0.68; 95% CI, 0.31–1.46)]. The
risk of elevated AST, abdominal pain, fatigue and nausea were
higher in the sorafenib with TACE group compared with the
monotherapies examined. A markedly higher risk of elevated AST,
abdominal pain, fatigue and nausea was observed in the lenvatinib
with TACE group compared with the lenvatinib monotherapy group
(Table SIII).
Quality assessment, convergence,
global inconsistency, local inconsistency and heterogeneity
analyses
Overall, the risk of bias in the included trials was
low for most of the seven domains of source of bias. However,
regarding performance bias, a high risk of bias related to double
blinding in five of the eight trials was observed (REFLECT, STAH,
Ding et al, TACTICS and LAUNCH) (Fig. S1). The open-label assessment of the
aforementioned five trials may have caused a potential
overestimation of treatment efficacy. In all analyses, the
preferred model convergence was confirmed using the
Brooks-Gelman-Rubin method. The potential scale reduction factor
was limited to one, which demonstrated that the analysis possessed
good convergence (Fig. S2). No
global inconsistencies were detected (Table SIV). Local inconsistencies between
direct and indirect comparisons of the outcomes in terms of OS, ORR
and DCR were not observed using the node-splitting method (Fig. S3). However, due to the lack of a
closed loop in the PFS network, an inconsistency assessment was not
applicable in this particular analysis. Given the limited number of
studies included, the analyses did not include funnel plots to
detect publication bias. No heterogeneous outcome measures emerged
between all pairwise comparisons, except for PFS, owing to the lack
of a closed loop in the network (Fig.
S4).
Discussion
With the use of sorafenib and lenvatinib as
first-line treatments in patients with uHCC, combination therapy
with TACE has gradually been applied. A previous meta-analysis
pooled the results of four retrospective studies and one RCT, and
showed that the lenvatinib with TACE combination therapy had better
long-term survival in OS (HR, 0.48; 95% CI, 0.39–0.59) and PFS (HR,
0.47; 95% CI, 0.40–0.56) (27). For
patients with HCC with portal vein tumor thrombus, a single-center
RCT compared the efficacy of lenvatinib with TACE to sorafenib with
TACE combination therapies and reported that the use of lenvatinib
with TACE significantly improved TTP, ORR and DCR but did not
significantly improve OS (14).
Lenvatinib with TACE therapy had a markedly longer OS compared with
the sorafenib with TACE combination therapy (14.5 vs. 10.8 months),
however, the study was limited by a small sample size. Another
retrospective cohort study that enrolled 253 patients with advanced
HCC demonstrated a significantly improved OS and tumor response in
the lenvatinib with TACE group compared with that in the sorafenib
plus TACE group (28). In addition,
our previous meta-analysis reached the same conclusion (15). However, the number of head-to-head
studies comparing the two aforementioned combination therapies, in
particular large-scale RCTs, are limited. In the present study, an
NMA that focused on including RCTs landmark phase III randomized
clinical trials to explore the efficacy of the two combination
therapies was performed. However, several differences among
clinical characteristics could also affect the results of the
present NMA. The proportion of patients with hepatitis B virus or
hepatitis C virus infection, portal vein tumor thrombosis and
extrahepatic metastasis varied greatly among the different included
trials, which could have led to potential heterogeneity. The Ding
et al (14) trial was the
only direct comparison between the two combination therapy groups,
however the sample size of this trial was relatively small thus
limiting the accuracy of the survival outcome analysis and the
overall results. Due to the control of included trials (focus on
two combination therapies and their monotherapy), which excluded
the studies on treatments unrelated to the present study resulting
in a reduction in possible confounding factors, the heterogeneity
analysis did not detect significant heterogeneity.
A recent NMA included 10 RCTs and 35 cohort studies
and compared the efficacy and safety of TKIs in combination with
TACE in the treatment of uHCC (16). The lenvatinib with TACE combination
therapy had a significantly longer PFS (HR, 0.53; 95% CI,
0.32–0.88) but not OS (HR, 0.88; 95% CI, 0.65–1.13), when compared
with sorafenib with TACE group. However, significant inconsistency
was observed between direct and indirect comparisons of OS between
the two treatment groups. Moreover, heterogeneity in comparisons
between the different treatment groups had not been reported.
Furthermore, the inclusion of different types of studies, both RCT
and retrospective cohort studies in the aforementioned NMA, may
have contributed to a potential risk of reduced similarity and
transitivity. Therefore, the credibility and accuracy of the
results presented in the aforementioned NMA were limited. Another
NMA comparing TKIs in combination with TACE included two RCTs and
39 cohort studies (29). No
significant difference was found between lenvatinib with TACE and
sorafenib with TACE, in terms of OS (HR, 0.54; 95% CI, 0.26–1.07).
High heterogeneity (consistency model, I2=69%; inconsistency model,
I2=66%) was observed in OS under the fixed-effects model. The
commonality between the two aforementioned NMAs was that non-RCTs
accounted for the majority of the included studies, which may be
the underlying cause of the inaccurate results (16,29).
In addition, a previous NMA included 23 RCTs
comparing the combination of different first-line TKIs and
transarterial therapies in uHCC and the pooled outcomes showed that
lenvatinib with TACE did not provide any significant advantage in
terms of OS, PFS, TTP, ORR or DCR (30). In addition, no inconsistency was
observed, but heterogeneity was observed in several pairwise
comparisons. The results of the pooled outcomes were inconsistent
with those of previous head-to-head studies, including RCTs and
real-world cohort studies (14,28,31,32).
Therefore, a possible reason may be that this NMA included too many
treatment types (additional inclusion of hepatic arterial infusion
chemotherapy, selective internal radiation therapy and their
combination therapy with sorafenib), which could have led to a
heterogeneous emergence and unavoidable confounding factors, thus
decreasing the level of evidence of pooled results of the mixed
comparisons. The present NMA, by contrast, mainly included landmark
phase III RCTs closely related to target treatments with low
heterogeneity and good global and local consistency. Previous
research on combination therapies of TACE and TKIs have mainly
focused on sorafenib with TACE, and the currently available
literature analyzing the efficacy of lenvatinib with TACE for uHCC
treatment was limited. Even if the present NMA had expanded the
scope of types of other combination treatments, the further
additional closed loops may not have significantly enhanced the
robustness of the present model or have had a significant impact
comparing the efficacy between lenvatinib with TACE and sorafenib
with TACE, but instead may have increased the risk of biases. More
large-scale RCTs are needed to further confirm the efficacy of
lenvatinib or sorafenib with TACE combination therapies with
uHCC.
The present study compared lenvatinib or sorafenib
with TACE based only on RCTs. We narrowed down the scope of the
included studies to include only lenvatinib, sorafenib, TACE and
their combination therapies to reduce possible confounding bias and
heterogeneity, without oversimplification of the network. The
results of the present study demonstrated that the combination of
lenvatinib with TACE was significantly superior in terms of OS, ORR
and DCR in the treatment of patients with uHCC. The present results
were similar to those of our previous meta-analysis (15). However, in the present NMA, no
significant difference in PFS between lenvatinib with TACE and
sorafenib with TACE was observed. Given the lack of closed loops in
the present NMA in terms of PFS, this result should be interpreted
with caution. In addition, global inconsistency, local
inconsistency and heterogeneity were not observed in the present
NMA. By contrast, sorafenib with TACE ranked 3rd for PFS, ORR and
DCR, while lenvatinib monotherapy ranked 2nd. Furthermore, there
were no significant differences in the survival advantage between
sorafenib with TACE and lenvatinib monotherapy in the present NMA.
Similar results have been reported by other studies (16,30,33).
However, considering the increased survival outcomes of lenvatinib
monotherapy compared with sorafenib monotherapy and the lack of a
direct comparison between lenvatinib and sorafenib with TACE in the
currently available studies, more head-to-head research is needed
to further explore the superiority of survival outcomes (12,34).
The analysis of AEs in the present study showed no
difference between lenvatinib with TACE and sorafenib with TACE
regarding grade 3/4 AEs. Similarly, according to the NMA by Long et
al, no significant difference was reported between the two
combination therapies in terms of all-grade AEs or ≥ grade 3 AEs
(16). However, due to lack of the
data regarding total AEs in the SPACE, TACTICS and LAUNCH trials,
the present NMA only compared separate AEs, and total AE analysis
was not performed. In the REFLECT trial (12), hypertension and HFSR were the most
common AEs. Sorafenib showed a higher rate of HFSR (11 vs. 3%) and
a lower rate of hypertension (14 vs. 23%) than lenvatinib. In our
previous meta-analysis, there were significant differences
regarding HFSR (HR, 0.51, 95%; CI, 0.27–0.95) and hypertension (HR,
3.05; 95% CI, 1.45–6.39) between the two combination therapy
groups, and the differences in incidence of HFSR and hypertension
were similar to results of the REFLECT trial (12,15).
However, the present NMA found no significant difference regarding
HFSR and hypertension between the two combination therapy groups.
In the RCT conducted by Ding et al (14), the incidence of hypertension in the
lenvatinib with TACE group was notably higher compared with the
sorafenib with TACE group, but no differences were observed
regarding total AEs, grade 3/4 AEs or HFSR. The occurrence of HFSR
and hypertension was attributed to lenvatinib and sorafenib
treatment in the aforementioned RCT, but the different results
regarding the incidence of HFSR and hypertension between the
present NMA and our previous meta-analysis may be attributed to the
involvement of real-world studies. Therefore, head-to-head RCTs are
needed to compare the two combination therapies. The present
analysis of the included studies demonstrated that the incidence of
total AEs and grade 3/4 AEs of the combination therapy group were
more likely to be significantly higher compared with those of the
monotherapy group, and most AEs could be controlled by the use of
symptomatic treatment.
TKIs inhibit tumor growth and angiogenesis mainly by
targeting the VEGFR, platelet-derived growth factor receptor and
EGFR signaling pathways (35,36).
Although TACE can cause necrosis of liver tumor cells by
selectively blocking the nutrient arteries for HCC cells and
depositing chemotherapy drugs in tumor cells, TACE can also create
a hypoxic tumor microenvironment, which leads to the upregulation
of VEGF, fibroblast growth factor (FGF) and angiopoietin-2,
increasing the risk for cancer recurrence and metastasis (11,37,38). A
previous study administered sorafenib treatment combined with TACE
for patients with HCC and the results showed that the circulating
levels of plasma VEGF did not significantly increase after
combination therapy (39). A phase
II single-arm trial that enrolled 50 patients with uHCC showed
promising survival efficacy and manageable safety with the
combination of sorafenib with TACE therapy (40). Previous studies have indicated that
lenvatinib possesses a stronger affinity for VEGFR2 and inhibits
more targets, including FGF, compared with sorafenib, which results
in a better efficacy of lenvatinib in combination with TACE
(28,41–43).
Another factor contributing to the superior efficacy of lenvatinib
with TACE rather than sorafenib with TACE therapy is the
therapeutic advantage of lenvatinib monotherapy over sorafenib
monotherapy, in that lenvatinib targets more signaling pathways,
including FGFR-MAPK, ERK/MAPK and EGFR-PI3K-AKT (44–46).
Notably, a novel combination therapy of TACE with lenvatinib and
programmed death-1 inhibitors has emerged as a treatment for
patients with uHCC, and previous meta-analysis showed that this
triple combination therapy has achieved a superior OS advantage and
better tumor response than lenvatinib with TACE (47).
Nevertheless, the present NMA has some limitations.
First, the number of included studies was limited; therefore,
publication bias was not analyzed which may have caused a potential
overestimation of positive results. Second, due to the lack of AE
comparisons, the results were insufficient for guidance of clinical
treatment strategy selection in terms of safety. Third, although
significant heterogeneity was not observed, potential confounding
factors may have originated from the region, portal vein invasion,
extrahepatic metastasis and etiology that were overlooked due to
the limited report on the aforementioned factors for inclusion in
the study.
The present NMA showed that combination therapy of
lenvatinib with TACE had significantly increased treatment efficacy
compared with sorafenib with TACE, TACE or sorafenib and lenvatinib
monotherapy in terms of OS, ORR and DCR. Therefore, the results of
the present NMA indicated that lenvatinib with TACE may be
potentially preferrable in the consideration of TACE treatment in
combination with either lenvatinib or sorafenib, or their
monotherapy, for patients with uHCC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the Bureau of Science and Technology
Nanchong City (grant nos. 22SXQT0052 and 22SXQT0057).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YL, XL and PY conceived and designed the study. YL,
XL and JuL acquired patient data. JuL, LY, SW, JiL, HG and TM
analyzed and interpreted the data. YL, XL, PY, HG and TM drafted
and revised the manuscript. All authors read and approved the final
version of the manuscript. YL, XL and PY confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vogel A, Meyer T, Sapisochin G, Salem R
and Saborowski A: Hepatocellular carcinoma. Lancet. 400:1345–1362.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reig M, Forner A, Rimola J, Ferrer-Fàbrega
J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V,
Salem R, et al: BCLC strategy for prognosis prediction and
treatment recommendation: The 2022 update. J Hepatol. 76:681–693.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park JW, Chen M, Colombo M, Roberts LR,
Schwartz M, Chen PJ, Kudo M, Johnson P, Wagner S, Orsini LS and
Sherman M: Global patterns of hepatocellular carcinoma management
from diagnosis to death: The BRIDGE study. Liver Int. 35:2155–2166.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Benson AB, D'Angelica MI, Abbott DE, Anaya
DA, Anders R, Are C, Bachini M, Borad M, Brown D, Burgoyne A, et
al: Hepatobiliary cancers, version 2.2021, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 19:541–565. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang C, Zhang H, Zhang L, Zhu AX, Bernards
R, Qin W and Wang C: Evolving therapeutic landscape of advanced
hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol.
20:203–222. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singal AG, Llovet JM, Yarchoan M, Mehta N,
Heimbach JK, Dawson LA, Jou JH, Kulik LM, Agopian VG, Marrero JA,
et al: AASLD practice guidance on prevention, diagnosis, and
treatment of hepatocellular carcinoma. Hepatology. 78:1922–1965.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sergio A, Cristofori C, Cardin R, Pivetta
G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A and
Farinati F: Transcatheter arterial chemoembolization (TACE) in
hepatocellular carcinoma (HCC): The role of angiogenesis and
invasiveness. Am J Gastroenterol. 103:914–921. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiong XX, Qiu XY, Hu DX and Chen XQ:
Advances in hypoxia-mediated mechanisms in hepatocellular
carcinoma. Mol Pharmacol. 92:246–255. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shimose S, Kawaguchi T, Tanaka M, Iwamoto
H, Miyazaki K, Moriyama E, Suzuki H, Niizeki T, Shirono T, Nakano
M, et al: Lenvatinib prolongs the progression-free survival time of
patients with intermediate-stage hepatocellular carcinoma
refractory to transarterial chemoembolization: A multicenter cohort
study using data mining analysis. Oncol Lett. 20:2257–2265. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ding X, Sun W, Li W, Shen Y, Guo X, Teng
Y, Liu X, Zheng L, Li W and Chen J: Transarterial chemoembolization
plus lenvatinib versus transarterial chemoembolization plus
sorafenib as first-line treatment for hepatocellular carcinoma with
portal vein tumor thrombus: A prospective randomized study. Cancer.
127:3782–3793. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu JN, Li JJ, Yan S, Zhang GN and Yi PS:
Transarterial chemoembolization combined with lenvatinib versus
transarterial chemoembolization combined with sorafenib for
unresectable hepatocellular carcinoma: A systematic review and
meta-analysis. Front Oncol. 13:10747932023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Long J, Chen B and Liu Z: Comparative
efficacy and safety of molecular targeted agents combined with
transarterial chemoembolization in the treatment of unresectable
hepatocellular carcinoma: A network meta-analysis. Front Oncol.
13:11794312023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343:d59282011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kudo M, Imanaka K, Chida N, Nakachi K, Tak
WY, Takayama T, Yoon JH, Hori T, Kumada H, Hayashi N, et al: Phase
III study of sorafenib after transarterial chemoembolisation in
Japanese and Korean patients with unresectable hepatocellular
carcinoma. Eur J Cancer. 47:2117–2127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lencioni R, Llovet JM, Han G, Tak WY, Yang
J, Guglielmi A, Paik SW, Reig M, Kim DY, Chau GY, et al: Sorafenib
or placebo plus TACE with doxorubicin-eluting beads for
intermediate stage HCC: The SPACE trial. J Hepatol. 64:1090–1098.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meyer T, Fox R, Ma YT, Ross PJ, James MW,
Sturgess R, Stubbs C, Stocken DD, Wall L, Watkinson A, et al:
Sorafenib in combination with transarterial chemoembolisation in
patients with unresectable hepatocellular carcinoma (TACE 2): A
randomised placebo-controlled, double-blind, phase 3 trial. Lancet
Gastroenterol Hepatol. 2:565–575. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park JW, Kim YJ, Kim DY, Bae SH, Paik SW,
Lee YJ, Kim HY, Lee HC, Han SY, Cheong JY, et al: Sorafenib with or
without concurrent transarterial chemoembolization in patients with
advanced hepatocellular carcinoma: The phase III STAH trial. J
Hepatol. 70:684–691. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kudo M, Ueshima K, Ikeda M, Torimura T,
Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, et al:
Final results of TACTICS: A randomized, prospective trial comparing
transarterial chemoembolization plus sorafenib to transarterial
chemoembolization alone in patients with unresectable
hepatocellular carcinoma. Liver Cancer. 11:354–367. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao
C, Huang F, Tang R, Cheng Y, Huang Z, et al: Lenvatinib combined
with transarterial chemoembolization as first-line treatment for
advanced hepatocellular carcinoma: A phase III, randomized clinical
trial (LAUNCH). J Clin Oncol. 41:117–127. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan D, Liu H, Ma X, Qu P, Cao M, Qin X,
Tang J, Pan R, Huang Q and Han Z: Safety and efficacy of TACE +
lenvatinib in treating advanced hepatocellular carcinoma: A
systematic review and meta-analysis. J Gastrointestin Liver Dis.
32:222–229. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xue M, Wu Y, Zhu B, Zou X, Fan W and Li J:
Advanced hepatocellular carcinoma treated by transcatheter arterial
chemoembolization with drug-eluting beads plus lenvatinib versus
sorafenib, a propensity score matching retrospective study. Am J
Cancer Res. 11:6107–6118. 2021.PubMed/NCBI
|
|
29
|
Zhang Z, Wu Y, Zheng T, Chen X, Chen G,
Chen H, Guo X, Zheng S, Xie X and Zhang B: Efficacy of
transarterial chemoembolization combined with molecular targeted
agents for unresectable hepatocellular carcinoma: A network
meta-analysis. Cancers (Basel). 14:37102022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu L, Lin J, Shi X and Wang A: Efficacy of
transarterial therapy combined with first-line tyrosine kinase
inhibitors for unresectable hepatocellular carcinoma: A network
meta-analysis. World J Surg Oncol. 21:2082023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang JX, Chen YX, Zhou CG, Liu J, Liu S,
Shi HB and Zu QQ: Transarterial chemoembolization combined with
lenvatinib versus transarterial chemoembolization combined with
sorafenib for unresectable hepatocellular carcinoma: A comparative
retrospective study. Hepatol Res. 52:794–803. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang B, Jie L, Yang T, Chen M, Gao Y,
Zhang T, Zhang Y, Wu H and Liao Z: TACE plus lenvatinib versus TACE
plus sorafenib for unresectable hepatocellular carcinoma with
portal vein tumor thrombus: A prospective cohort study. Front
Oncol. 11:8215992021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
An J, Han S, Kim HI and Shim JH: Ranking
of transarterial and targeted therapies for advanced hepatocellular
carcinoma in the era of immuno-oncology: A network meta-analysis of
randomized sorafenib-controlled trials. Hepatol Commun.
6:2886–2900. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luo J, Gao B, Lin Z, Fan H, Ma W, Yu D,
Yang Q, Tian J, Yang X and Li B: Efficacy and safety of lenvatinib
versus sorafenib in first-line treatment of advanced hepatocellular
carcinoma: A meta-analysis. Front Oncol. 12:10107262022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mou L, Tian X, Zhou B, Zhan Y, Chen J, Lu
Y, Deng J, Deng Y, Wu Z, Li Q, et al: Improving outcomes of
tyrosine kinase inhibitors in hepatocellular carcinoma: New data
and ongoing trials. Front Oncol. 11:7527252021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang L and Fu L: Mechanisms of resistance
to EGFR tyrosine kinase inhibitors. Acta Pharm Sin B. 5:390–401.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schicho A, Hellerbrand C, Krüger K, Beyer
LP, Wohlgemuth W, Niessen C, Hohenstein E, Stroszczynski C, Pereira
PL and Wiggermann P: Impact of different embolic agents for
transarterial chemoembolization (TACE) procedures on systemic
vascular endothelial growth factor (VEGF) levels. J Clin Transl
Hepatol. 4:288–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Petrillo M, Patella F, Pesapane F, Suter
MB, Ierardi AM, Angileri SA, Floridi C, de Filippo M and
Carrafiello G: Hypoxia and tumor angiogenesis in the era of
hepatocellular carcinoma transarterial loco-regional treatments.
Future Oncol. 14:2957–2967. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dufour JF, Hoppe H, Heim MH, Helbling B,
Maurhofer O, Szucs-Farkas Z, Kickuth R, Borner M, Candinas D and
Saar B: Continuous administration of sorafenib in combination with
transarterial chemoembolization in patients with hepatocellular
carcinoma: Results of a phase I study. Oncologist. 15:1198–1204.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Park JW, Koh YH, Kim HB, Kim HY, An S,
Choi JI, Woo SM and Nam BH: Phase II study of concurrent
transarterial chemoembolization and sorafenib in patients with
unresectable hepatocellular carcinoma. J Hepatol. 56:1336–1342.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Okamoto K, Ikemori-Kawada M, Jestel A, von
König K, Funahashi Y, Matsushima T, Tsuruoka A, Inoue A and Matsui
J: Distinct binding mode of multikinase inhibitor lenvatinib
revealed by biochemical characterization. ACS Med Chem Lett.
6:89–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Matsuki M, Hoshi T, Yamamoto Y,
Ikemori-Kawada M, Minoshima Y, Funahashi Y and Matsui J: Lenvatinib
inhibits angiogenesis and tumor fibroblast growth factor signaling
pathways in human hepatocellular carcinoma models. Cancer Med.
7:2641–2653. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yamamoto Y, Matsui J, Matsushima T,
Obaishi H, Miyazaki K, Nakamura K, Tohyama O, Semba T, Yamaguchi A,
Hoshi SS, et al: Lenvatinib, an angiogenesis inhibitor targeting
VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft
models associated with microvessel density and pericyte coverage.
Vasc Cell. 6:182014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hoshi T, Watanabe Miyano S, Watanabe H,
Sonobe RMK, Seki Y, Ohta E, Nomoto K, Matsui J and Funahashi Y:
Lenvatinib induces death of human hepatocellular carcinoma cells
harboring an activated FGF signaling pathway through inhibition of
FGFR-MAPK cascades. Biochem Biophys Res Commun. 513:1–7. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
He X, Hikiba Y, Suzuki Y, Nakamori Y,
Kanemaru Y, Sugimori M, Sato T, Nozaki A, Chuma M and Maeda S: EGFR
inhibition reverses resistance to lenvatinib in hepatocellular
carcinoma cells. Sci Rep. 12:80072022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Romei C, Ciampi R and Elisei R: A
comprehensive overview of the role of the RET proto-oncogene in
thyroid carcinoma. Nat Rev Endocrinol. 12:192–202. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu J, Wei S, Yang L, Yu J, Yan D and Yi
P: Efficacy and safety of transarterial chemoembolization plus
lenvatinib with or without programmed death-1 inhibitors in the
treatment of unresectable hepatocellular carcinoma: A systematic
review and meta-analysis. J Cancer Res Clin Oncol. 149:14451–14461.
2023. View Article : Google Scholar : PubMed/NCBI
|